COVID-19 and hepatic involvement: The liver as a main actor of the pandemic novel
Abstract
In the natural history of SARS-CoV-2 infection, liver injury is frequent but quite mild and it is defined as any liver damage occurring during disease progression and treatment of infection in patients with or without pre-existing liver diseases. The underlying mechanisms for hepatic injury in patients with COVID-19 are still unclear but the liver damage in SARS-CoV-2 infection seems to be directly caused by virus-induced cytopathic effects. In this review, we will summarize all data of updated literature, regarding the relationship between SARS-CoV-2 infection, acute response and liver involvement. An overview will be given on liver injury, liver transplant and the possible consequences of COVID-19 in patients with pre-existing liver diseases.
1 COVID-19, LIVER DAMAGE AND CYTOKINE STORM
The worldwide breakdown due to novel coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has attracted the interest of the scientific community. Current research is also focusing on the liver as site of ongoing SARS-CoV-2 infection. In this review, we will summarize all data of updated literature, regarding the relationship between SARS-CoV-2 infection and liver involvement. An overview will be given on liver injury, liver transplant and the possible consequences of COVID-19 in patients with pre-existing liver diseases.
In the natural history of SARS-CoV-2 infection, liver injury is frequent but quite mild1-3 and it is defined as any liver damage occurring during disease progression and treatment of infection in patients with or without pre-existing liver diseases. An epidemiological study on the geographical distribution of COVID-19 outbreak describes that in the areas with a higher incidence, such as Wuhan in China, the rate of COVID-19 patients with liver injury is also higher.4 The proportion of infected hospitalized COVID-19 patients with abnormal levels of liver biomarkers ranges from 14% to 53%, mainly aminotransferase and bilirubin.5, 6 In most cases, the increased levels in bilirubin are observed in patients with severe COVID-19 in the setting of multiorgan failure.
Chen et al7 observed that 43 out of 99 COVID-19 patients had differing degrees of liver function impairment with mild-to-moderate increase of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels and one patient with severely elevated serum aminotransferases (ALT: 7590 U/L, AST: 1445 U/L). The occurrence of infected patients with abnormal liver enzymes is more frequent in adults than in children and in men than in women4; in addition, liver injury is prevalent with in severe than in mild cases of COVID-19 infections.6, 8, 9 Analysing clinical features of COVID-19 patients, Huang et al10 observed that the increase of AST occurred in the 62% of patients in the intensive care unit (ICU), while it was abnormal in 25% of patients who did not require care in the ICU. However, it is important to consider that AST elevation may derive from muscle origin in the setting of myositis in these patients.11 Zhang et al6 found that the gamma-glutamyl transferase (GGT), a diagnostic biomarker for cholangiocyte injury, was elevated in 30 out of 56 (54%) COVID-19 hospitalized patients and only 1/56 patients (1.8%) had increased level alkaline phosphatase. SARS-CoV-2 infected patients also report low levels of albumin that correlated with severe infection and poor prognosis.5 The occurrence of abnormal high ALT levels together with a reduction in platelet count and in albumin levels at the time of admission has been associated with higher mortality,12 although not all these alterations are independent risk factors for the disease.13 During the clinical course of infections sustained by other respiratory viruses, hepatitis is a collateral event mediated by virus-specific effector cells generated in response to pulmonary infection. Nowadays, the underlying mechanisms for hepatic injury in patients with COVID-19 are still unclear but the liver damage in SARS-CoV-2 infection seems to be directly caused by virus-induced cytopathic effects. Approximately 2%-10% of patients with COVID-19 complaint diarrhoea as first symptom of, and, in these cases, virus genome may be detected in stool and blood samples.14 SARS-CoV-2 binds to target cells through receptor angiotensin converting enzyme II (ACE2)15; the RNA-seq data in the human protein atlas database demonstrated the expression of ACE2 in the liver that, in all respects, could be considered a potential target.5, 16, 17 In particular, ACE2 expression is limited to the epithelial cells of the bile duct of normal hepatic tissue and, in minimal part, in the hepatocytes.17, 18 Chai et al performed an unbiased evaluation of cell type specific expression of ACE2 in healthy hepatic tissues employing single cell RNA-seq data of two independent cohorts. This evaluation revealed significant enrichment of ACE2 expression in cholangiocytes cluster (59.7% of cells) compared to hepatocytes (2.6% of cells) suggesting that SARS-CoV-2 might directly bind to ACE2-positive cholangiocytes and the liver abnormalities of COVID-19 patients may be due not to a direct hepatocyte damage but, probably, to cholangiocyte dysfunction.16 However, the first postmortem liver biopsy of one 55 years old who died from COVID-19 did not report viral inclusions. But the histological analysis of hepatic tissue showed moderate microvascular steatosis and mild lobular and portal activity19 together with an overactivation of T cells, indicating that the liver injury could have been caused by either SARS-CoV-2 infection or treatment.5, 20 Liver autopsies of several Chinese patients revealed the presence of hepatomegaly with hepatocyte degeneration accompanied by lobular focal necrosis, exudation of neutrophils, lymphocytes and monocytes in the portal area, and congestion of hepatic sinuses with microthrombosis. However, neither histological features of liver nor bile duct injuries have been observed.21, 22 These data suggest that liver injury in COVID-19 is likely inflammatory and immune mediated rather than direct cytopathic damage as described in other viral respiratory diseases.19 In fact, one of the major determining mechanisms for COVID-19 progression is the cytokine release occurrence, the so-called ‘cytokine storm syndrome’ (CSS) (Figure 1).23
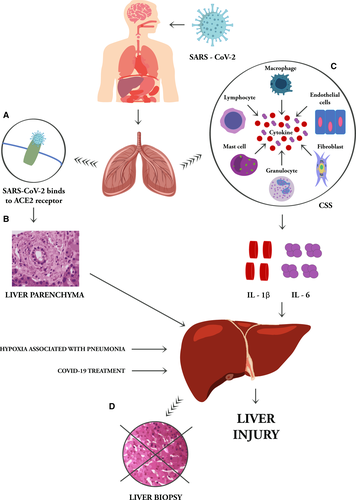
Cytokine storm syndrome consists a set of different conditions identified by a clinical phenotype of systemic inflammation, multiorgan failure, hyperferritinemia and, if untreated, often fatal. This clinical picture is a consequence of the release into the circulation of a large amount of inflammatory mediators resulting from the activation and amplification of the unmanaged immune feedforward.24, 25 When SARS-CoV-2 infects the upper and lower respiratory tract, it can cause a range from asymptomatic, mild, moderate, up to severe disease resulting in activation of intracellular inflammasomes and release of pro-inflammatory cytokines, including interleukin (IL)-1β and IL-6. IL-6 is a pleiotropic cytokine that exerts multiple functions in the body. In the liver, IL-6 is an important inducer of the acute phase response and infection defense.26 On target cells, IL-6 can bind to the signal transducing subunit gp130 either in complex with the membrane-bound or with the soluble IL-6 receptor to induce intracellular signalling. By the latter'trans-signalling’ mechanism, IL-6 can target monocyte chemotaxis and maintain sustained chronic inflammation towards any injured tissue.27-29 The hyperactivated inflammatory-immune responses, lymphopenia and CSS occurring in SARS-CoV-2 infection can impaired and damage many organs, including the gut and liver.22, 30 An increasing collection of data suggest that the treatment of cytokine storm, interrupting the signal transduction pathway of IL-6 by Tocilizumab, may be effective in patients with severe outcome.31
Analysing different factors of risk associated with liver injury in COVID-19 patients, Lu et al observed that lymphopenia and C reactive protein (CRP) level were independently associated with liver injury, suggesting that the CSS may play a major prominent role in liver damage in these patients.32, 33 Under responses to stress conditions, the immune balance is altered, as well as during the COVID-19 infection and the homoeostatic role of the liver in maintaining immune tolerance through the intestine-liver axis is altered indeed. Liver contains most macrophages (Kupffer cells) in the body and is a potent cytokine producer. Concurrent hepatic disease states that dysregulate liver innate immune status might play a critical role in COVID-19 outcome and worsening evolution, even if not directly linked.19 Specifically, obesity and Non-Alcoholic Fatty Liver Disease (NAFLD) have been associated with an increased production of pro-inflammatory cytokines Kupffer cells.34 In NAFLD patients the polarization of macrophagic state is altered and could affect the host's inflammatory or tolerant response to SARS-CoV-2 signals activated by the intestine-liver axis.19 Through the analysis of postmortem liver biopsies in patients with NAFLD affected by COVID19, Ji et al described liver lesions as likely immune mediated rather than the result of a viral direct cytopathic damage.20 Stemming from this evidence, a condition of increasing global prevalence as NAFLD, appears to represent a risk factor for progression of COVID-19 to a worsening severe condition.
2 COVID-19 AND LIVER TRANSPLANT
SARS-CoV-2 infects all age groups. Organ transplant recipients are a susceptible population.30, 35 Whether organ transplantation should be carried out during this pandemic is still controversial. If transplantation surgery could be carried out after careful risk assessments, or it should be suspended or limited to patients whose conditions is deemed life threatening, is still an open debate.36, 37
The number of confirmed COVID-19 cases after liver transplantation is limited. There is no evidence that the incubation period in liver transplant recipients is different from general population: there may be only low-grade fever or no fever at all38; dry cough, loss of smell and taste, and other common symptoms are also present.8
There is a risk of COVID-19 transmission from donor to recipient, both from deceased donors and living donors. The risk of donor-derived infection would depend upon donor exposure, infectivity during incubation period, degree and duration of viremia, and viability of the virus within blood or specific organ compartments.39 The virus was also isolated from the blood in up to 15% of cases and therefore, hypothetically, all organs may be at risk of infection.10 For these reasons, all donors and recipients should be tested for SARS-CoV-2.40 This approach to donation may differ in countries depending on the degree of community transmission of COVID-19.
In Italy, where a significant community transmission has been increasing, deceased donor screening by nucleic acid testing for SARS-CoV-2 on bronchoalveolar lavage sample has become mandatory since February 23, 2020.37
The Tertiary Liver Transplant Center in France suggests that all liver transplant donors and candidates without symptoms or diagnosis of COVID-19 in an epidemic area should be tested for SARS-CoV-2 in the bronchoalveolar lavage or nasopharyngeal swab specimens collected before validating organ procurement and transplant procedure in order to assure recipient's and organ procurement teams’ safety. Liver transplant candidates diagnosed with COVID-19 are temporary suspended for liver transplantation and are re-tested 8 days later by nasal-swab.41
Recent guidelines from National Health Service Blood and Transplant (NHSBT) recommend that all potential donors have to be tested for SARS-CoV-2 and donation is suspended in case of positivity. Asymptomatic individuals, monitored following contact with a proven case of COVID-19, are excluded from donation.42 Moreover, the urgency for transplant is dependent on individual patient features as well as on the specific organ.
The American Society of Transplant Surgeons has disclosed best practice guidelines for transplantation in the COVID-19 era. They state that living donation should be suspended unless necessary.43 However, each case must be individually evaluated, with a tailored balance between risks and benefits, considering the risk of donor transmission, assessing the severity of disease in the recipient, and recognizing the potential for transmission to healthcare workers.
Unrecognized SARS-CoV-2 infection of recipients largely increased the potential for development of severe immune suppression and postsurgical infection, which may lead to multisystem organ damage or death. The therapeutic paradox is challenging in such patients: insufficient immunosuppression results in graft loss due to rejection, whereas excessive immunosuppression results in severe infections.44
The management of post-transplant patients who develop COVID-19 is still uncertain. Although comorbid conditions may increase the risk of severe disease, it is unknown whether transplant patients are at increased risk.
A case report describing the fatal outcome in a liver transplant recipient with COVID-19 does not clarify if organ transplant status may increase the risk of COVID-19 as an opportunistic infection.45 Anyhow, the dramatic combination of liver dysfunction, multiple secondary bacterial infections, kidney and respiratory failure rapidly led to death. Patient was in a state of chronic rejection. He developed multiple nosocomial infections despite changes in treatment. Following a retrospective analysis of disease course, an earlier and more aggressive approach with antiviral and antibacterial drug treatment should be warranted. Early blood cultures in similar patients are mandatory for guiding treatment decisions. The immunosuppressive drugs for suspected chronic rejection in this patient likely contributed to his increased risk of infection.45
Another case report describes the clinical outcome of a patient who received liver transplantation for hepatitis B virus-related hepatocellular carcinoma and was confirmed to have COVID-19 nine days later. The test for SARS-CoV-2 of the donor was negative. Temporary withdrawal of immunosuppressive drugs allowed patient to reacquire anti-infection immunity, which is devoted to eliminating the virus. However, while the patient was healing from pneumonia, risk of acute rejection also increased. The appropriate doses of corticosteroids throughout the process are crucial in suppressing inflammatory storms and promoting the recovery from pneumonia, without severe side effects.46
A report of two confirmed COVID-19 cases suggested that the outcome in post-transplant population might be very poor. Both patients developed a nosocomial bacterial infection during hospitalization, which warrants more careful use of steroid than usually employed in COVID-19 infection. Furthermore, the number of T cells was significantly decreased. T cell reduction is frequently observed in severe COVID-19 cases, indicating coronavirus might mainly act on T lymphocytes. The pre-exposure to the immunology impairment may exacerbate the severity of COVID-19 infection and thus impair the course of disease.47
Bhoori et al reported their experience in a transplant centre in Lombardy (Italy): three out of 111 long-term liver transplant survivors (transplanted more than 10 years ago) died following severe COVID-19 disease. All three patients were male and older than 65 years, on minimal immunosuppressive regimens and with metabolic comorbidities. They died between 3 and 12 days after the onset of pneumonia. Conversely, 3 out of 40 recently transplanted (ie, within the past 2 years) patients, fully immunosuppressed, were SARS-CoV-2 positive, and although quarantined, are all experiencing an ordinary course of disease. Given that a reactive innate immune response might be responsible for severe clinical manifestations, immunosuppression might be protective. The authors suggested that immunosuppression should not be reduced or stopped in asymptomatic liver transplant recipients.48
The results from the ELITA/ELTR COVID-19 registry suggest that mortality in liver transplant recipients might be higher in older recipients than in younger patients and could be worse in patients with longer time since transplantation.49
Antiviral drugs for COVID-19 will be available in very near future, but drug-drug interactions with immunosuppressive drugs will also need to be considered.39
3 COVID-19 AND PRE-EXISTING LIVER DISEASE
Upon the worldwide epidemiological impact of chronic liver disease, a deeply analysis of the interactions between pre-existing liver disease and COVID-19 is widely desired. However, the exact cause of the pre-existing liver conditions has not yet been outlined in literature, so a careful analysis of correlation between prognosis and SARS-CoV-2 infection is still not available. Preliminary results from an international registry highlighted that mortality correlates strongly with baseline Child-Turcotte-Pugh class and model for end-stage liver disease (MELD) score.50
Patients with advanced chronic liver diseases are undoubtedly at increased risk of infection due to immune dysfunction associated with cirrhosis and/or immunosuppressive therapies employed for autoimmune liver diseases.13 The current guidelines of European Association for the Study of the Liver (EASL) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) recommend not to reduce immunosuppressive therapy in patients with autoimmune liver disease.13 Although, in these patients, the effects of glucocorticoid administration on disease prognosis are not clear at all. In a cohort of 1099 COVID-19 patients, 2.1% displayed hepatitis B virus (HBV) infection.8 Guan et al showed that more severe cases were more likely to display HBV infection (2.4% vs 0.6%) than non-serious cases8 but chronic viral hepatitis does not appear to increase the risk of a severe course of COVID-19.13
As ACE2 receptors are expressed on cholangiocyte surfaces, it is necessary to study if SARS-CoV-2 infection worsens cholestasis in patients with primary biliary cholangitis.6 To date, the occurrence of a pre-existing liver disease has not been outlined in most studies on COVID-19 and the interaction between existing liver disease and SARS-CoV-2 infection must be deeply analysed. However, SARS-CoV-2 infection and related immune modifications could be considered a ‘second hit’ to simple fatty liver and might induce liver injury and steatohepatitis. The immunological stress is particularly problematic in cirrhotic patients, as it can trigger acute-on-chronic liver failure.22 Individuals with a more severe prognosis for COVID-19 are typically of advanced age and/or have metabolic diseases such as diabetes, cardiovascular disease and hypertension, a profile like those at increased risk of non-alcoholic fatty liver disease. So, a better understanding of the correlation between NAFLD and COVID-19 can helpful to clarify the pathogenesis with therapeutic implications. Patients with liver cirrhosis may be more susceptible to infections due to their systemic immunocompromised state.51
The 30-day mortality rate is higher in patients with moderate/severe respiratory failure and in those who have a more deteriorated liver function, as indicated by the increased MELD and CLIF-OF scores at COVID-19 diagnosis. Moreover, infection with SARS-CoV-2 led to rapid clinical deterioration in otherwise stable cirrhotic patients, independently of the aetiological agent.52
These data highlight the importance of preventing SARS-CoV-2 infection above all in patients with liver disease.
4 COVID-19 AS A CAUSE OF LIVER DISEASE
To date, it is not clear whether COVID-19-related liver dysfunction is provoked by the pathogenetic mechanism that leads to CSS or is due to the employment of hepatotoxic drugs.4, 19 Liver damage in mild COVID-19 cases is often transient and may be restored to an integrum state without any special treatment.6 Otherwise, the severe hypoxia associated with pneumonia, could also contribute to liver injury, or even develop into liver failure in patients who are seriously ill by pre-existing chronic conditions of impairment, as it happens in other clinical settings.53
Liver and kidney functions, devoted to pharmacological de-toxification and excretion, may be compromised by therapeutic agents for COVID-19 treatment. It is also possible that liver failure is due to the drug's hepatotoxicity, which can be explained based on the variability observed in the various cohorts analysed until now. It has been observed that the incidence of liver disease increases after SARS-CoV-2 infection, during the course of the disease and, above all, is due to the collateral effects of drugs used by patients.6 Most of the antipyretic agents used in the COVID-19 treatment consist of recognized acetaminophen known as inducing of the liver failure.19
The therapeutic agents for COVID-19 include: Remdesevir, Lopinavir/Ritonavir, Oseltamivir, Ribavirin and Chloroquine phosphate or hydroxychloroquine (HCQ) sulphate,54 all these drugs are all currently tested. Tocilizumab can effectively block IL-6 signal transduction pathway and is likely to become an effective drug for patients with severe COVID-19.31
Remdesivir is a nucleotide analogue that inhibits viral RNA polymerase by preventing replication. Grein et al, although in a limited cohort show that Remdesivir can be effective in severely affected patients with COVID-19 according to numerous recent studies. In addition, hepatotoxicity cannot be directly related to the use of the antiviral due to the high percentage of liver dysfunction in patients with COVID-19.55
Chloroquine and HCQ are used for chemoprophylaxis and treatment for malaria and several inflammatory diseases. With the approval of the Food and Drug Administration (FDA) they have been tested and currently used for anti- SARS-CoV-2 therapy. They are not actually used for the prevention of infection because of different harmful side adverse effects as decreased vision, nausea, digestive disorders and in more serious cases heart failure56; but a final conclusion cannot be yet reached because several clinical trials are ongoing.
Falcão MB and collegues described a case of hepatotoxicity following the intake of HCQ in a hospitalized patient with acute respiratory syndrome due to COVID-19. On day 7 of hospitalization, the patient had a transaminase level increased up to 10-fold from the normal value and rapidly decreased within 5 days after drug withdrawal. Furthermore, bilirubin, alkaline phosphatase and GGT values, prothrombin time and renal function were re-enter in the normal range. These data show that even a high dose of HCQ may display an increase of side effects.9 Rismanbaf A et al stated that synergy between adverse reactions to HCQ reactive metabolites and inflammatory processes caused by COVID-19 contributes to liver toxicity.54
Liver function should be assessed and monitored in appropriate tests in COVID-19 patients and in patients with previous liver disease to identify the cause of liver dysfunction. Additionally, hepatoprotective treatments may be needed to reduce liver damage.57
5 CONCLUSIONS
This review represents a picture of the pathophysiology and clinical manifestations of liver disorders occurring in course of COVID-19 outbreak. We focused here on the liver involvement to contribute to the understanding of COVID-19 and to management and treatment.
Abnormalities in the distribution of biochemical markers of inflammation, cardiac, muscle injury, kidney and liver function and coagulation parameters, have been highlighted in patients with COVID-19, which would lead to cataloging COVID-19 as a systemic pathology. The dramatic release of cytokines and the inflammatory-immune responses is crucial for disease progression, altering different pathophysiological circuits related to the onset and disease severity; it is currently deeply analysed to identify its diagnostic and prognostic role and to drive the therapy choice and the appropriate diagnostic tests. Clinical and laboratory parameters could be integrated in clinical prognosis scores and should be helpful in understanding the pathogenesis, as already demonstrated in other clinical settings.58
Laboratory medicine evidences and the radiological (Computer Tomography) picture could be fundamental to discriminate between severe and non-severe COVID-19 patients. The large variation in clinical features, spanning from asymptomatic to fatal, could be dealt addressed with novel laboratory biomarkers that predict promptly and more easily the COVID-19 prognosis in liver diseases patients.
ACKNOWLEDGMENT
Thanks to Ms Simona Troiano for the graphic support.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors have contributed to the work, read, approved and agreed to submit the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.