Application of double-tube negative pressure drainage in repair of refractory wounds
Abstract
Objective
To observe the application effect of double-tube negative pressure drainage in the repair of refractory wounds.
Methods
From January 2020 to April 2023, 50 patients undergoing refractory wound repair in the Department of Burn and Plastic Surgery of Jingjiang People's Hospital, Jiangsu were selected. According to different treatment methods, they were divided into an observation group and a control group, with 25 patients in each group. The observation group was treated with double-tube negative pressure drainage inside and outside the wound, while the control group was treated with negative pressure drainage inside the wound. By two-week observation, the wound healing status and complication rate after treatment, as well as the wound bacterial clearance rate, wound pain score and patient satisfaction 3, 7 and 14 d after treatment were compared between the two groups. Statistical analysis was carried out to determine the efficacy.
Results
After treatment for two weeks, the observation group showed a higher grade A healing rate (92% vs. 60%, X2 = 7.018, P = 0.008), a higher wound bacterial clearance rate (100% vs. 76%, X2 = 6.818, P = 0.03), a lower pain score (1.44 ± 0.51 vs. 2.36 ± 0.49, t = -6.53, P < 0.01), a higher patient satisfaction score (8.48 ± 0.96 vs. 6.64 ± 0.95, t = 6.80, P < 0.01), and a lower complication rate (8% vs. 40%, X2= 7.018, P = 0.008) compared with the control group.
Conclusion
Double-tube negative pressure drainage has a significant application effect in the repair of refractory wounds. It can accelerate wound healing, shorten treatment time, effectively eliminate bacteria, relieve wound infection, reduce complications, alleviate pain and improve patient satisfaction. Therefore, the application, promotion and research of double-tube negative pressure drainage should be strengthened in clinical practice.
1 INTRODUCTION
Chronic refractory wounds refer to those that are not fully healed according to biological laws after treatment for more than 1 month,1 or those that shrink by <15% per week or <50% per month,2 also known as chronic wounds. Chronic refractory wounds are a long-term treatment problem that is difficult to solve in surgery, resulting in a high disability rate.3 Due to the high therapeutic difficulty, prolonged course of disease and high treatment costs, chronic refractory wounds have become a chronic disease that seriously reduces patients’ quality of life, endangers their physical and mental health, and increases social and family burdens.1, 4 The main goal of treating chronic refractory wounds is to achieve wound healing in the shortest possible time.5
At present, there are a variety of methods to treat refractory wounds, including physical therapy using localized hyperbaric oxygen therapy on legs,6 ultrasound, infrared ray, laser and electrical stimulation, negative pressure drainage, growth factor therapy represented by fibroblast growth factor (FGF) and epidermal growth factor (EGF), traditional Chinese medicine therapy represented by Jingwanhong ointment, novel wound dressings represented by synthetic polymer dressings and artificial biological dressings, and biotherapy with platelet-rich plasma, stem cells and genes.7 Doppler ultrasound can not only evaluate large blood vessels, but also high frequency ultrasound (>18 Mhz and above) is useful in evaluating skin microvessels.8 Among them, vacuum sealing drainage (VSD) with double-tube internal and external negative pressure is a relatively new therapeutic concept, which uses a medical foam dressing for wrapping and multi-side hole drainage, and then utilizes a permeable adhesive film to seal the drainage area, connecting to a negative pressure source to form an efficient drainage system. It adopts negative pressure suction both inside and outside the wound after debridement and suturing, so that the sutured wound can be healed successfully. Therefore, it has significant advantages in infection control and wound repair.9, 10
A technique similar to VSD is negative pressure drainage inside the wound. Compared with VSD, negative pressure drainage inside the wound only provides drainage inside the wound, which can prevent bacteria from invading inside of the wound, but cannot guarantee the external safety of the wound.11 There is currently little comparison between these two techniques. On this basis, this study included patients with refractory wound repair from January 2020 to April 2023. In addition, the wound healing status, wound bacterial clearance rate, pain score, patient satisfaction and complication rate of the patients treated with the two methods were compared, and the application effect of double-tube negative pressure drainage inside and outside the wound on refractory wounds was explored.
2 MATERIALS AND METHODS
2.1 Clinical data and grouping
From January 2020 to April 2023, 50 patients with refractory wounds admitted to the Department of Burn and Plastic Surgery in Jingjiang People's Hospital, Jiangsu were selected as subjects. According to different treatment methods, they were divided into an observation group treated with double-tube negative pressure drainage (n = 25) and a control group treated with negative pressure drainage inside the wound (n = 25). This study protocol was implemented after approval by the Medical Ethics Committee of our hospital, and all patients and their family members signed the informed consent. The inclusion criteria were as follows: (1) meeting the diagnostic criteria for refractory wounds,12 with excessive suture tension on the wound surface; (2) good blood supply to the limbs on the wound side in color Doppler ultrasound; (3) wound defect area ≥4 cm2. The exclusion criteria included: (1) artery occlusion in color Doppler ultrasound; (2) complicated with hepatic, renal, cardio cerebral, and vascular diseases; (3) inability to cooperate with treatment due to psychological or intellectual factors; (4) complicated with connective tissue, hematological and immune diseases.
2.2 Treatment methods
2.2.1 Observation group
Wound specimens were retained for examination before initial debridement. Debridement was performed using an MUI-T8 multifunctional ultrasonic debridement machine (produced by Chongqing Tengyue Medical Equipment Co., Ltd.) or surgically as thoroughly as possible. After thoroughly rinsing the wound with 0.5% iodophor disinfectant, 3% hydrogen peroxide solution and 0.9% normal saline, the infected, necrotic, inactivated, aged, edematous, and granulation tissues were removed. Skin flaps on both sides along the wound margin were released and freed, and then the wound margin was pulled towards the wound center after being trimmed neatly for relaxation suture. In the case of excessive tension, a regional rotation flap was designed to cover the defective wound. At the deepest point of the wound cavity or below the skin flap, a trimmed suction tube with side holes was indwelt and attached with a disposable venous infusion needle (needle head removed) with two side holes cut at the distal end for easy rinsing. The skin around the wound was disinfected with 75% alcohol to remove dirt and oil on the skin's surface. After the skin was dried, the wound was completely covered using a VSD biological dressing (Wuhan VSD Medical Science & Technology Co., Ltd.) trimmed to a suitable size according to the size and shape of the wound surface or wound cavity, extending about 3 cm beyond the wound margin, and then sealed and fixed with a sterile transparent film. The suction tube and VSD device supporting compression-resistant tube were connected to the central negative pressure suction system through the “Y”-shaped joint inside the device, with the negative pressure adjusted to −80∼−125 mmHg. After the negative pressure was loaded, whether the negative pressure drainage device was sealed, whether there was hematocele and effusion under the film, and whether the suction tube was blocked were regularly observed. The negative pressure was effective with significant shrinkage and collapse of the VSD biological dressing. If the dressing was restored to its original state, there was hematocele and effusion under the film, or the suction tube was still not unobstructed after rinsing with a disposable venous infusion needle, it should be removed and replaced in a timely manner. The drainage tube was rinsed with sterile normal saline within 2 d after surgery. One week later, the suction tube and flushing pipe were removed, and the VSD device was replaced for continuous use for another week. The wound healing status, wound bacterial clearance rate, wound pain score, patient satisfaction and complication rate were observed during the treatment.
2.2.2 Control group
The control group was treated with single-tube negative pressure drainage inside the wound. Wound specimens were retained for examination before initial debridement, and the debridement procedure was the same as the observation group. Skin flaps on both sides along the wound margin were released and freed, and then the wound margin was pulled towards the wound center after being trimmed neatly for retention suture. In the case of excessive tension, a regional rotation flap was designed to cover the defective wound. At the deepest point of the wound cavity or below the skin flap, a trimmed drainage tube with side holes was indwelt and connected to a negative pressure drainage ball for suction. When the amount of drainage fluid in the drainage ball was less than 5 mL within 24 h, the drainage tube was removed. The wound healing status, wound bacterial clearance rate, wound pain score, patient satisfaction and complication rate during the treatment were observed.
2.3 Observation indicators and evaluation criteria
-
Wound healing status: After treatment for two weeks, the wound healing status of the two groups was divided into grade A: good wound healing without adverse reactions such as redness, swelling and exudation, grade B: not good healing with mild inflammatory responses, and grade C: poor healing with wound redness, swelling, and suppuration.13
-
Wound bacterial clearance rate: Wound pathogen detection was carried out by sampling with sterile cotton swabs and bacterial culture before treatment, 3, 7, and 14 d after treatment, respectively. On the 3 d of treatment, drainage fluid was sampled after rinsing. On the 7 d of treatment, the secretions at the end of the drainage tube were sampled. On the 14 d of treatment, the secretions at the VSD biological dressing close to the wound were sampled. The number of bacteria per unit area (m2) was calculated based on colony count. Wound bacterial clearance rate = (average colony count per unit area before treatment—average colony count per unit area after treatment)/average colony count per unit area before treatment.
-
Wound pain score: The wound pain was evaluated using the numerical rating scale (NRS), with a score of 0 indicating no pain, 1–3 indicating mild pain, 4–6 indicating moderate pain, and 7–10 indicating severe pain.
-
Patient satisfaction: Patient satisfaction was totally scored as 10. The higher the score, the higher the satisfaction.
-
The occurrence of complications was recorded.
2.4 Statistical processing
The data were processed using SPSS 16.0. The comparisons of general data, wound healing status, bacterial clearance rate and complication rate between the two groups were conducted by the X2 test. Pain score and patient satisfaction were measurement data, which all conformed to the normal distribution and were expressed as mean ± standard deviation (x ± s). Their comparisons between the two groups were performed with the t test. P < 0.05 was considered statistically significant.
3 RESULTS
3.1 General data
In the observation group, there were 13 males and 12 females, aged 40–78 years (average, 60.80 ± 12.28 years), including five with diabetic foot ulcers, eight with pressure ulcers, six with postoperative incisional infection and six with traumatic ulcers. The wound area ranged from 2.0 cm × 2.2 cm to 7.2 cm × 9.5 cm, with an average of (42.70 ± 22.25) cm2, and the wound formation time was (31.84 ± 8.00) d. The control group included 14 males and 11 females, with an age of 36–80 years (average, 59.44 ± 13.05 years). Among them, three patients suffered from diabetic foot ulcers, nine had pressure ulcers, eight presented postoperative incisional infection and five showed traumatic ulcers, with a wound area of 1.5 cm × 2.8 cm–7.8 cm × 9.0 cm (average, 40.81 ± 21.77 cm2) and a wound formation time of (33.68 ± 7.51) d. The general data such as age, gender, nutritional status, wound type, wound area and wound formation time showed no statistically significant differences between the two groups (P > 0.05), indicating comparability (Table 1).
| Indicator | Observation group | Control group | P value | |
|---|---|---|---|---|
| Average age (year) | 60.80 ± 12.28 | 59.44 ± 13.05 | 0.706 | |
| Gender (n) | Male | 13(52%) | 14(56%) | 0.777 |
| Female | 12(48%) | 11(44%) | ||
| Nutritional status (n) | Excellent | 4(16%) | 8(32%) | 0.185 |
| Good | 16(64%) | 13(52%) | 0.39 | |
| Poor | 5(20%) | 4(16%) | 0.713 | |
| Wound type (n) | Diabetic foot ulcers | 5(20%) | 3(12%) | 0.7 |
| Pressure ulcers | 8(32%) | 9(36%) | 0.765 | |
| Postoperative incisional infection | 6(24%) | 8(32%) | 0.529 | |
| Traumatic ulcers | 6(24%) | 5(20%) | 0.733 | |
| Wound area (cm2) | 42.70 ± 22.25 | 40.81 ± 21.77 | 0.762 | |
| Wound formation time (week) | 31.84 ± 8.00 | 33.68 ± 7.51 | 0.406 |
3.2 Comparison of wound healing status
After treatment for two weeks, the observation group had a higher grade A healing rate compared with the control group (92% vs. 60%, X2 = 7.018, P = 0.008), while the control group presented a higher grade B healing rate compared with the observation group (32% vs. 8%, X2 = 4.500, P = 0.034). The grade B healing rate was higher in the control group than that in the observation group, but no difference was found in the grade C healing rate between the two groups (P > 0.05) (Table 2).
| Group | Observation group | Control group | X2 value | P value |
|---|---|---|---|---|
| Grade A healing | 23(92%) | 15(60%) | 7.018 | 0.008 |
| Grade B healing | 2(8%) | 8(32%) | 4.5 | 0.034 |
| Grade C healing | 0 | 2(8%) | 2.083 | 0.47 |
3.3 Comparison of wound bacterial clearance rate
The wound bacterial clearance rates on the 3rd, 7th, and 14th d were 52%, 28% and 0 in the observation group, and 68%, 48%, and 24% in the control group, respectively. The wound bacterial clearance rates showed no statistically significant differences between the two groups on the 3rd and 7th d (P > 0.05). On the 14th d, namely after treatment for two weeks, the wound bacterial clearance rate in the observation group was higher than that in the control group (100% vs. 76%, X2 = 6.818, P = 0.03) (Table 3).
| Group | Observation group | Control group | X2 value | P value |
|---|---|---|---|---|
| Before treatment [n(%)] | 25(100) | 25(100) | – | – |
| Day 3 [n(%)] | 13(52) | 17(68) | 1.333 | 0.248 |
| Day 7 [n(%)] | 7(28) | 12(48) | 2.122 | 0.145 |
| Day 14 [n(%)] | 0 | 6(24) | 6.818 | 0.03 |
| Negative conversion ratio | 100% | 76% | 6.818 | 0.03 |
3.4 Comparison of pain score and patient satisfaction
The pain scores on days 3, 7, and 14 were (3.96 ± 0.79), (2.64 ± 0.70) and (1.44 ± 0.51) in the observation group, and (5.16 ± 0.75), (4.00 ± 0.76) and (2.36 ± 0.49) in the control group, respectively. The pain scores in the observation group were all lower than those in the control group on days 3, 7 and 14 (t = −5.52, −6.56, −6.53, all P < 0.01) (Table 4).
| Group | Observation group | Control group | X2 value | P value |
|---|---|---|---|---|
| Day 3 | 3.96 ± 0.79 | 5.16 ± 0.75 | −5.52 | <0.01 |
| Day 7 | 2.64 ± 0.70 | 4.00 ± 0.76 | −6.56 | <0.01 |
| Day 14 | 1.44 ± 0.51 | 2.36 ± 0.49 | −6.53 | <0.01 |
The patient satisfaction scores were (4.36 ± 0.70), (5.76 ± 0.78) and (8.48 ± 0.96) in the observation group, and (3.12 ± 0.67), (4.84 ± 0.69) and (6.64 ± 0.95) in the control group at day 3, 7, and 14, respectively. In the observation group, the patient satisfaction scores were all higher compared with the control group on days 3, 7, and 14 (t = 6.42, 4.43, 6.80, all P < 0.01) (Table 5).
| Group | Observation group | Control group | t value | P value |
|---|---|---|---|---|
| Day 3 | 4.36 ± 0.70 | 3.12 ± 0.67 | 6.42 | <0.01 |
| Day 7 | 5.76 ± 0.78 | 4.84 ± 0.69 | 4.43 | <0.01 |
| Day 14 | 8.48 ± 0.96 | 6.64 ± 0.95 | 6.8 | <0.01 |
3.5 Comparison of complication rate
The overall incidence of complications was 8% (n = 2) in the observation group and 40% (n = 10) in the control group. The overall incidence of complications in the observation group was lower than that in the control group (X2= 7.018, P = 0.008) (Table 6).
| Group | Observation group | Control group | X2 value | P value |
|---|---|---|---|---|
| Hematoma [n(%)] | 0 | 3(16) | – | – |
| Poor wound healing [n(%)] | 2(8) | 3(16) | – | – |
| Wound infection [n(%)] | 0 | 2(12) | – | – |
| Skin flap necrosis [n(%)] | 0 | 2(12) | – | – |
| Overall incidence [n(%)] | 2(8) | 10(40) | 7.018 | 0.008 |
3.6 Typical case
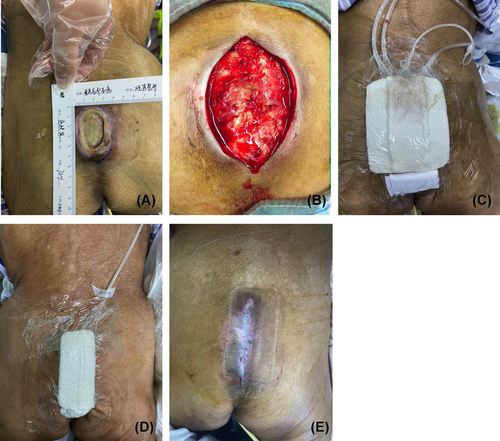
A female patient aged 72 years was admitted because of sacrococcygeal pressure ulcers for 40 d. Specialized examination at admission showed an ulcerated wound area of approximately 6.0 × 5.0 cm2 in the sacrococcygeal region, reaching the periosteum, mostly with turbid inflammatory exudate, yellowish-white necrotic tissue attachment, poor granulation tissue growth and slight surrounding tissue protrusion (Figure 1A). After admission, wound bacterial culture suggested “Acinetobacter baumannii”. Fluid replacement and anti-infective therapy were provided based on drug sensitivity test results. In addition, surgical debridement was conducted by releasing and freeing skin flaps on both sides along the wound margin and then pulling the wound margin towards the wound center after trimming neatly for retention suture (Figure 1B). After debridement, double-tube negative pressure drainage inside and outside the wound was carried out for one week (Figure 1C). One week later, the suction tube and flushing pipe were removed, and the VSD device was replaced for continuous use for another week (Figure 1D). Two weeks after surgery, the suture was removed and the wound healed well (Figure 1E).
4 DISCUSSION
Refractory wounds are chronic wounds that are difficult to heal and caused by excessive tension for various reasons. Most of the patients with initially delayed treatment due to various causes are accompanied by severe infections, pathogenic bacterial invasion, skin barrier damage, local blood circulation disorders, and tissue and cellular hypoxia, resulting in prolonged wound healing. Their treatment is time-consuming and difficult, and untimely or inappropriate treatment will lead to various complications, bringing heavy economic and psychological burdens to patients and their families. Consequently, active and effective treatment measures should be taken for patients with refractory wounds.
At present, there are numerous ways to treat refractory wounds in clinical practice, each with its own advantages and disadvantages. VSD was for the first time applied in clinical practice by Dr. Fleischmann from Germany in 199214 and first applied in China by Qiao Jianguo et al. in 1994. It has become an advanced technique for treating various acute and chronic wounds, with simple operation, and low requirements for operating conditions, which can also be performed at the bedside.15, 16 Its mechanisms in promoting wound healing are as follows17-20: (1) It timely removes exudate and necrotic tissues from the drainage area, contributing to a clean wound environment and reducing the number of bacteria, which can effectively prevent the spread of infection and absorption of toxins. (2) Continuous negative pressure can improve local wound microcirculation, promote the continuous flow of body fluid in the wound tissue towards the drainage tube, provide continuous and effective dynamics for wound blood circulation and stimulate granulation tissue growth, as well as high negative pressure can also accelerate the narrowing of large cavities, eliminate potential cavities, shorten wound healing time and improve healing efficiency. (3) It effectively reduces vascular permeability and wound tissue edema. (4) In addition, VSD also achieves autolytic debridement on the wound by promoting the release of fibrinolytic protein activators and other enzymes in the body.21
In this study, the wound bacterial clearance rate, wound healing status and complication rate of the observation group were significantly better compared with the control group (P < 0.05). This may be resulted from: (1) Negative pressure suction inside the wound can prevent bacteria from invading the interior of the wound and timely drain exudate inside the wound. At the same time, negative pressure drainage is used outside the wound. Their combined effect can effectively reduce wound bacterial infection and timely remove harmful metabolites and inflammatory factors. (2) Negative pressure suction outside the wound can fix the wound, reduce the tension around the skin flap at the wound margin and relieve tissue edema, thus promoting wound healing.11 (3) A relevant study has shown that appropriate negative pressure can promote the reopening of previously occluded micro vessels in the wound, forming new capillaries, which significantly improves local microcirculation. Moreover, negative pressure can also relieve tissue edema. As a result, the blood supply to the wound is improved, bringing more oxygen, nutrients and various growth factors, and taking away metabolites such as lactate, which is conducive to effective wound healing.22 (4) It has been reported that adjustable closed negative pressure suction can regulate the activities of active gelatinases MMP-2, MMP-9 and fibronectin in chronic wounds, activate all growth factors inside the wounds, and promote the rapid healing of chronic wounds.23 (5) Simultaneously negative pressure suction inside and outside the wound can promote timely and effective drainage of wound exudate, and attach the flap more closely to the wound, which is beneficial for the blood supply to the flap and greatly reduces the incidence of flap necrosis.24 (6) Early flushing with a disposable venous infusion needle within the wound can avoid the accumulation of exudate and the occurrence of hematoma. In addition, negative pressure suction outside the wound can ensure unobstructed drainage throughout the wound, thereby greatly reducing the incidence of complications such as hematoma and infections.
The advantages of VSD with double-tube negative pressure inside and outside the wound compared with negative pressure drainage inside the wound are as follows: (1) VSD with double-tube negative pressure inside and outside the wound can effectively remove harmful metabolites and bacteria from the wound. (2) Its combined effect can adhere the skin flap tightly to the wound, and reduce the incidences of dead space and hematoma, which is more conducive to skin flap survival and promoting wound healing. (3) It can significantly improve wound microcirculation, accelerate wound tissue growth, and facilitate wound healing. (4) Negative pressure drainage inside and outside the wound can provide throughout drainage, reduce exudate accumulation, maintain a clean and moist wound environment, thus providing favorable conditions for wound growth, and accelerating wound healing. (5) It can isolate the external environment in a closed manner, avoid external bacterial contamination, effectively drain necrotic tissues and exudate, and prevent bacteria from invading the interior of the wound, thereby reducing bacterial infection on the wound.25 Therefore, it can reduce antibiotic use and bacterial resistance. (6) Negative pressure drainage inside and outside the wound can relieve tissue edema and greatly reduce the incidence of flap tip necrosis. (7) Its treatment is safe and reliable, with no obvious stimulation to the wound tissue, minimal damage, good wound healing, reduced pain and increased satisfaction.
During the treatment, the author believes that the following points should be noted: (1) Thorough debridement is needed to remove the infected, necrotic, edematous, aged and granulation tissues on the wound. After debridement, retention suture or skin flap transfer should be chosen based on wound tension. (2) Intraoperative thorough hemostasis by ligation or suturing is needed to ensure safety and reliability and avoid postoperative wound re-bleeding due to negative pressure suction. (3) The drainage tube should be rinsed with sterile normal saline 2 d before surgery, and it should be stopped on the 3rd d to avoid affecting the reliability of the flap attachment to the wound and wound healing. (4) During treatment, attention should be paid to maintaining the sealing of negative pressure and the effectiveness of central negative pressure, as well as closely observing the patency of negative pressure drainage. (5) During treatment, attention should be paid to the properties of drainage fluid. If the patient experiences increased pain or fever in the wound, it is necessary to remove the negative pressure suction device timely and check the wound condition.
This study still has certain limitations. Firstly, the samples discussed in our study are patients undergoing refractory wound repair, and the scope of the samples is relatively small. In the future, patients with wounds at different parts and depths can also be included in the study for discussion. Additionally, our study only explores the effectiveness of double-tube negative pressure drainage alone in repairing refractory wounds. In future research, the combination of double-tube negative pressure drainage with other techniques such as platelet-rich plasma therapy and growth factor therapy can be considered to explore the effects of various therapies on the repair of refractory wounds.
In conclusion, double-tube negative pressure drainage in the treatment of refractory wounds can relieve tension at the wound margin, improve local blood supply, decrease wound infection rate, reduce complication rate and alleviate pain, with high safety. Therefore, it is worthy of clinical promotion and application.
ACKNOWLEDGMENTS
The authors have nothing to report.
CONFLICT OF INTEREST STATEMENT
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
ETHICS STATEMENT
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Jingjiang People's Hospital. We obtained signed informed consent from the participants / legal guardians in this study.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article




