Topical dexpanthenol effects on physiological parameters of the stratum corneum by Confocal Raman Microspectroscopy
Abstract
Background
Topical use of dexpanthenol presents well-established moisturizing properties and maintenance and repair of the skin barrier function, however, its exact action mechanisms are not completely elucidated. In this context, Confocal Raman Microspectroscopy is an optical method that enables non-invasive and non-destructive in vivo analysis with the sensitive acquisition of molecular changes in different skin layers. Herein, the aim was to evaluate the effects of topical dexpanthenol on the components and physiological parameters of the stratum corneum (SC).
Materials and methods
Ten healthy female subjects underwent skin evaluation by means of a Confocal Raman Spectrometer Skin Analyzer 3510. Spectral data were obtained from the skin of the anterior forearm region, before and 2 h after applying a cosmetic formulation containing or not containing 5% dexpanthenol.
Results
Semiquantitative analysis of the natural moisturizing factor showed a significant decrease in content after 2 h of topical dexpanthenol application, while the analysis of the lamellar organization of intercellular lipids and the secondary structure of keratin showed a significant increase in hexagonal organization of lipids at the first half of the SC and a significant increase in β-pleated sheet conformation of keratin.
Conclusion
Effects of topical dexpanthenol on SC suggest a contribution in increasing fluidity of both lipidic and protein components of the SC and are compatible with dexpanthenol activity in maintaining adequate physiological conditions and preventing transepidermal water loss. This study also contributes to the elucidation of action mechanisms and other concurrent biochemical processes.
1 INTRODUCTION
Moisturizing formulations are among the most relevant classes of cosmetic products, among which topical dexpanthenol has well-established moisturizing and skin barrier protective functions being widely used in clinical practice. Dexpanthenol, the stable alcohol form of Vitamin B5, is well absorbed through the skin where it is rapidly converted enzymatically to pantothenic acid, a component of coenzyme A, which is important in cellular skin metabolism.1 Besides exhibiting skin permeability, it has anti-inflammatory and wound-healing properties and acts to restore the physiological condition of the skin barrier function by promoting cell renewal.2, 3 It has also been pointed out to promote epidermal regeneration by increasing epidermal differentiation and lipid synthesis.4 As a humectant, it has been shown to retain water more efficiently than other humectants during the last stages of dehydration, in fact, dexpanthenol solutions, of both high and low molar fractions, assume a density close to the density of the pure substance alone.5 This water-binding ability is related to greater flexibility and mobility of water molecules through the SC.5, 6 However, despite its widespread applications, dexpanthenol's precise action mechanisms are not completely elucidated.
In this context, Confocal Raman Microspectroscopy (CRM) stands out as a method widely used in dermatology7-18 for analysis of the concentration of skin components and penetration depth of cosmetic formulations in the human stratum corneum (SC) in vivo. CRM is an optical method that allows non-invasive and non-destructive analysis of biological samples and the sensitive acquisition of subtle molecular changes occurring in different layers of the skin with semiquantitative insight.19, 20 In recent years, CRM has also been applied for in vivo determination of SC physiological parameters, such as the natural moisturizing factor (NMF) content, lateral organization of intercellular lipids (ICLs), keratin folding properties, water mobility, and its binding states,17, 21 while being suitable for real-time characterization of molecular conformational changes.
Considering dexpanthenol water binding properties and ability to regenerate the epidermal barrier, the evaluation of the composition and molecular organization of the SC extracellular lipid matrix and keratin would support the understanding of the subtle molecular effects of this active ingredient in improving the epidermal barrier function and skin hydration. It would also allow assessing its possible effects in terms of skin permeability, and transepidermal water loss, as well as a better understanding of skin homeostasis. Thereby, this study proposed a qualitative and semiquantitative analysis of SC components and physiological parameters as immediate clinical effects of the topical use of dexpanthenol-containing formulations.
2 METHODS
2.1 Study design
A single-center, double-blind clinical study was conducted to evaluate the effects of the topical use of dexpanthenol on skin components and physiological parameters. For this purpose, 10 healthy women, aged between 25 and 30 years, phototypes II and III, according to skin color and reaction to sun exposure22 were recruited adopting the following exclusion criteria: presence or history of skin disease, irritation or sensitivity to active ingredients, pregnancy or lactation, use of drugs that may produce abnormal skin response and use of the cosmetic product in the measurement area in the last 7 days. All participants signed a consent form approved by the Ethics Committee on Research involving Human Beings of the School of Pharmaceutical Sciences of Ribeirão Preto, CEP/FCFRP Protocol No. 490 - CAAE: 00669818.7.0000.5403, in accordance with the Brazilian Resolution No. 466/12 and the Helsinki Declaration,23, 24 prior to the beginning of the study. Each participant was previously acclimatized for a period of 20 minutes in a controlled environment of a temperature of 20–22°C and relative humidity of 45%–55%. Data acquisition was performed with randomization on the skin of the forearm anterior region, immediately before (T0) and after 2 h (T2) of the application of 2 mg/cm2 (FDA, 2012) of a cosmetic formulation, containing or not (vehicle) 5% dexpanthenol.
2.2 Cosmetic formulations
Formulations were based on triglycerides of capric and caprylic acid, polyacrylamide (e) C13-14 isoparaffin (e) laureth-7, cyclopentasiloxane (e) dimethicone, ammonium acryloyldimethyltaurate/copolymer VP, cyclomethicone, phenoxyethanol and parabens, disodium EDTA, butylhydroxytoluene, propylene glycol, glycerine, and water, contained (F2) or not (F1) 5% of dexpanthenol.
2.3 Confocal Raman Microspectroscopy
For the non-invasive skin evaluation, a 3510 Confocal Raman Spectrometer Skin Analyzer (RiverD International B.V.) was installed with a 785 excitation laser and 25 mW power, and a charge-coupled device was used to collect Raman signals. Spectral data were acquired from the low-frequency region (400–1800 cm−1) of the Raman spectrum, known as the fingerprint region, and analyzed at 16 different depths up to 46.3 μm. A step size of 1.9 μm was used for the acquisition of frames 2–4, 0.3 μm for frame 5, 4 μm for the following five frames (frames 6–10), 2 μm for frame 11, and a step size of 4 μm was used for the following five frames (12–16). In order to compare the relative intensities at specific depths across groups, depth profiles were projected onto the same skin depth axis values (%) by interpolating measured data points onto a common axis.26 The integration time was 86 s for each depth.
2.4 Data pre-processing
Using Labspec software (Horiba JobinYvon, France) the Confocal Raman spectra were smoothed to minimize noise interference using the Savitzky-Golay filter, then subjected to linear baseline correction and normalized by vectorial normalization.
2.5 Data analysis
The determination of the relative amounts of SC components at different depths was performed using Skin Tools 2.0 (River Diagnostics) by the non-restricted multiple least squares fitting method to evaluate NMF, ceramides, and fatty acids (treated by the software as a single variable),25 cholesterol and keratin. The skin surface was determined with respect to the depth-dependent keratin Amide I profile at half the value of its maximum intensity at 1650 cm−1.25
Unsupervised hierarchical clustering analysis (HCA) was performed on pre-processed data aiming to group data from each data point analyzed, based on the distance measure, and the identification of similarities and differences between spectra groups.27, 28 Spectra were grouped with regard to the 1070–1800 cm−1 spectral range, relevant to the analysis of lipids and keratin. By means of the cluster analysis, the surface and average SC thickness were estimated and applied to determine the limits of integration of the areas under the curves (AUCs) of the average semiquantitative profiles of each component.
The secondary structure of the keratin depth profile was determined relative to the intensities (I1670 + I1685)/I1655 of the Amide I band related to β-sheet and random turns and coils/α-helix conformation, while the buried/exposed tyrosine configuration was determined relative to the I830/I850 intensities ratio.16, 21 The depth profile relative to the lamellar organization of ICLs was determined with respect to the I1080/(I1130 + I1060) intensities ratio related to lipids trans-gauche conformation.21
Depth profiles were drawn with the mean values along with each respective AUC numerical distribution and standard deviation bars were omitted for clarity. Paired sample t-test was used to evaluate the statistical significance of treatment (before and after applying each formulation), between AUC groups. Analysis of variance and multiple comparison tests were applied to compare differences between depth data points between different groups. Data processing, descriptive statistics, analysis of variance, and multivariate analysis were performed using the Origin Pro 2023 software, p-values < 0.10 were considered statistically significant.
3 RESULTS AND DISCUSSION
3.1 SC surface and thickness
By means of the HCA, it was possible to identify the distribution of spectral data obtained in depth in two main groups according to similarity27, 28 (Figure 1) comprised of frames 1–8 and frames 9–16. Figure 1 also shows the keratin profile in relative amounts varying as a function of depth at basal conditions (T0), since keratin is synthesized in the basal layer of the epidermis and the accumulation of its disulfide bonds occurs with the proliferation of keratinocytes from the lower regions of the SC to the outermost7, 29 while it assumes different secondary and tertiary structures through the SC.16, 21 Based on these results and due to the presence of signal relative to the optical window, frame 1 was not considered for the comparative evaluation, but rather frames 2–8, accounting for an average SC thickness of 16.3 μm. In fact, Richters et al. also reported an average SC thickness of 16.6 μm (13.5–24.2) for subjects with non-sensitive skin25 on a confocal Raman spectroscopy characterization study, which agrees with the results reported here. Thus the AUC integration of each component profile comprised the area from 0 to 16.3 μm depth.
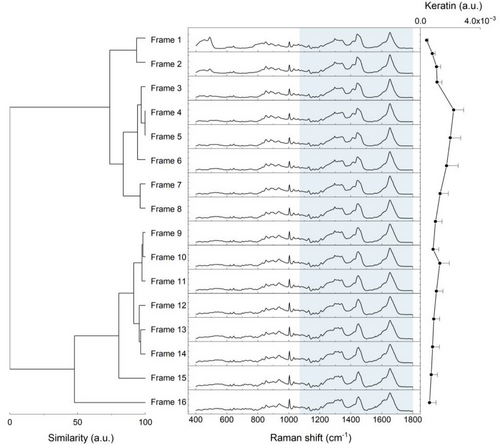
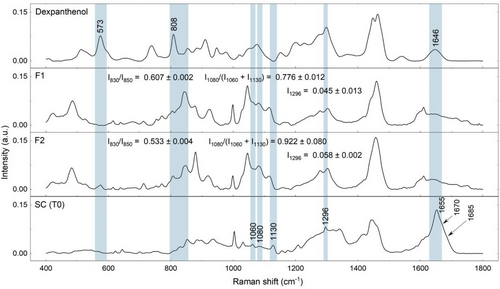
Figure 2 presents the spectra of dexpanthenol, the neat formulations, and the SC at baseline conditions, regarding some spectral contributions arising from vehicle composition and dexpanthenol to the Amide I Raman band at 1650 cm−1 related to hydrogen bonds between N–H and C = O of α-helix keratin, as well as to the ICLs bands at 1060 and 1080 cm−1, related to the trans and gauche conformation of ICLs, that were considered in the interpretation of keratin conformation and lipid lamellar organization parameters. It was not possible to establish a significant contribution arising from F1 and F2 for the SC surface.
3.2 Natural moisturizing factor
Figure 3A shows the mean depth profile of NMF components for five out of the ten subjects, at baseline and after 2 h of applying F1 and F2, along with each respective AUC (Figure 3A,B). First, it was possible to observe a maximal content at 0%–20% SC depth, where the NMF is mainly responsible for binding water.8-13 The NMF is a complex of hygroscopic components products of filaggrin degradation, consisting mainly of free amino acids and their derivatives. Besides preventing water loss, the NMF modulates and balances skin barrier conditions along with other physiological parameters. Physiologically, as NMF content gradually decreases at the intermediate SC region (20%–60% SC depth), the binding of water is compensated by keratin unfolding and increased packing order of ICLs (Figures 4 and 5), while at 80–100% SC depth the NMF reaches a minimum (Figure 3A) near the viable epidermis where water content is increased.
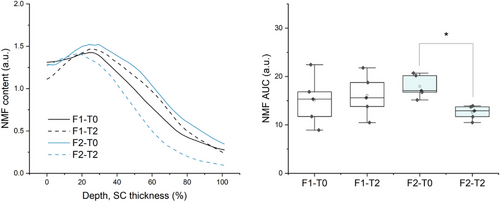
These results also showed a non-significant increase in NMF content in F1-treated skin as well as a significant decrease in F2-treated skin after 20% SC depth. Although non-significant, it is possible that the increase in NMF content verified for F1 is related to an increase in water activity at the SC due to modest occlusion by F1. Harding et al. and Rawlings and Harding explained that filaggrin hydrolysis is facilitated by humidity and only occurs within a narrow water content range (70%–95% humidity),9, 10 referring to different instances where skin occlusion leads to filaggrin persistence in the SC.11-13 The author further discussed the role of proteases catalyzing the final stages of filaggrin hydrolysis, as they seem to be sensitive to changes in water activity. In regard to F2, it is possible that the decrease in NMF content is related to higher water activity in the SC. Notably, Choe et al. reported a significant reduction in NMF content between 50% and 70% SC depth, while evaluating occlusion effects by different oils, which she attributed to NMF dilution due to increased water in corneocytes.
3.3 Keratin secondary and tertiary structure
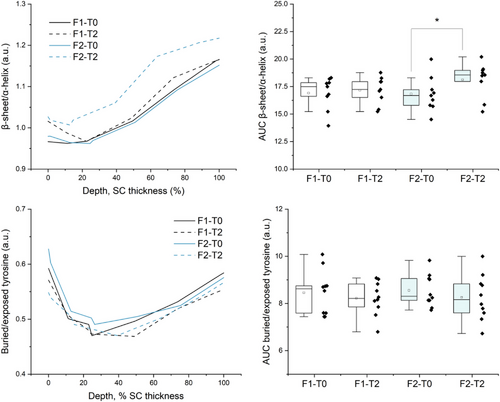
Regarding keratin conformation, both baseline and treated skin showed a prevalence of α-helix keratin at the SC surface and a gradual increase in β-sheet conformation after 20% SC depth (Figure 4A,B). It also observed a significant increase in the mean intensities related to the β-sheet conformation of keratin after 2 h of F2 application. In this regard, Figure 2 addresses the superposition of the Amide I band at 1650 cm−1 related to α-helix keratin by the dexpanthenol band at 1646 cm−1. Since there was no other superposition for the I1070 and I1085 intensities, dexpanthenol intensity at 1646 cm−1 contributes to the (I1670 + I1685)/I1655 parameter towards lower values, therefore the higher relative amounts verified for F2-treated skin could be related to the transformation of α-helix towards β-sheet keratin.
Indeed, secondary and tertiary structures of keratin act in the regulation of water binding in the SC,21 so an increase in the β-sheet conformation in relation to the α-helix, its most stable form, would suggest a greater interaction of keratin with water molecules through hydrogen bonds. Björklund and colleagues reported increased mobility of glycine and serine residues, which are abundant in the terminal domains of keratin filaments, after 8 h of soaking dermatomed skin samples in 5% dexpanthenol solutions, when compared to the control.6 Yamada et al. also pointed out how hydration in SC skin models treated with humectants caused the loosening of the protruding keratin filament chains into mobile inter-twined peptide filaments.53 The author further discussed how different humectants affected the decrease in soft keratin spacing upon drying as a function of the water-binding capability of different humectants.53 Notably CRM studies performed by Darvin and Choe16, 21 et al. characterized SC composition and attributed a higher swelling capacity of corneocytes at 30%–70% SC depth to the prevalence of β-sheet keratin at this same region when compared to both upper and deeper SC layers. At the upper layers, rigid and stable keratin α-helix prevails in highly cornified corneocytes that withstand NMF osmotic pressure and do not swell, while near the viable epidermis, the bidding sites of keratin, ICLs, and NMF are fully saturated.16, 21 In fact, Choe et al. also reported an increase in β-sheet conformation at different SC depths after one hour of petrolatum application—the author described an increase in weaker hydrogen-bound water in the upper layers of the SC, which was attributed to the accumulation and diffusion of high mobility weakly bound and free water types towards SC surface.17
In terms of tyrosine configuration, for all groups, it was possible to observe a decrease in the I830/I850 intensities at 20–50% SC depth, as tyrosine residues become more exposed. This configuration enables the residues to interact and form hydrogen bonds with surrounding molecules, while buried tyrosine forms hydrogen bonds with keratin side chains and increases keratin folding,21, 17 thereby a reduction in I830/I850 intensities ratio at the intermediate SC region inferring a higher relative amount of exposed tyrosine, which was not significantly affected by F1 or F2.
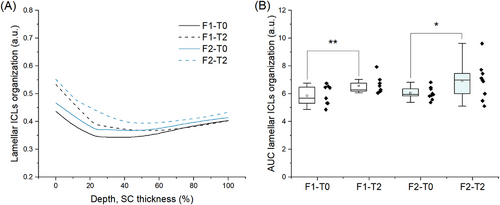
3.4 Lamellar organization of intercellular lipids
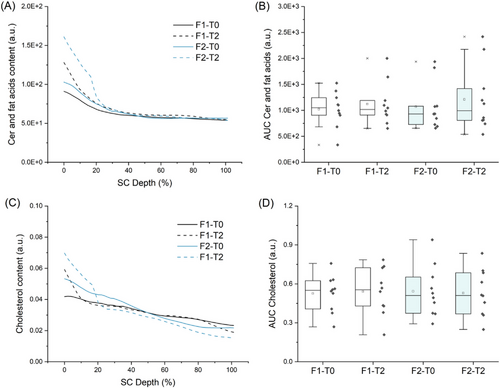
In terms of ICLs lamellar organization, both baseline and treated skin presented decreased I1080/(I1130 + I1060) intensities ratio at 20–60% SC depth (Figure 5A,B). Recent studies suggest the use of intensities I1130 and I1060 for characterization of the alkyl chains in the trans conformation (ordered state, densely packed arrangement, orthorhombic phase), while the intensity at 1080 cm−1 point to the presence of gauche conformation of the alkyl chains (ordered state, less densely packed, and hexagonal phase).15, 21, 52 Therefore the decrease in this parameter for both baseline and treated skin groups indicates the prevalence of the trans conformation of ICLs at the intermediate SC region. Yet the analysis of the gauche-trans conformation also pointed to a significant increase in gauche conformation at the first half of the SC after 2 h of applying both F1 and F2 (Figure 5A,B). In this regard, spectral contributions arising from the vehicle and dexpanthenol (Figure 2) should be considered, as they superimpose SC bands at 1080, 1060, and 1130 cm−1, contributing to both gauche and trans components. Besides the contributions arising from the vehicle composition (F1), compared to F1, neat F2 assumed a 15,8% higher value for gauche-trans conformation (Figure 2), which indicates that permeation of dexpanthenol in F2-treated skin could influence the intensities ratio towards higher relative amounts of gauche conformation ICLs. This effect should also be considered for the depth profiles of ceramides and fatty acids (Figure 6A,B) and cholesterol (Figure 6B,C) obtained by Skin Tools. Figure 6 shows an apparent increase in the relative amounts of lipids for F2-treated skin at the uppermost SC region (0%–20% SC depth), but no significant changes were observed in terms of the AUC. Both composition and organization of the extracellular space are fundamental for the regulation of transepidermal water loss and skin permeability33, 36, 48-51 and it is mainly composed of long-chained ceramides, free fatty acids, and cholesterol.47 It is possible that the apparent increase in lipid content verified for F2 (Figure 6A,C) is related to the higher increase in gauche conformation (Figure 5A) at the upper non-swelling region of the SC and to ICLs mobility. However, the difference between F1 and F2 I1296 intensities should also be considered, as the Raman peak at 1296 cm−1, related to –CH2 twist, is present for both skin fatty acids and ceramides. Nevertheless, since it was possible to verify dexpanthenol permeability up to the maximum depth analyzed in the study, it is possible to infer that the differences between F1 and F2 effects on SC components and physiological parameters are related to dexpanthenol and that the study of dexpanthenol effects on skin lipids poses a limitation.
Compared to F2, F1 showed a lower increase in gauche conformation at the uppermost SC region, which could be related to occlusion and permeation of the excipient molecules. Choe et al. also verified a significant decrease in ICLs packing order at 0–30% SC depth, which was attributed to the accommodation of diffused water among ICLs at the upper non-swelling SC region, which could also indicate the widening of the intercellular space between corneocytes.17
Since F2-treated skin showed significant ICL disorganization, it is also possible to assume higher mobility of lipids within the extracellular matrix. Compared to the orthorhombic phase, the reduced packing order found in the hexagonal organization allow the lipid chains some rotational mobility along their long axes, although their translational mobility is restricted.32 Notably, dexpanthenol has been shown to interact with lipid segments of the extracellular lamellae and protein residues in the SC corneocytes, compensating for reduced hydration by retaining/increasing molecular fluidity.3, 6 Besides, the reduced packing order of ICLs could also be related to a decrease in cohesion between corneocytes, since intermolecular forces between lamellar lipids and covalently bound lipids of the cornified envelope provide some tissue cohesion.34, 35
Björklund et al. investigated how transcutol and dexpanthenol influenced the mobility of SC molecular components and the barrier function by solid-state NMR.6 The authors reported an increase in the NMR signal corresponding to the trans-gauche conformation of SC lipids when treated with both active substances, compared to the control, implying modestly higher mobility of the SC lipids.6 The same effect was observed for ceramides headgroups and cholesterol, with a more pronounced effect for the SC samples treated with the solution containing 5% dexpanthenol.6
Yamada et al. pointed out that hydrating skin models SC with humectants led to different transient stages of the hydrocarbon chain packing structures upon drying, the first state being the fully hydrated one, marked by keratin loosening reduction—and a gradual shrinkage of the ICLs packing structure into orthorhombic phase, followed by the more organized state, marked by an increased and sustained orthorhombic packing, with a subsequent reduction in both ICLs packing and keratin mobility.53 Yamada also relates the increased mobility of the hydrocarbon chain and ceramide head groups after hydration by different hygroscopic molecules to the rate of spacing shrinkage observed between the fully hydrated and the near-physiological stage, as well as to the longer water retention by the SC upon drying.53 Complementarily, Iwai et al. also observed, by static X-ray diffraction experiments, that the orthorhombic packing of ICLs would be reduced at low SC water content and, with increasing water content there would be an increase in the organization of hydrocarbon chains near a maximum, which would be followed by a decrease in the orthorhombic packing order as the water content was further increased.54
These results also agree with the SC lipid model proposed by Froebe et al. and Mattai et al., in which the SC lipid model containing glycerol would undergo a phase change that prevents the formation of an ordered hydrocarbon chain packing structure and improves SC flexibility.55, 56 Still, it is important to consider the dynamic nature of these systems. Whitesides and Boncheva notably draw attention to the fact that self-assembling structures such as SC lipid lamellae are formed by minimizing the free energy of the system as it appears to be driven from one equilibrium state to, forming their characteristic order after energy dissipation.32, 58 Thereby, we believe that this study would largely benefit from a thorough evaluation involving a larger cohort over a longer period of time, not only to characterize other equilibrium states of this microenvironment but also to overcome the interpersonal variability of the skin that is emphasized evaluating reduced panels immediate effects.
4 CONCLUSION
Overall, these results suggest the contribution of dexpanthenol in increasing fluidity of both lipid and protein components of the SC, as the evaluation of SC components and physiological parameters pointed to a decrease in packing order of ICLs lamellar organization and an increase in β-sheet conformation after 2 h of topical application of dexpanthenol. The effects reported here support the activity of dexpanthenol in maintaining adequate physiological conditions and preventing transepidermal water loss and also contribute to the elucidation of the mechanisms of action of dexpanthenol and other biochemical processes underlying its moisturizing function.
ACKNOWLEDGMENTS
Vitoria Tonini Porto Ferreira acknowledges the financial support of Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2019/26836-4) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (001). Airton Abrahão Martin thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (309966/2021-3) and Gustavo Carlos Silva thanks the CAPES (88882.365434/2019-01).
CONFLICT OF INTEREST STATEMENT
Gustavo Carlos Silva is a PhD student at Dermo PROBES—Skin and Hair Technology and received a scholarship for this study. Airton Abrahão Martin is CEO of Dermo PROBES—Skin and Hair Technology. The authors acknowledge no other actual or potential conflict of interest with individuals or entities.
ETHICS STATEMENT
The study was carried out according to and after approval of the Ethics Committee on Research involving Human Beings of the School of Pharmaceutical Sciences of Ribeirão Preto, CEP/FCFRP Protocol No. 490 - CAAE: 00669818.7.0000.5403, in compliance with the Brazilian Resolution No. 466/12 and the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
We declared that the research data are not shared.




