Cathelicidin Antimicrobial Peptide LL-37 in Cholesteatoma Enables Keratinocyte Reactivity with Cytosolic DNA
Abstract
The purpose of this study was to determine whether self-DNA can trigger the inflammatory response in cholesteatoma. Specimens were collected from nine patients with invasive cholesteatoma, nine patients with attic-type cholesteatoma (pars flaccida was perforated in five patients and intact in four) and four healthy skins. Expression and localization of LL-37 and interferon-alpha were detected by immunofluorescence and immunoblot analysis. Cultures of human cholesteatomatous keratinocytes were exposed to CpG DNA, LL-37 or CpG DNA complexed to LL-37 for 24 h. Expression of interferon-alpha was detected by RT-PCR. We detected abundant cytosolic DNA, increased LL-37 and interferon-alpha in keratinocytes in invasive cholesteatoma and attic-type cholesteatoma with pars flaccida perforation, but not in attic-type cholesteatoma with pars flaccida intact and normal skin. In cultured keratinocytes, LL-37–DNA complexes induced IFN-α expression. These data suggest that cytosolic DNA is an important disease-associated molecular pattern that triggers the inflammation response in cholesteatoma. Furthermore, LL-37 played an important role in DNA-triggered inflammation. Thus, we have identified a link between cytosolic DNA, LL-37 and autoinflammation in cholesteatoma, providing new potential targets for the treatment of this disease.
Introduction
Cholesteatomas are epidermal inclusion cysts of the middle ear or mastoid. They are composed of desquamated debris (principally keratin), an epithelial matrix and connective tissue (perimatrix), including granulation tissue and an inflammatory cell infiltrate 1. Cholesteatoma is categorized as congenital or acquired 2. Acquired cholesteatoma is generally subdivided into primary and secondary.
The most common cause of acquired primary cholesteatoma is the progression of disease from a retraction pocket in the eardrum. Retraction pockets can occur in several locations. The pars flaccida and the posterosuperior portion of the pars tensa are the most common sites of origin. One reason for acquired secondary cholesteatoma is from migration of squamous epithelium inward from the margin of a perforation to invade and displace mucosal lining of the middle ear. It is hypothesized that in some tympanic membrane perforations, inflammation damages the inner mucosal lining of the tympanic membrane, allowing the outer keratinizing epithelium to migrate inward and generate a cholesteatoma 3, 4. Acquired secondary cholesteatoma is generally associated with an intense inflammatory reaction; acquired primary cholesteatoma is not associated with any infection or inflammatory reaction. Understanding the mechanism of the inflammatory reaction in cholesteatoma seems to be crucial; however, the mechanisms underlying the inflammatory reaction in cholesteatoma remain unknown.
Apoptosis has been noted in cholesteatoma 5. Park 6 noted a greater number of apoptotic cells in the cholesteatoma epithelium than in normal retroauricular skin. During apoptotic and necrotic cell death, host-derived (self)-DNA is released into the extracellular environment 7, where it may modulate immune responses, stimulating and inhibiting immune cell activity 8. The activity of extracellular DNA depends on concentration and context, with DNA in the form of immune complexes potently stimulating the production of interferon (IFN)-α 9-11.
To preserve the integrity of the organism, there should be barriers that prevent immune response to extracellular self-DNA. However, binding of self-DNA with LL-37 can break these barriers by forming aggregated and condensed structures that are protected from nuclease degradation, delivered to endocytic compartments of the mammalian cell and retained within early endosomes to sustain TLR9 activation. This leads to robust production of type I IFNs that initiate an antiviral-like immune response 12.
Antimicrobial peptides are major participants in the defence system 13. LL-37, also known as human cationic antimicrobial protein (hCAP18), is the only identified member of the cathelicidin family and plays a vital role in cutaneous innate immunity 14-19. Many cell types produce LL-37, including keratinocytes 20, neutrophils 21, monocytes 22 and macrophages 23. LL-37 is expressed scarcely, if at all, in normal skin, but expression of these peptides increases when skin is affected by external stimulation such as trauma, inflammation and infection 16.
LL-37 may have a dual role in immunity because it can kill microbes and trigger various host tissue responses 24. LL-37 acts as a chemoattractant for neutrophils, monocytes and T cells by binding N-formyl peptide receptor-like 1 25. LL-37 also induces interleukin-1β in monocytes via activation of P2X7, a nucleotide receptor 26. A major link between LL-37 and the initiation of immunity was revealed by the discovery that LL-37 can convert otherwise inert extracellular self-DNA into a potent trigger of the IFN response in pDCs 12. Thus, LL-37 can break innate tolerance to self-DNA and initiate an antiviral-like immune response. If cholesteatomatous keratinocytes can release LL-37, it may provide a critical cofactor for recognition of self-DNA; this, in turn, can be a critical element in the pathogenesis of the inflammatory reaction for cholesteatoma.
Myxovirus resistance A (MxA) is specifically induced by IFN-α/β. Other cytokines, including IFN-γ, are poor inducers of MxA 27. Moreover, MxA protein is very stable, making it detectable even weeks after IFN production has ceased 28, 29. In contrast, direct measurement of IFN is difficult because of its low concentration and short half-life. Therefore, MxA is an ideal marker of type I interferon signalling.
Here, we investigated self-DNA as a trigger for the inflammatory response in cholesteatoma. We also studied the role of the cathelicidin peptide LL-37 in DNA-triggered inflammation.
Materials and methods
Patients
All specimens including cholesteatomatous matrix, perimatrix and their contents were obtained from nine patients (4 males and 5 females) with invasive cholesteatoma (mean age: 49 ± 14 years) and nine patients (pars flaccida in five patients was perforated and intact in four) with attic-type cholesteatoma (4 males and 5 females, mean age: 44.7 ± 11 years) during tympanoplasty. The otoscopic appearance of the attic-type cholesteatoma is retraction of the pars flaccida and debris, both of which are characteristic of cholesteatoma of the attic portion. In patients with invasive cholesteatoma, keratinizing epithelium has migrated through a perforation into the middle ear. None of these patients had received steroids prior to surgery. In addition, tiny biopsy-obtained specimens from the external canal skin of four patients undergoing tympanoplasty for chronic otitis media served as a control group. The study was performed in accordance with the Helsinki Declaration, and written informed consent was given by all patients.
Immunofluorescence
Each specimen was fixed overnight in 10% buffered formalin at room temperature and embedded in paraffin. Serial sections were cut at 7-μm thickness and placed on 3-aminopropyltriethoxysilane-coated glass slides. Immunofluorescence was performed to examine the expression of LL-37 (Abcam, Cambridge, MA, USA) and MxA (Genetex, Irvine, CA, USA) in tissue sections. Paraffin sections were deparaffinized with toluene and rehydrated with serially graded ethanol solutions. After rinsing in PBS, the sections were preincubated with 10% normal goat serum for 1 h to block non-specific reaction with the first antibody. The sections were incubated with the working solution of antibodies against LL-37 (1:100) and MxA (1:250) at 4 °C overnight. After washing with PBS three times, the sections were incubated with Alexa 546-labelled goat anti-rabbit/mouse antibody for 1 h at room temperature. The slides were washed with PBS, and blue fluorescent DAPI was used for nuclear counterstaining. Sections were incubated with PBS instead of the primary antibody (against LL-37 and MxA) as negative control.
Western blotting
Tissue samples were homogenized in ice-cold lysis buffer (Beyotime Biotechnology, Haimen, China) with 1% PMSF (phenylmethylsulfonyl fluoride). Samples were centrifuged at 10,000 × g for 20 min at 4 °C, and the protein concentration in the supernatant was determined by the BCA (bicinchoninic acid) protein assay kit (Beyotime, China). Proteins were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were washed with Tris-buffered saline–Tween-20 (TBST; 20 mm Tris, 500 mm NaCl and 0.1% Tween-20) and blocked with 5% non-fat dry milk in TBST for 1 h. After washing three times with TBST, membranes were incubated with LL-37/MxA overnight at 4 °C. The membranes were washed three times with TBST and incubated with HRP-conjugated secondary antibody in blocking buffer for 1 h. After washing three times with TBST, immunoreactive bands were visualized by incubation with ECL plus detection reagents (GE Healthcare, Piscataway, NJ, USA) for 5 min and exposure to ECL Hyperfilm (GE Healthcare). Densitometry was performed with NIH Image 1.63 software (National Institutes of Health, Bethesda, MD, USA). Protein expression was normalized to the quantity of β-actin. The grey values were expressed relative to the control and presented as means ± SD.
TUNEL staining
Paraffin sections of the attic-type cholesteatoma, invasive cholesteatoma and healthy skin were deparaffinized and rehydrated, and antigen retrieval was performed in citrate buffer (10 mm, pH 6). For positive controls, some sections were treated with DNase I (1500 U/ml; Thermo Fisher) before staining. TUNEL was performed according to the manufacturer's instructions (In situ Cell Death Detection Kit, TMR red; Roche, Indianapolis, IN, USA).
Cell culture and stimuli
Tissue from the attic-type cholesteatoma with an intact pars flaccida was hand-carried to the laboratory 2 h after resection and transferred into 5 ml Hanks' balanced salt solution (HBSS). The tissue was cut into small pieces with scissors and digested with 20 U/ml collagenase IV (Sigma Aldrich, St Louis, MO, USA) at 4 °C overnight. The digested cells were washed twice with HBSS and centrifuged for at 1500 rpm for 5 min. The pellet was removed, added to 10 ml keratinocyte-free media (KSFM) with 100 μl Pen Strep (Invitrogen, Carlsbad, CA, USA) and cultured in humidified CO2 at 37 °C. The KSFM and antibiotics were changed every 3 days. Cells at 60% confluence were stimulated with CpG oligodeoxynucleotides M362 (2 μm/l, Invivogen, San Digo, CA, USA) and synthetic cathelicidin peptide LL-37 (10 μg/ml; Innovage) in six-well flat-bottom plates (Coring incorporated Life Sciences) for 24 h.
Quantitative real-time PCR
Total RNA from cultured keratinocytes was extracted using Trizol (Invitrogen) according to the manufacturer's instructions. After purification, complementary DNA (cDNA) was synthesized from 1 μg total RNA using the first-strand cDNA synthesis kit (TaKaRa, Otsu, Japan) according to the manufacturer's instructions. IFN-α and β-actin were amplified by quantitative real-time RT-PCR. qRT-PCR was performed with SYBR® Premix Ex Taq GC (TaKaRa) in a LightCycler (Roche Diagnostics, Indianapolis, IN, USA). Individual RT-PCR tests were repeated on at least three independent occasions. Melting curve analysis was performed to verify that only one product was amplified. Data were analysed by the 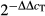 method with β-actin for normalization.
method with β-actin for normalization.
Primer: IFN-α: 5′-GACTCCATCTTGGCTGTGA-3′, 5′-TGATTTCTGCTCTGACAACCT-3′; β-actin: 5′-CTCTTCCAGCCTTCCTTCCT-3′, 5′-AGCACTGTGTTGGCGTACAG-3′.
Statistical analysis
Statistical analysis was performed using independent-sample t-test provided in SPSS (version 11.5). Statistical significance was defined as P < 0.05 for all analyses, and data are presented as mean ± standard deviation (M ± SD).
Results
TUNEL staining of cytosolic DNA in keratinocyte in cholesteatoma
To detect cytosolic DNA in middle ear cholesteatoma, we stained paraffin sections using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labelling (TUNEL). DNA fragments were detectable in the cytosol of keratinocytes in all patients with middle cholesteatoma (Fig. 1H–M), but not in sections from healthy skin (Fig. 1A–C). A summary of these findings is shown in Table 1.
| Cytosolic DNA | LL-37 | MxA | |
|---|---|---|---|
| Normal skin | − | 0.21 ± 0.19 | − |
| Attic-type cholesteatoma with an intact pars flaccida | + | − | − |
| Attic-type cholesteatoma with pars flaccida perforation | + | 0.96 ± 1.32a,b | 0.39 ± 0.22a,b |
| Invasive cholesteatoma | + | 1.07 ± 1.15a,b | 1.27 ± 0.73a,b,c |
- a P < 0.05: compared with the normal skin group.
- b P < 0.05: compared with the attic-type cholesteatoma with an intact pars flaccida group.
- c P < 0.05: compared with the attic-type cholesteatoma with pars flaccida perforation group.
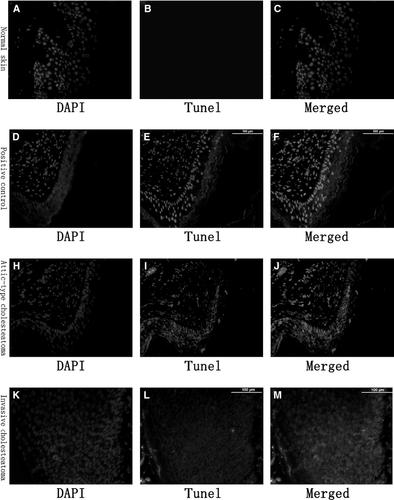
Immunostaining of LL-37 and MxA in cholesteatoma and normal skin tissues
Immunofluorescence staining showed the expression of LL-37 in the cytoplasm mainly in the granular, prickle and basal layers in invasive cholesteatoma epithelium (Fig. 2K–M) and attic-type cholesteatoma with pars flaccida perforation (Fig. 2H–J). However, we could not detect the expression of LL-37 in attic-type cholesteatoma with an intact pars flaccida (Fig. 2D–F). The normal skin of the external ear canal revealed that LL-37 expression was localized to granular and upper prickle layers (Fig. 2A–C), but its expression was lower than the expression of invasive cholesteatoma epithelium and attic-type cholesteatoma with pars flaccida perforation. LL-37 expression was much greater in invasive cholesteatoma epithelium and attic-type cholesteatoma with pars flaccida perforation than in normal ear canal skin, and the expression was extended to the basal layer.
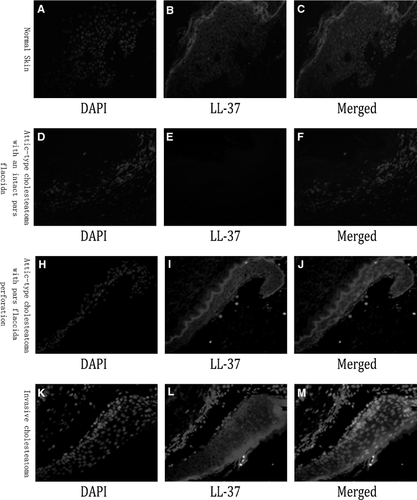
In invasive cholesteatoma (Fig. 3K–M) and attic-type cholesteatoma with pars flaccida perforation (Fig. 3H–J), MxA was expressed mainly in the epidermis. Intracellular MxA was predominantly localized to the cytoplasm in the epidermis. However, no MxA expression was detected in attic-type cholesteatoma with intact pars flaccida (Fig. 3D–F) and normal skin (Fig. 3A–C).
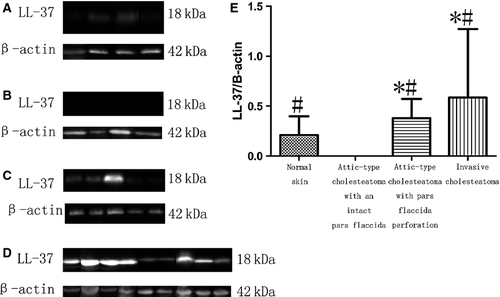
Immunoblot of LL-37 and MxA in cholesteatoma and normal skin tissues
Western blotting revealed that LL-37 expression differed in these specimens. The ratio of β-actin to LL-37 in invasive cholesteatoma (Fig. 4D), attic-type cholesteatoma with pars flaccida perforation (Fig. 4C) and normal skin (Fig. 4A) was 1.07 ± 1.15, 0.96 ± 1.32 and 0.21 ± 0.19, respectively. However, we did not detect LL-37 expression in attic-type cholesteatoma with an intact pars flaccida (Fig. 4B). The LL-37 level in invasive cholesteatoma and attic-type cholesteatoma with pars flaccida perforation was significantly higher than in attic-type cholesteatoma with an intact pars flaccida and normal skin (P < 0.05) (Fig. 4E). However, LL-37 concentration did not differ significantly between attic-type cholesteatoma with pars flaccida perforation and invasive cholesteatoma (P = 0.91). A summary of these results is shown in Table 1.
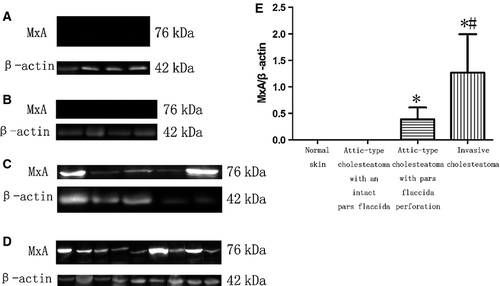
Myxovirus resistance A levels in these specimens varied. There was almost no expression in normal skin (Fig. 5A) and attic-type cholesteatoma with an intact pars flaccida (Fig. 5B). The ratio of β-actin to MxA in invasive cholesteatoma (Fig. 5D) and attic-type cholesteatoma with pars flaccida perforation (Fig. 5C) was 1.27 ± 0.73 and 0.39 ± 0.22, respectively. A consistently higher degree of cytoplasmic MxA expression was observed in invasive cholesteatoma than in attic-type cholesteatoma with pars flaccida perforation (P < 0.05) and normal skin (Fig. 5E). A summary of these findings is shown in Table 1.
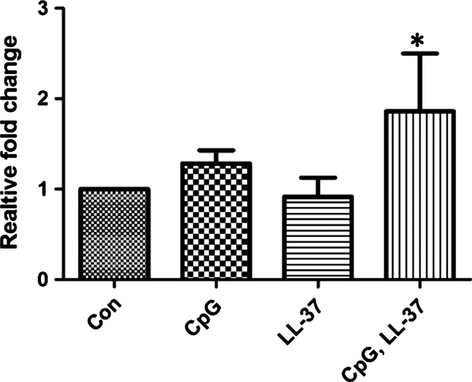
LL-37 complexed to CPG DNA induces expression of IFN-α messenger RNA in cholesteatomatous keratinocytes
LL-37 and DNA are thought to form a complex between the peptide and nucleic acids to induce IFN-α expression in pDCs (Lande et al.). We next evaluated whether the presence of LL-37 could influence IFN-α expression in cholesteatomatous keratinocytes. Cultures of human cholesteatomatous keratinocytes were exposed to CpG DNA, LL-37 or CpG DNA complexed to LL-37 for 24 h. Keratinocytes cultured with CpG DNA complexed to LL-37, but not CpG DNA or LL-37 alone, increased the expression of IFN-α mRNA (Fig. 6).
Discussion
We observed increased expression of cytosolic DNA in middle ear cholesteatoma. Although eukaryotic cells store DNA primarily in the nucleus and mitochondria, we detected DNA fragments in the cytosol of keratinocytes in cholesteatoma. Cytosolic DNA was identified as a trigger for TLR9 activation with an antiviral-like IFN response. Thus, cytosolic DNA may provide a major disease-associated molecular pattern that might promote inflammation in middle ear cholesteatoma. However, the source of cytosolic DNA in cholesteatomatous keratinocytes remains unknown. Proliferation and apoptosis are both present in cholesteatoma 5. During apoptotic cell death, host-derived DNA can be released into the extracellular environment 7, so it is possible that extracellular DNA from surrounding apoptotic cells may be taken up. It may thus constitute a source of cytosolic DNA in cholesteatomatous keratinocytes.
The proinflammatory mechanism in psoriasis was recently attributed to self-DNA: complexes of extracellular DNA with LL-37 activated TLR9 in pDCs, generating a type I IFN response 12. Because LL-37 and IFN-α were strongly expressed in acquired secondary cholesteatoma and primary cholesteatoma with tympanum perforation, we hypothesized that LL-37 might contribute to IFN-α activation in the same way. Indeed, we have shown that cholesteatomatous keratinocytes will respond to DNA with an LL-37-dependent increase in IFN-α. These data suggest that LL-37 could serve as a proinflammatory factor in cholesteatoma by promoting uptake of self-DNA into keratinocytes, leading to increased IFN-α. Thus, LL-37 might act as a physiological trigger of inflammation in cholesteatoma.
LL-37 is a carboxy-terminal peptide fragment derived from the human cathelicidin precursor protein hCAP18. This peptide has the capacity to kill a wide variety of microbes and can modify host immune and growth responses. In this study, LL-37 formed a complex with DNA fragments to induce IFN-α in cholesteatomatous keratinocytes. Thus, the abundant keratinocytes expressing LL-37 in cholesteatoma might play a critical role in inflammation.
LL-37 is produced and secreted by keratinocytes and neutrophils. Expression of LL-37 is specific to the cell, tissue and disease state. LL-37 was found at low levels in normal skin 30. In contrast, excess LL-37 is observed in the inflammatory skin disease rosacea 30. LL-37 expression in keratinocytes was also strongly induced by skin injury. In this study, LL-37 was strongly expressed in primary cholesteatoma with tympanum perforation, but not in primary cholesteatoma with an intact tympanum. We suggest that the difference is attributable to rupture of the cholesteatomatous sac, which might induce strong expression of LL-37 in primary cholesteatoma with tympanum perforation. Rupture of the cholesteatoma matrix might induce the inflammatory response 31. Thus, our results indicated that rupture brought on inflammation that might have an indirect effect on the expression of LL-37.
In summary, activation of keratinocytes by LL-37–DNA complexes greatly increases the production of IFN-α. In addition, cytosolic DNA fragments were present in the cholesteatomatous epithelium. These events suggest that persistent overexpression of LL-37 in cholesteatoma leads to breakdown of innate tolerance to self-DNA, which leads to keratinocyte recognition of self-DNA and initiation of the immune response.
Acknowledgment
We would like to thank all patients for their collaboration. This work was supported by the Shanghai Shen-kang Medical Science Project SHDC12010119 and Ministry Clinical Disciplines Project 2010-439.
Competing interests
The authors declare that they have no competing interests.




