IL-17 and Glutamate Excitotoxicity in the Pathogenesis of Multiple Sclerosis
Abstract
Immunoinflammatory-mediated demyelination, the main pathological feature of multiple sclerosis (MS), is regularly accompanied by neurodegenerative processes, mostly in the form of axonal degeneration, which could be initiated by glutamate excitotoxicity. In the current study, the relationship between Th17-mediated inflammatory and excitotoxic events was investigated during an active phase of MS. Cerebrospinal fluid (CSF) of patients with MS and control subjects was collected, and IL-17A and glutamate levels were determined. IL-17A level was significantly higher in patients with MS; whereas no statistically significant changes in glutamate concentrations were found. There was a direct correlation between IL-17A and glutamate levels; IL-17A levels were also associated with the neutrophil expansion in CSF and blood–brain barrier disruption. However, IL-17A level and the number of neutrophils tended to fall with disease duration. The results suggest that Th17 cells might enhance and use glutamate excitotoxicity as an effector mechanism in the MS pathogenesis. Furthermore, Th17 immune response, as well as neutrophils, could be more important for MS onset rather than further disease development and progression, what could explain why some MS clinical trials, targeting Th17 cells in the later stage of the disease, failed to provide any clinical benefit.
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disorder of the central nervous system (CNS) characterized by episodic neurological deficits disseminated in time and a neuroanatomical location 1. There seems to be an extensive heterogeneity in disease aetiopathogenic mechanisms, but an autoimmune origin of demyelinating processes is postulated in the majority of cases.
Traditionally, MS was considered to be a Th1-cell-driven autoimmune disease that develops in genetically susceptible individuals 2. However, many features of MS could not be explained solely by Th1-mediated brain inflammation, but they rather point to a more elaborate pathogenesis engaging different Th cell subsets. Almost a decade ago, Th17 cells were identified as a novel IL-17-producing subset of CD4+ T cells 3. Further studies disclosed that Th17 cells are characterized by much larger cytokine secretion milieu, including IL-17A, IL-17F, IL-22, IL-26, granulocyte–macrophage colony-stimulating factor (GM-CSF) and tumour necrosis factor α (TNF-α) 4-6 and the expression of retinoic acid-related orphan receptor γt (RORγt) as a major transcription factor 4, 7. The possible pathogenic role and involvement of Th17 cells in MS pathogenesis has been strongly emphasized, mainly based on the data obtained from experimental autoimmune encephalomyelitis (EAE), a rodent model of MS 8, 9. In humans, in MS plaques isolated at autopsy, an increase in IL-17 mRNA is detected 10, and IL-17-producing cells are indeed identified in lesions of MS brain sections, but not in normal-appearing white matter or non-inflamed brain specimens 11. Moreover, a significant increase in the number of Th17 cells is detected in the cerebrospinal fluid (CSF) during a disease exacerbation that was not observed in the Th1 cells 12.
Besides the immunoinflammatory-mediated demyelination, which is considered to be pathological hallmark of MS, glutamate excitotoxicity has recently emerged as a valid mechanism involved in MS pathogenesis. It is a phenomenon that takes place when an excessive amount of excitatory neurotransmitter, glutamate overactivates its glutamate receptors, inducing the augmentation of intracellular Ca2+ level and leading to cell death 13. In EAE model, this mechanism is recognized as a major instrument of axonal damage, but most importantly it is also implicated in oligodendrocyte depletion and demyelinating processes 14, 15, what summarily identifies glutamate excitotoxicity as a tool that could cause the entire pathological substrate of MS. Although today glutamate excitotoxicity is considered to be important in the MS development, there are insufficient data about relationship between immunoinflammatory and excitotoxic events observed in MS.
In the current study, we aimed to investigate the relationship between Th17-mediated inflammatory and excitotoxic events in MS pathogenesis. To assess Th17 cell expansion in encephalic compartment, we estimated the level of IL-17A (the major secretion product of Th17 cells) in CSF and correlated it with CSF glutamate level, as a parameter of excitotoxic damage. Furthermore, we also explored the association of CSF IL-17A and glutamate levels with clinical and biochemical parameters of patients with MS.
Patients and methods
Patient and sample characterization
The study included 79 subjects, median age 54 (18–73), both males and females, divided into two groups: a control and MS group.
The control group included 40 patients, 29 men and 11 women, with urologic pathology, admitted to the University Clinic of Urology, Clinical Center Nis, Serbia, between October 2011 and June 2012. Selected patients required surgical procedures planned to be performed under spinal anaesthesia and had no previous medical history of neurological disorders. During pre-operative preparation, after an overnight fasting, under aseptic conditions, CSF was collected by lumbar puncture from L4/L5 intervertebral space, in the seating position, before injecting the anaesthetic for spinal anaesthesia.
The MS group included 39 patients, 13 men and 26 women, examined at the University Clinic of Neurology, Clinical Center Nis, Serbia between October 2011 and August 2012. All patients were diagnosed with MS based on the 2010 revised McDonald criteria and underwent lumbar puncture for diagnostic purposes. Immediately after L4/L5 lumbar puncture in the seating position, 500 μl of collected CSF sample was separated for the study analyses while the rest was used for standard diagnostic panels. Only the patients that exhibited oligoclonal IgG bands in CSF, but did not receive any immunosuppressive or immunomodulatory therapy for at least 3 months, and did not have an acute infection according to blood analyses (Table 1), were included in the study. Also, all selected patients did not meet diagnostic criteria for neuromyelitis optica (NMO) 16, 17. Patients' age, gender, duration of the disease, clinical state and standard biochemical blood and CSF analyses were also examined based on the medical records of the hospitalization. The duration of the disease was defined as the interval between the first signs of the disease onset and the hospitalization when CSF was obtained. A patient's clinical state was accessed using The Expanded Disability Status Scale (EDSS) score 18. Standard biochemical blood and CSF analyses were performed by the referent laboratory (Central Biochemical Laboratory, Clinical Centre Nis, Nis, Serbia), and blood–brain barrier (BBB) permeability was estimated by albumin CSF/serum ratio. Detailed patient characteristics are shown in Table 1.
| MS (n = 39) | Control (n = 40) | ||
|---|---|---|---|
| Clinical characteristics | Median (range) | Median (range) | |
| Gender (male/female) | 13/26 | 29/11 | |
| Age (years) | 40 (18–64) | 65 (31–73) | |
| Duration of the disease (years) | 1 (0.08–11) | / | |
| EDSS score | 3.5 (1–7) | / | |
| Annualized relapse rate | 1 (0.36–2) | / | |
| Phase of the disease | Relapse | / | |
| Days elapsed between initiation of relapse and CSF withdrawal | 7 (1–14) | / | |
| Blood analyses | Median (range) | Reference rangea | |
| WBC (×109/l) | 7.20 (4.14–11.20) | 3.90–10.00 | / |
| Neutrophils (×109/l) | 4.15 (1.92–8.80) | 1.60–7.00 | / |
| Monocytes (×109/l) | 0.55 (0.28–1.20) | 0.10–1.00 | / |
| Lymphocytes (×109/l) | 2.20 (1.11–3.36) | 0.80–5.00 | / |
| CRP (mg/l) | 0.60 (0.20–7.40) | 0.00–5.00 | / |
| CSF analyses | Median (range) | Reference rangea | |
| WBC (/μl): | 6 (0–26) | <5 | |
| Neutrophils (%) or (/μl) | 6 (0–23) or 1 (0–6) | 0–3 or 0 | / |
| Monocytes (%) or (/μl) | 20 (0–42) or 1 (0–8) | 20–40 or 0–2 | / |
| Lymphocytes (%) or (/μl) | 67 (0–96) or 2 (0–17) | 60–80 or 0–4 | / |
| BBB permeability (%) | 1.16 (0.49–2.21) | <0.70 | / |
| Oligoclonal IgG bands | Present | Absent | / |
- BBB, blood–brain barrier; CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; WBC, white blood cells.
- a Used by referent laboratory (Central Biochemical Laboratory, Clinical Centre Nis, Nis, Serbia).
All patients were promptly informed about the relevant details concerning their participation in the study and gave written informed consents. This study was approved by the Ethic Committee of Medical Faculty, University of Nis, Serbia under the number 01-7289-5.
Sample analyses
Immediately after the collection, CSF samples used for IL-17A and glutamate determination were aliquoted into Eppendorf tubes, centrifuged at +4 °C for 5 min. at 1000 g and within 15 min frozen at −80 °C until the analysis.
Cerebrospinal fluid glutamate levels were determined by high-performance liquid chromatography (HPLC) using Agilent 1200 Series Rapid Resolution LC System with the 1200 series fluorescence detector (FLD), set at 340 nm (excitation) and 445 nm (emission wavelength) (Agilent, Santa Clara, CA, USA). Stationary phase was a Zorbax SB-C18 column (3.5 μm particle size), 3.0 × 150 mm (Agilent). Mobile phases were solvent A (phosphate buffer: 50 mm, pH 6.8) and solvent B (methanol: HPLC purity). After thawing, CSF samples were deproteinized by vortexing 150 μl of sample with 30 μl of perchloric acid (1 m) and incubation lasting for 2 min. Samples were then neutralized with 20 μl of potassium carbonate (1 m), vortexed and centrifuged for 5 min at 10,000 g. Afterwards, derivatization was performed by mixing 100 μl of supernatant with 100 μl of ortho-phthaldialdehyde (OPA) and incubation for 2 min. After the injection of 10 μl of the sample, a stepped gradient at a flow rate of 0.6 ml/min was applied (0–4 min/12–15% of solvent B; 4–8 min/15–80% of solvent B; 8–11 min/80% of solvent B; 11–15 min/80–15% of solvent B; 15–16 min/15–12% of solvent B). The temperature of the column was 40 °C, and the sample analysis lasted for 16 min in total. A standard mixture of l-Glutamic Acid ReagentPlus ≥99% TLC (Sigma-Aldrich, St. Louis, MO, USA) was analysed as an external standard before CSF samples. A sample peak area was compared with the area of glutamate standard of a known concentration, which allowed calculating the measured sample concentrations.
Cerebrospinal fluid IL-17A levels were determined by quantitative enzyme-linked immunosorbent assay (ELISA), using the Human IL-17A High Sensitivity ELISA kit (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions. The assay sensitivity was 0.01 pg/ml.
Cell counting in CSF of patients with MS was carried out manually by referent laboratory, Central Biochemical Laboratory, Clinical Centre Nis, Serbia. Total leucocyte count was performed in Fuchs–Rosenthal chamber: the cells were counted in at least five group squares of the chamber; if there were fewer than 20 cells in a group square, the cells in the entire chamber were counted (3.2 μl) to improve the quality of the count results. Results were expressed as the number of cells per μl. Differential leucocyte count was performed on stained cytological smears. Briefly, CSF samples were cytospined (800 rpm or 110 g) onto poly-L-lysine-coated slides and stained by May–Grunwald–Giemsa stain. Under the microscope, the number of each leucocyte type was determined, percentage calculated, and according to total leucocyte count, the results were also presented in the number of cell per μl.
Statistical analyses
The whole statistical analysis of data was carried out using the SPSS 17.0 statistical software system (SPSS Inc., Chicago, IL, USA). Due to the uneven distribution of our data (Shapiro–Wilk test), the statistical analysis was performed with nonparametric tests. Descriptive analysis of parameters measured in CSF and blood, included the median value and range. Differences between two groups were evaluated by Mann–Whitney U-test. The interdependence of the data from different groups was assessed with Spearman nonparametric correlation test. Reported P-values, <0.05, were considered statistically significant.
Results
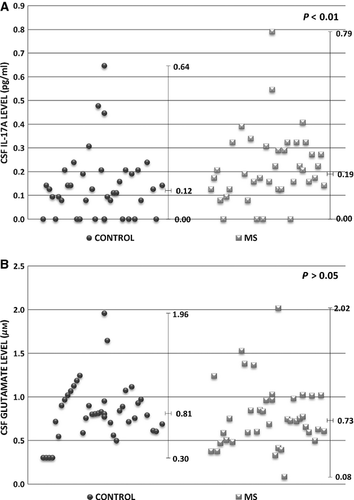
To assess Th17 cell expansion in the control and MS group, the level of IL-17A was determined in CSF. Generally, IL-17A level was significantly higher in CSF of the patients suffering from MS when compared with the control subjects (P = 0.003; Fig. 1A). However, no statistically significant changes in CSF glutamate level were found (Fig. 1B).
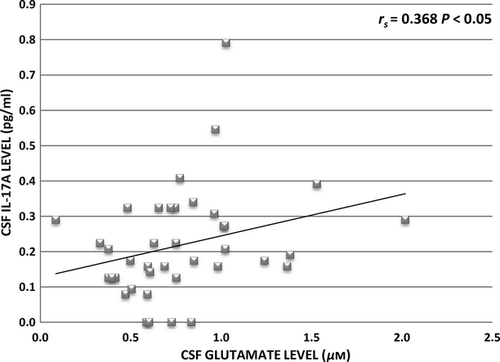
To analyse the relationship between inflammatory and excitotoxic processes in MS, CSF IL-17A level was compared with CSF glutamate level, and a significant positive correlation was found in the MS group (rs = 0.368; P < 0.05; Fig. 2).
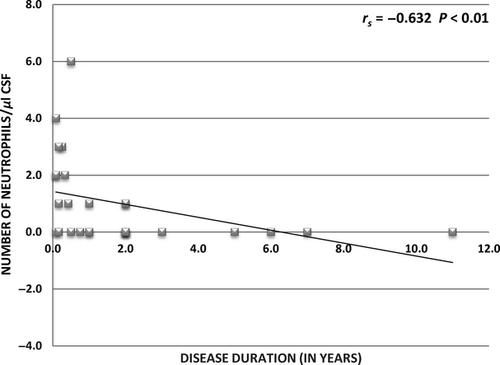
The relationship between the level of IL-17A and glutamate in CSF, patient clinical characteristics and CSF parameters (cytological examination and BBB permeability) were also examined. Statistically significant positive correlation was found between IL-17A level, number of neutrophils in CSF and BBB disruption, whereas an inverse correlation between IL-17A level and the disease duration was observed. The results are summarized in Table 2. Furthermore, disease duration was also in inverse correlation with the number of neutrophils in CSF (rs = −0.632; P < 0.01; Fig. 3).
| CSF IL-17A level | CSF glutamate level | |
|---|---|---|
| Clinical characteristics | ||
| Gender (male versus female) | ND | ND |
| Age (years) | NC | NC |
| Duration of the disease (years) | rs = −0.466 (P < 0.01) | NC |
| EDSS score | NC | NC |
| CSF analyses | ||
| Cytology (WBC): | ||
| Neutrophils (/μl) | rs = 0.426 (P < 0.05) | NC |
| Monocytes (/μl) | NC | NC |
| Lymphocytes (/μl) | NC | NC |
| BBB permeability (%) | rs = 0.440 (P < 0.05) | NC |
- ND, no difference (P > 0.05); NC, no correlation (P > 0.05).
- BBB, blood–brain barrier; EDSS, Expanded Disability Status Scale; CSF, cerebrospinal fluid; MS, multiple sclerosis; WBC, white blood cells.
Discussion
A widely accepted view of the demyelinating processes suggests that encephalitogenic myelin-reactive T cells have a crucial role in MS pathogenesis. In the current study, we focused exclusively on Th17 cell subset and their major secretion product IL-17A in CSF, and did find a significant difference between the MS and control group. Similar findings were reported earlier by Ishizu et al. 19 what reconfirms substantial role of IL-17A in MS development. However, in contrast to previously publish data 20, we found no statistically significant difference in CSF glutamate level between MS and control group. Possible explanation is that glutamate uptake decreases with normal brain ageing 21, and our control group subjects were indeed elder than those in MS group (Table 1).
More importantly, one of the most intriguing results of our study is a direct correlation between IL-17A and glutamate levels in CSF of patients with MS. This correlation, although moderate, implicates Th17-mediated inflammation in a glutamate excitotoxicity concept, suggesting that Th17 cells parallel to inflammation might use glutamate excitotoxicity as an effector mechanism in MS pathology development. This fact should not be underestimated considering that glutamate excitotoxicity results in oligodendroglial and neuronal degeneration 22 and cumulative axonal injury is largely responsible for the development of permanent neurological disability in EAE and MS 23, 24. The possible means, by which Th17 cells could promote excitotoxicity, as our results suggested, are variable.
Firstly, via IL-17A secretion, activated Th17 cells support the expansion, maturation and recruitment of innate immune cells such as neutrophils to enhance inflammatory reactions 25, 26. Encephalic compartment is not privileged, considering direct association between IL-17A levels and the number of neutrophils in CSF of patients with MS (Table 2). Due to the activation, infiltrated neutrophils release large quantities of glutamate by upregulating the glutamate-producing enzyme glutaminase and promote excitotoxic damage 27. Indeed, during the peak of EAE, profound neutrophil infiltration is found in spinal cord parenchyma, where prominent demyelination, axonal loss or axonal degeneration occur 28. IL-17A also favours BBB permeability disruption (Table 2), another important pathological feature of MS, which could be an additional manner of promoting neutrophil recruitment and excitotoxic damage. IL-17, by inducing generation of reactive oxygen species (ROS) in brain endothelial cells, leads to the activation of the contractile apparatus, responsible for the incompetence of tight junction proteins, and consecutively BBB disruption. However, ROS also lead to the overexpression of endothelial adhesion molecules and an increased transmigration of inflammatory cells including neutrophils via the BBB, thus promoting excitotoxicity 29.
Considering that our study excluded NMO patients, who typically have neutrophils in CSF and can be easily misdiagnosed as MS in early stage, neutrophil finding in CSF of patients with MS is unusual. However, it appears that the CSF amount of neutrophils tends to fall with disease duration (Fig. 3), and the same is observed in paediatric MS patients 30. CSF neutrophil count is also increased in mildly versus severely disabled patients 31. Our patients were in the early stage of the disease (median: 1 year) and had mild disability score what could explain the increased neutrophil count and at the same time suggest a potential involvement of these innate immune cells during the earlier stages of the disease development. In concordance to this, the inverse correlation between the duration of MS and CSF IL-17A level (Table 2) indicates that, as well as neutrophils, Th17 immune response could be more important for MS onset rather than further disease development and progression. The similar observations were reported by others, in particular peripheral blood mononuclear cells (PBMC) from patients with early MS produce significantly higher levels of IL-17 than PBMC from patients with established MS 32. The initial Th17 cell and neutrophil engagement in MS pathogenesis needs to be further analysed and reconfirmed with a larger number of patients with MS, considering that it could be the reason why some MS clinical trials, targeting Th17 cells in the later stage of the disease, failed to provide any clinical benefit. Vollmer and colleagues found no therapeutic efficacy of the monoclonal antibody against the p40 subunit of IL-23, which promotes and stabilizes Th17 cells, in MS patients with mean duration of the disease of 8 years 33. Newsworthy, CSF glutamate level appears not to be in correlation with the duration of the disease (Table 2). It is possible that glutamate excitotoxicity and the subsequent neurodegeneration, at the later stage of the disease, may persist in an inflammatory-independent manner, and some supporting data were reported 34-36.
Besides the neutrophil recruitment, Th17 cells could enhance extracellular glutamate to excitotoxic levels by other means. The recent findings suggest that in demyelinating lesions, Th17 cells can directly interact with neurons, in a manner that remarkably resembles immune synapses, and release important amount of glutamate, proposing Th17 cells as a considerable glutamate source in excitotoxicity concept 37. The interplay between Th17 cells and astrocytes is poorly studied, but, considering exclusive role of astrocytes in glutamate metabolism, it could be of great importance in the MS excitotoxic damage. There are some indications that activated Th17 cells could promote glutamate release from astrocytes by secreting or stimulating production of pro-inflammatory cytokines, including TNF-α 38, 39. Interestingly, astrocytes also express IL17A receptor 40, but IL-17A signalling in astrocytes in a concept of glutamate metabolism is still unknown.
In conclusion, patients with MS generally have higher CSF IL-17A levels when compared to the control group. Direct correlation between the Th17 polarized environment and glutamate increase suggests that Th17 cells, at last partially, might enhance glutamate level in the encephalic compartment and thus use excitotocity as a potential effector mechanism in the disease pathogenesis. Th17 cells and neutrophils appear to be predominantly implicated in the early stage of MS development, so therapeutic strategies targeting these cells in the later stage of the disease might be unsuitable.
Acknowledgment
This paper was supported by The Ministry of Education and Science of the Republic of Serbia under the project number 41018.




