Shortcomings in the Application of Multicolour Flow Cytometry in Lymphocyte Subsets Enumeration
Abstract
The lymphoid system is composed of numerous phenotypically distinct subsets of cells, each of which has a unique role in the effectiveness of an immune response. To distinguish specifically between these subsets, it is mandatory to detect simultaneously different cell surface antigens. This became feasible by the development of multicolour flow cytometric technologies. With these techniques, researchers now have the opportunity to study individual cells in far greater detail than previously possible. However, proper data analysis, interpretation and presentation of results will require a high level of understanding of the intricacies of the technology and the inherent limitations of the acquired data. The present report is intended to contribute to the better understanding of how the flow cytometer operates. This report may help new and inexperienced users to work appropriately with the flow cytometer.
Introduction
Flow cytometry is a technique used to measure antigen expression on populations of cells. In the flow cytometry, fluorochrome associated with a cell is excited by a laser, and the fluorescence emitted by the fluorochrome is collected by the detector assigned to that fluorochrome 1. In the multicolour flow cytometry system, each detector accepts light in a particular range of emission wavelengths optimized to detect a specific dye. However, each dye whose excitation is not zero at the excitation wavelength and whose emission is not zero in the detector's emission band will contribute to the signal on that detector. While fluorescent dye combinations used in flow cytometry are selected to minimize spectral overlaps in multicolour measurements, each dye can contribute to the signal on several detectors. Therefore, although the multicolour flow cytometry has the potential of enhancing the resolving power for important subpopulations, allowing the use of smaller amounts of sample and decreasing the cost of specimen processing by reducing the quantity of antibodies used and the number of tubes analysed, several challenges are associated with the use of this advanced flow cytometer design. These challenges result not only from an increased number of spectral overlaps 1, 2, but also from measurement errors generated by the presence of multiple fluorochromes on a cell. For instance, steric hindrance 3 or antibody coblocking 4, 5 may be a problem when two or more antibodies are used to label antigens that are in close proximity to each other on the cell surface. This will lead to reduced binding of the hindered antibody and eventually to a reduction in the associated fluorescence, resulting to a lower-than-expected signal. Also, fluorescence resonance excitation transfer may occur between dyes in close proximity and has been reported when cells are labelled with phycoerythrin (PE) conjugates and tandem dye conjugates such as PE-cyanine-5 (PE-CY5), PE-CY7 or allophycocyanin-CY7 (APC-CY7) 3. In such a condition, the fluorescence signal of the tandem-conjugated antibody is enhanced and that of the PE-conjugated antibody is quenched 6. Moreover, when a PE-conjugated antibody is adjacent to an APC-CY7-conjugated antibody, fluorescence resonance excitation transfer would result in a false PE-CY7 signal 3.
Flow cytometry has several uses beyond that of measurement of expression of antigens on populations of cells. Cell sorting is another aspect that has greatly extended the research potential of flow cytometry. Flow sorting enables the isolation of specific subpopulations of cells, which are then made available for morphological or genetic examination, as well as functional assays. For example, on the basis of differences in morphological, biochemical and molecular changes occurring in dying cells, flow cytometry can be applied to distinguish the two distinct types of cell death, that is, apoptosis and necrosis 7. This technique can also be employed to measure cellular DNA content in order to estimate cell cycle distribution and to analyse cell aneuploidy associated with chromosomal abnormalities 8. Virtually, every event that occurs during the process of cellular activation can be measured by flow cytometry. For instance, the use of flow cytometry to measure the reduction in fluorescence signal on cells as they divide could enable researchers to calculate cellular activation and proliferation. Likewise, flow cytometry can be used to monitor the flux of calcium into cells to measure the extent to which cells respond to stimuli 9.
In this article, we review reports pertaining to the flow cytometry technique with as goal to illustrate applications particularly well suited to the multicolour flow cytometry. We focus on the complexities of spectral overlaps and the intrinsic limitations of compensation and the subsequent compensated data. Further, we discuss the necessity of controlling background fluorescence and the usefulness of including appropriate controls and optimal fluorochrome combinations. Finally, we propose recommendations to untangle these limitations.
Spectral overlaps
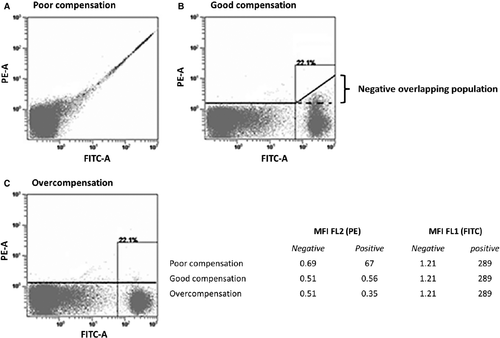
The optical pathway of flow cytometric instruments is arranged in an attempt to separate signals from different fluorochromes by directing them into dedicated detectors. However, due to the similarities and overlaps in the emission spectra of different dyes, it is not possible to choose emission filters that uniquely measure light from a single fluorochrome. Because of these spectral overlaps between dyes, the fluorescence emission of one fluorochrome will be detected in a detector designed to measure emission from another fluorochrome. This phenomenon is an important obstacle in multicolour flow cytometry because spectral overlaps and measurement errors worsen as the number of fluorochromes used to label cells increases. Figure 1A 10 shows this effect for human peripheral blood lymphocytes labelled with a fluorescein isothiocyanate (FITC)-conjugated antibody. Cells labelled with FITC appear to also have PE fluorescence. Therefore, to obtain a correct estimate of the amount of signal produced by the PE dye, the contribution of FITC signal in the PE detector must be measured and subtracted from the total signal in the PE detector. The measurement of spectral overlap requires particles that are stained with only a single colour. A series of calibration particles including single-stained cellular controls and various beads could be used to measure spectral overlap. These spillover values are subsequently converted to compensation values, which are then used by the flow cytometer to subtract out the contributions of non-primary colours overlapping into a given detector 2. Table 1 shows a guideline for where to expect important spectral overlap in a five-colour staining panel. As expected, the different dyes portray different spectral characteristics in terms of spillover into secondary detectors. For example, the measured signal of FITC in the PE channel is 21.4% of the signal in the FITC channel.
| Blue laser (488 nm) | |||||
|---|---|---|---|---|---|
| FITC | PE | ECD | PC5 | PC7 | |
| Blue laser (488 nm) | |||||
| FITC (FL1) | |||||
| PE (FL2) | 21.4 | 29.1 | 8.6 | 3.5 | |
| ECD (FL3) | 2.2 | 12.3 | 2.5 | ||
| PC5 (FL4) | 1.5 | 16.2 | |||
| PC7 (FL5) | 6.9 | 41.1 | |||
- Table 1 shows a guideline for where to expect important spillover in a five-colour staining panel. Human peripheral blood mononuclear cells were stained with anti-CD45 conjugated to the indicated fluorochromes. The percentage of compensation required for each fluorochrome is shown for the five detectors. Each value is the percentage of a fluorochrome's signal measured in each of the detectors. Thus, each column is related to the emission spectrum of the fluorochrome named in the first row. For example, the measured signal of FITC in the PE channel is 21.4% of that in the FITC channel. For clarity, values <1% as well as the 100% expected in the diagonal area (grey) are not indicated. We can deduce from this table that the FITC channel does not receive important fluorescence spillover from the other channels. Also, the PC7 channel does not contribute significantly to the signals on the other channels.
One common strategy to mitigate the effect of spectral overlaps in multicolour settings is to choose fluorochromes with minimal spectral overlap. Quantum dots (QDs) are inorganic crystalline nanoparticles that are currently competing with organic fluorophores for an array of fluorescent applications as their spectral properties may confer significant advantages over organic dyes 11. First, QDs display a narrow emission spectrum, and thus, emissions from many QDs can be resolved over the same spectral range without substantial spectral overlap. This limited spectral overlap between QDs can improve detection and sensitivity, which is critical in situations where target cells need to be discriminated above background fluorescence. In contrast, organic fluorophores have broad emission spectra, which limit the number of fluorescent probes that can be simultaneously resolved. Second, whereas organic fluorophores are restricted by their narrow excitation spectra, QDs have broad absorption spectra 12 and can be excited with any wavelength – over the entire visual wavelength range as well as the ultraviolet – lower than their emission wavelength. This makes it possible to excite multiple colours of QDs simultaneously with a single excitation light source 11. Third, QDs exhibit remarkable photostability and are highly resistant to chemical and metabolic degradation, more than organic fluorophores. Most notably, QDs can be multiplexed with many of the organic dyes currently used for multicolour flow cytometry, significantly increasing the number of parameters that can be measured simultaneously on single cells.
Apart from QDs, other dyes with improved spectral characteristics also exist in the market nowadays. APC-H7 is an APC-cyanine tandem fluorochrome, which uses an analog of Cy7 and has spectral properties similar to APC-Cy7. APC-H7 conjugate is an excellent replacement for APC-CY7, in that it is more stable in light, heat and paraformaldehyde-based fixatives and has less spillover into the APC channel than APC-Cy7 conjugates. Also, PE-CF594 dye is a newly developed tandem conjugate that combines PE and CF594 and has significantly less spillover into many detectors including the PerCP and PE-CY7 detectors compared to conventional PE tandem conjugates. In addition, it has improved brightness and spectral characteristics over other dyes in the PE-Texas Red detector. PE-CF594 can be excited by the blue, green and yellow-green lasers, and it provides an additional advantage in that it exhibits consistent spectral overlap values between lots, minimizing the need for lot-specific compensation controls.
Recommendations with regard to spectral overlaps
Choice of filters
The appropriate choice of filters can greatly reduce spectral overlap and would depend on the available lasers in the cytometer. Conceptually simpler would be to use as many lasers as possible. For example, a combination of up to four dyes excited by three different lasers might not require any compensation. However, the necessity of having three lasers for a four-colour experiment is not justifiable.
Spectral overlap between fluorochromes
When designing experiments for the flow cytometer, careful consideration must be given to the degree of spectral overlap between fluorochromes. If staining combinations are chosen carefully such that they exhibit little or essentially no spectral overlap, problems due to spectral overlap could be significantly minimized. For example, PE-CF594 could be used instead of the conventional PE tandem conjugates in that it has less spillover into many detectors and it exhibits consistent spectral overlap values between lots, minimizing the need for lot-specific compensation.
Compensation
Fluorescence compensation is the mathematical process by which the signal of any given fluorochrome is removed from all detectors except the one devoted to measuring that fluorochrome 13, 14. While compensation is one of the most important processes required in flow cytometric data analysis, it is also undoubtedly one of the least well-understood processes. Customarily, a compensation matrix is computed from the fluorescence measured in each of the detectors, using cells separately labelled with a single fluorochrome 1, 15-18. These compensation control samples assure that compensation will be correct for test signal levels lower or equal to those of the compensation controls. Indeed, when the compensation control signals are higher than those of the test samples – and compensation appropriately applied – the intensity of the overlapping population measured by a particular detector is reduced to the same intensity as the negative population measured by that detector 2 (see Fig. 1B), where the mean fluorescence intensities of FITC-positive and FITC-negative cells in the PE detector are equal. In other words, a cell population that is negative for a given dye of interest, but which stains positively with one or more spectrally overlapping dyes will have a near-zero-compensated mean for the dye of interest 1. However, computational errors in correcting for the other dyes may lead to important spread in dye estimates for individual cells, and the degree of spreading would depend on the intensity of the dye to be corrected. The end result is that the negative overlapping population will portray more spread in compensated data values than would be observed for completely unstained cells, located within the first decades of the axes (see Fig. 1B). This phenomenon becomes important when many colours are used, and often results in misinterpretation of the overlapping population as a weak positive subset (undercompensation) 19 or in overcompensation of the entire data set in an attempt to force the overlapping population on the other side of the axis 16 (see Fig. 1C).
Another issue of major concern regarding compensation is the difficulty to accurately set compensation using antibodies to markers that are dim and/or found only on rare subsets of cells. In such cases, a different antibody conjugated to the same fluorochrome is often used as a compensation control. In fact, some investigators routinely use an antibody which is very bright and present on a reasonable number of cells to set compensation in each channel 20, regardless of what antibody will be used experimentally in that channel. In many cases, this is an acceptable practice, because the use of a signal that is equal or brighter in intensity to the signal in the test samples will allow for accurate calculation of the compensation for a given fluorochrome 20. A serious problem arises when certain tandem dyes are used (e.g. APC-Cy7, PE-Cy7 and PE-CY5). Tandem constructs are covalently linked combinations of donor (e.g. APC, PE) and acceptor (e.g. CY5, CY7) dyes. And the ratio of donor to acceptor is usually in the range of 1:3 to 1:10, depending on the optimization procedures used by the manufacturer 21. In fact, different tandem constructs, even from the same manufacturer, often portray slightly different donor-to-acceptor ratios 21, 22. This inability to produce constructs that have exactly the same donor-to-acceptor ratio will lead to an inadequate or variable energy transfer – from the donor to the acceptor – and consequently to a variable fluorescence intensity, resulting in constantly changing compensation requirements 21. Therefore, regarding APC-Cy7, PE-Cy7 and PE-CY5 tandem-conjugated antibodies, the use of a tandem conjugate – other than is present in the test samples – for compensation could result in an inadequate compensation 20.
Currently, the most popular way to set compensation matrices is by using beads labelled with the same specific fluorochromes to be used in the multicolour set-up 22. Two types of beads can be used for this purpose: prestained beads or capture beads that can be stained with a labelled antibody of interest. The former are suitable for many applications where emission characteristics of the dyes are stable. However, when certain tandem dyes are of concern, these prestained beads, like single-stained cells, may not be able to accurately set compensation for every tandem conjugate lot that can be used in an experiment. The most flexible and reliable way of setting compensation is by performing single colour staining of antibody capture beads 17. Capture beads are better in that they can be stained with antibodies much like cells, but because the antibodies are captured regardless of their specificity, they can provide a very bright and uniform signal for each antibody irrespective of how bright that antibody would stain cells. This makes them more suitable where the fluorescence signal is very dim or the antigen is expressed on a rare population of cells. Some capture beads can be used to capture different lots of a given dye to enable their separate measurements. This could be very useful in situations where tandem dyes are being employed, because they may exhibit lot-to-lot variations. In general, spectral overlap values determined from single-stained beads closely match those from single-stained cells. However, beads do have much smaller background fluorescence than cells, indicating that the spillover computation would be far more precise with beads. The only major hurdle in using capture beads is that they might require a different scatter gate than cells, and they will only capture antibodies of a certain class. Thus, knowledge of the species and class of antibody used in the experiment is mandatory to successfully use capture beads as compensation control. If particles have different spectral characteristics than stained cells, the bead-based compensation settings would be different than those achieved with stained cells. As a result, it is always useful to verify bead-based settings using stained cells.
Recommendations with regard to compensation
Add the correct compensation controls
Fluorescence compensation being paramount to correct analysis of flow cytometry data, it is imperative to add the correct compensation controls necessary for the experiment being planned. Keep in mind that the ability to estimate proper compensation is compromised by using compensation controls that are less bright than experimental stains 23. Therefore, cells stained with an antibody against CD4, for example, might not be optimal for the generation of compensation matrix, compared to CD45 or CD8, because the expression levels of CD4 are relatively weak 24.
Compensation with the tandem dyes
Normally, the compensation matrix for a particular tandem construct is stable for a couple of months 25 provided that the reagents are properly stored and not exposed to bright light. However, light exposure and other adverse storage conditions may break down the tandem construct leading to the loss of some Cy7 molecules on each PE molecule in the case of PE-CY7 conjugates. This will alter the fluorochrome, particularly the tandem conjugation ratio, and might result in an inefficient energy transfer to the CY7 molecule with a consequent increased PE leakage. The end result might be emission in two channels and this would invalidate the compensation matrix. Such an occurrence would show up during periodic evaluation, as controls would fall outside acceptable thresholds established for each tandem-conjugated antibody. Therefore, it is recommended to check the compensation matrix of antibody panels regularly (e.g. monthly) especially when they contain certain tandem dyes or have been stored for a few months 25. Obviously, anytime a new batch of tandem dye is initiated, a new compensation matrix should be generated because it is unlikely that compensation requirements will be the same. It is also crucial to understand that some tandem dyes are not totally stable to the fixation/permeabilization procedures required for the staining of intracellular markers, leading to compensation problems 26. To determine whether that is the case, run two parallel samples with all the surface markers to be included in the test and submit one of them to an additional fixation/permeabilization step.
Apply appropriate concentration and amount of antibody
A proper titration assay should be applied to determine the antibody concentration resulting in the highest signal of the positive population and the lowest signal of the negative population 27. Product quality changes (different lots, different clones or variances due to storage) have to be considered and standardized to enable reproducible results. This can be done by parallel measurements of the old and new lots. Of course, the new lot should first be titrated and the optimal concentration – that is, where the ratio of the measured signal from stained and unstained cells is maximal – considered for the experiment. As mentioned above in this manuscript, light exposure and other adverse storage conditions can lead to changes in the fluorescence intensities of certain antibodies. If changes in antibody fluorescence occur even when the same antibody lot is used, a different compensation measure might be necessary to obtain comparable results. This will not affect the usefulness of the antibody lot. However, the usefulness of the particular antibody vial that has been exposed to light would, of course, depend on the experiment in question and on the degree of antibody degradation. For example, such antibodies could be used safely alone, to detect a parent population of cells amongst other antibodies, or in an antibody panel that does not contain the parent fluorochrome.
Software compensation
Researchers, nowadays, highly recommend software compensation because a matrix can be prepared for each antibody combination and then applied to the sample during analysis of the data. As such, uncompensated data can be retrospectively compensated in software. But, overcompensated data obtained by inefficient initial compensation might be problematic in that by overcompensating, one can instigate a decrease in sensitivity with as consequence the inability to resolve weak positive signals from background fluorescence 28. While compensation softwares automatically calculate accurate compensation values, errors in compensation can occur if the wrong compensation controls are used, if there is contamination from fluorophore carried over from the previous sample or if the compensation controls are not gated properly. Therefore, software-generated compensation matrices as well as the associated compensated data should be carefully examined.
Background fluorescence
The causes of background fluorescence can be categorized into three main domains: undesirable antibody binding, autofluorescence and spectral overlap 29. In each of these domains, the level of background depends in turn on several components of the measurement including the choice of antibody, the antigen of interest, the protocol used, the choice of fluorochrome, the optical configuration of the flow cytometer and, of course, the type of cells being investigated.
Undesirable antibody binding may arise in several ways. For example, an antibody that has been generated against an epitope on a given antigen can non-specifically bind to a similar epitope present on another antigen. It is also possible that non-specific binding occurs at the antigen of interest, but to a different epitope from the one it was generated against. Undesirable binding may also occur through the fluorochrome that is conjugated to the antibody 30, 31. For example, a high number of FITC molecules per immunoglobulin have been reported to result in a highly charged antibody that binds non-specifically to cytoplasmic elements 29. This undesirable binding of antibodies to cells is particularly troublesome when a rare cell population is being studied or if the antibodies are of low affinity.
In addition to the fluorescence emission of antibody-bound dyes, excitation sources in flow cytometers can also excite naturally occurring cellular components resulting in some levels of fluorescence. This fluorescence emission by endogenous fluorophores is an inherent property of cells and is called autofluorescence. Autofluorescence is another factor that can contribute importantly to the presence of background staining. The most important endogenous fluorophores are nicotinamide adenine dinucleotide phosphate (NADPH), riboflavins, flavin coenzymes and flavoproteins bound in the mitochondria 32, 33. These autofluorescent molecules are excited by a broad range of wavelengths, and their emission wavelengths overlap the emission spectra of commonly used fluorescent dyes 33-35. Therefore, different cell types that conceivably have dissimilar levels of these compounds would portray relatively different levels of autofluorescence. Noteworthily, while the reduced NADPH responds to excitation, it is the oxidized flavin adenine dinucleotide that is excited, indicating that any change in morphological or physiological state that affects the reduced/oxidized equilibrium of these metabolites would shift a cell's autofluorescence signal 36. Autofluorescence is also caused by collagen, elastin, fibronectin and lipo-pigments. For example, the fluorescent pigment lipofuscin that accumulates with age in the cytoplasm of cells has emission wavelengths that overlap those of most commonly used fluorescent dyes 37. This can particularly result in an increase in the autofluorescence of certain cells as they age. Taken together, in vitro-cultured cells, tumour cells, aged cells or cells high in granule-associated flavoproteins may have relatively higher autofluorescence when compared to other cells.
Recommendations with regard to background fluorescence
Establish antibody specificity and quality
It is important to first ensure the specificity of the antibody intended to identify the protein of interest. Although commercially available antibodies are expected to have undergone extensive quality control programmes to meet the manufacturer's strict release criteria, antibodies with poor performance characteristics do exist. It is the user's responsibility to verify the manufacturer's proclaimed antibody-binding characteristics. Equally important in establishing a reliable antibody labelling protocol is the verification of the antibody specificity by other techniques. Because the flow cytometer measures fluorescence as total intensity of a cell, information about the antibody distribution on the cell is not available. Fluorescence microscopy can be used to examine the distribution of the fluorescence signal on or in a cell and often assists in distinguishing background from truly positive cells. In some cases, Western blot can be used to obtain confidence about the antibody's specificity 38.
Understand and minimize undesirable antibody binding
Non-specific binding of antibodies to the surface of cells reduces the sensitivity of specific epitope-binding immunofluorescence. This can be particularly troublesome when a weak or rare cell population is being studied 39. In addition, some antibodies – particularly the tandem-conjugated antibodies – tend to form aggregates over time, and Fc receptors non-specifically bind these antibody aggregates with greater avidity than they have for antibody monomers. For samples that contain sufficient negative and positive cell populations, the degree of undesirable antibody binding can be visually estimated by comparing the intensity of the internal negative population to that of the unstained control. If the background is unexpectedly high, the cell labelling protocols should include a step that involves blocking agents to reduce Fc receptor and other non-specific binding of antibodies. Otherwise, antibody aggregates can be removed by centrifugation in a microfuge at high speed for 10 min at 4 °C 26. It is not recommended that either PE-conjugated or IgM antibodies be centrifuged because these molecules are relatively large and may pellet out of the solution along with antibody aggregates.
Include a dump channel
Usually, we want to see data only from single, viable cells. Typically, one wishes to eliminate data from cell debris, dead cells and clumps of two or more cells. Cell debris and clumps can be distinguished from single cells by size (estimated by the intensity of low forward scatter). Dead cells have lower forward scatter and higher side scatter than living cells. These differences are accurately preserved following paraformaldehyde fixation (despite the fact that after fixation, all the cells are dead). However, it is almost impossible to completely eliminate these unwanted cells just by adjusting the forward and side scatter parameters 40. In most standard staining protocols, propidium iodide is included to identify the dead cells. But because propidium iodide will stain any cell whose membrane is compromised, it cannot be used to stain cells that have been fixed 41. Ethidium monoazide 40 is applicable for analysing human samples that require fixation. There is also a newly developed viability stain for the violet laser, FVS450, which is compatible with standard buffers used in surface and intracellular staining protocols, including fixation/permeabilization solutions. The use of a dump channel is a particularly good way for enhancing the fidelity of a given stain, because unstained cells, dead cells, sticky and highly autofluorescent cells can be clearly separated from the cells of interest. This procedure employs a combination of reagents conjugated to the same fluorochrome that are expressed on cells that are not of interest 42. PE-Cy5 is a recommendable channel for dump antibodies, because dyes that stain dead cells such as propidium iodide and 7AAD fluoresce in this channel 42. Noteworthily, no compensation is required from the antibody conjugates in the dump channel because all cells that fluoresce in this channel will be eliminated from analysis 42.
Use efficient antibody clones
In general, different clones of antibody are often generated to different epitopes on a single antigen. Because different antibody clones might portray different sensitivities – for instance, by having a better recognition site and/or giving less background noise – it may be wise to test different antibody clones in an attempt to improve sensitivity.
Determine the level of antigen expression separately for each cell subset being investigated
As mentioned above, spectral overlaps and the variation in the correction processes often result in considerable spread in the distribution of compensated background values for individual cells. This phenomenon is more pronounced in a multicolour flow cytometry study, where the expression of multiple markers by individual subsets would necessitate simultaneous compensation for many fluorescence signals. Accordingly, different subsets, which obviously express different epitopes, in a multicolour set-up may portray different amounts of variation in compensated background signal and typically show different levels of background fluorescence in any given detection channel (see Fig. 1B) 1. Further, autofluorescence is cell type dependent. Even within the same type of cells, different subpopulations portray different levels of autofluorescence. For instance, lymphocytes can be divided into a number of subsets including the large granular lymphocytes such as natural killer lymphocytes and smaller lymphocytes such as the T and B lymphocytes. Because autofluorescence is size and granularity dependent, the natural killer cells will portray a relatively higher autofluorescence compared to T and B cells. Therefore, determining the boundary between background levels and low expression of a particular determinant separately for each cell subset being investigated is critical to produce logical results.
Controls
To establish the level of background, control samples are essential. There are two major categories of controls: instrument set-up controls and specificity or gating controls. Certain aspects of cytometer set-up, such as the efficiency and performance of optical filters, mirror reflection and transmission, laser alignment, need to be checked periodically by an expert, but may not require controls in every experiment. Controls for checking and monitoring these parameters are well outlined elsewhere 43. Instrument set-up and optimization strategies can vary between different types of cytometers (analog versus digital) and for different types of experiments. For multicolour analyses in which spectral overlap occurs, the instrument set-up controls must include a proper measurement of the photomultiplier tube (PMT) voltage gains as well as the required compensation.
The cheapest way to set the PMT voltages is by using an unstained sample of the cells in question. In this approach, the voltages are adjusted such that the unstained cells appear in the first decade of a four-decade logarithmic scale for each fluorochrome to be measured 20. While this strategy can provide good results for some fluorochromes, it is not optimal in fluorochromes with longer wavelength emissions, such as APC-Cy7 and Alexa 700. This is because most cells emit very little autofluorescence at these wavelengths, so the variance in fluorescence intensity of unstained cells is principally dominated by photon counting statistics and electronic noise 20. As such, any attempt to adjust voltages in these channels based on visual placement of an unstained sample will be subjective and may not correlate with optimal detection of true signals in that channel. Bead particles are ideal in the calibration of PMT voltages because the dyes captured inside beads can be excited at many laser wavelengths with an analytical scale that covers the whole range from negative and very dim fluorescence to very bright fluorescence 22. For cytometers whose PMT voltages are not linear on the log scale, it might be necessary to evaluate the optimum PMT voltage to be used 42. Of course, the optimization of PMT voltages need not be carried out more than once for a particular instrument except when filters, PMTs, lasers or fluorochromes are changed.
After setting the PMT voltages, the compensation matrix is established using single-labelled cell suspensions. If particles have been used, the particle-based settings might be different from those achieved with stained cells. As such, it is recommended to validate particle-based settings using stained cells for biological significance 43. Whatever compensation measure considered, it is imperative that the compensation control samples are run after the PMT voltage gains for the experiment have been set, because the degree of compensation required is gain dependent 20. Of course, when the positive signals are excessively high as to be off-scale in a particular detector, the PMT voltage should be reduced to bring all events on-scale. This is essentially required; otherwise, the true extent of their fluorescence cannot be measured and both their brightness and compensation in other dye dimensions would be erroneous. On the other hand, if known negative events occur very high on the fluorescence scale, reducing the PMT voltage in that detector in an attempt to bring the negative population mean down might result in an increase in measurement error that can ultimately impact compensated populations. In situations of high background, when positive events are still visible, it is generally advised to first investigate and reduce the sources of the high background.
Once PMT voltages are set and the compensation matrix established, the experimental samples can be collected and analysed. At this point, specificity or gating controls may be required to confirm that the test sample was prepared properly and, if necessary, to establish the level of background fluorescence. These control stains are important for all experiments using flow cytometry, but they become particularly critical for multicolour stains, because the higher the number of fluorochromes used in each stain, the greater the risk for artefacts introduced by compensation errors and/or reagent interactions 21. When good-quality monoclonal antibodies are used at optimal concentrations, they tend to have relatively low background staining. Hence, in multicolour experiments, the major source of background staining is fluorescence spillover 20. Implying that the inclusion of controls that can measure the effects of spillover from populations in other dye dimensions on a particular channel of interest is indispensable to determine the threshold between positive and negative cells for all dyes where spectral overlap correction is of concern.
Fluorescein-minus-one (FMO) controls are highly recommended for multicolour flow cytometry, because they can account for fluorescence spillover from other channels 1, 2, 17. In an FMO control stain set, all reagents used in a given multicolour stain are included except the reagent for which the threshold is to be determined. Therefore, it does not provide a measure of the background staining that may be present when the antibody of interest is actually included. In this regard, FMO controls cannot be used to specifically expose any type of undesirable binding associated with the antibody of interest.
Isotype controls represent one way to determine whether there is undesirable antibody binding related to the antibody of interest 29. Isotype controls are antibodies of the same isotype as the specific antibody, but are either myeloma-derived hybridomas of unknown specificity 44 or are raised against an antigen, such as dinitrophenyl, that is supposed not to be present in the cells under investigation 45. Ideally, the isotype control should match the test antibody not only in light and heavy chain subclasses but also in protein concentration, fluorochrome type and fluorochrome/protein ratio. It should also have been derived by the same manufacturing process as the test conjugate under study and be obtainable in the same formulation 44. However, there are some concerns about the inefficiency of isotype controls to determine non-specific binding 44, 46. Because the variable regions (VH and VL) that determine the antibody-binding sites of the isotype control are different from those of the test antibody, the two antibodies may show different levels of non-specific binding through this part of the protein. Indeed, there are cases where an appropriately titred isotype and fluorochrome-matched control showed more cells non-specifically stained than specifically stained by the corresponding CD34 conjugate used 44, 47. This implies that even if the control antibody is isotype and fluorochrome-matched to the test antibody, it might not truly match the background staining of a particular test antibody because it might not stain exactly the same number of events that are non-specifically stained by the test antibody 44. Another major limitation of isotype controls is that, by themselves, they do not account for fluorescence spillover from other channels.
An alternative approach that is likely to provide more useful information than the conventional isotype control is to examine the negative populations within the investigated sample. An internal negative control is a population of cells that does not express the antigen of interest and thus remains unlabelled in the midst of cells that do express the antigen. Noteworthily, the only difference between these subpopulations should be the absence or presence of the fluorochrome of interest. The advantage of this type of control is the fact that it has been exposed to exactly the same antibodies, including the antibody of interest, as the positive population under study. As such, the effects of fluorescence spillover from populations in other dye dimensions would be identical in both subpopulations.
Recommendations with regard to controls
Collect data with a well-standardized instrument
Prior to data collection, standard reference particles or software should be used to adjust the PMT voltage gains so that the mean fluorescence intensity of the beads falls at approximately the same target value range as determined from the instrument calibration procedures 15, 43. Adjusting the instrument to the established setting each time data are collected will allow for optimum resolution sensitivity and data that are highly reproducible from one experiment to the next.
Biological comparison controls
In some situations, a biological control can be more appropriate for determining positivity of the test samples. For example, in flow cytometry assays for detecting post-treatment responses, the untreated sample can be far more relevant than an FMO control to distinguish positive from negative events. Biological controls are popular in these situations because, by including the same antibody conjugates in the untreated as for the treated samples, the untreated control samples will account for spillover effects in the channel of interest. Also, because the same antibodies are present in the untreated control as in the test sample, it will account for non-specific staining as well. In addition to untreated controls, positive biological comparison controls are also important. The purpose of the positive control is to ensure that the experiment was performed in such a way that responses could be detected, and that the cell sample in question underwent the specific treatment. This protects against misinterpretation of negative data in cases where a reagent was not added, the cells were non-viable, etc. Cell viability of the treated samples should also remain comparable to those of untreated samples.
Unstained controls
Many variables are involved in the sample preparation procedure that may influence background fluorescence of cells. Because the internal unstained control has been exposed to the same conditions as the cell population under study – including exposure to the antibody of interest – it is expected to have the same levels of background fluorescence like the test samples. Thus, background fluorescence can be optimally assessed if the negative population of a particular cell type is compared with the positive population within that same cell type. Unfortunately, not every application contains a positive as well as a negative subpopulation of the same cell type.
Fluorescein-minus-one versus isotype controls
Certainly, some gates can be drawn unambiguously, without reference to any control sample. For example, any marker that has a clearly bimodal expression, with no overlaps of positive and negative populations, does not require a control for accurate determination of positive and negative populations. However, when dealing with antigens that are expressed at low levels, in rare cells or in cell labelling procedures that easily result in overlapping antigen-positive and antigen-negative cell populations, controls are indispensable to be able to distinguish positive from negative cells. It is widely accepted that isotype controls are of little value in distinguishing positive from negative cell populations 19, 21, 26, 48-50 and should therefore not be used on their own to set positive gating regions for test antibodies. FMO controls are generally more relevant in multicolour experiments where the major source of background variance after compensation is spillover-induced.
Selection of fluorochrome combinations
Although fluorescence spillover is of major concern regarding quality results in multicolour flow cytometry, the careful selection of fluorochrome combinations can significantly mitigate its effects on the quality of data 51. As mentioned above, fluorochromes differ in relative brightness, meaning that the conjugation of the various fluorochromes to the same antibody and staining of the same cells can result in considerable differences in the resolution of positive and negative events. For example, FITC-conjugated anti-CD34 monoclonal antibodies poorly detect CD34 epitopes compared to their PE-conjugated counterparts 52. In addition, some reagents exhibit a certain degree of stickiness particularly with monocytes and B cells. Also, there are some indications that APC tandem degradation is cell dependent 53, and the degree of autofluorescence of cells might be different in the various regions of the light spectrum 21. In particular, autofluorescence interferes more with the FITC, Cascade blue and Cascade yellow detectors 21. Consequently, strongly autofluorescent cells might appear as dim positive in these detectors, despite the fact that they might be negative for all the reagents used. Therefore, the choice of fluorochrome conjugate used for identifying a given marker will depend not only on the biological features of the cells such as antigen expression characteristics and antigen patterns 54, but also on the types of cell that are being investigated, fluorochrome properties, photon counting statistics and hardware performance of the cytometer. To select a proper antibody–fluorochrome conjugate in a multicolour application, one should first identify which markers require the highest sensitivity. These are markers that are associated with weak positive populations and/or cells that are very rare. In either case, the brightest fluorescence channels will be required 28. After allocating the most sensitive antibody to the dim-positive population, it should be ensured that antibodies to bright markers are not used in channels that overlap importantly with the channel considered for high sensitivity 54. Noteworthily, if the bright marker is present on an entirely different subset of cells than on the subset that expresses the dim or rare marker, the data spread will not affect the cells for which high sensitivity is required. Thus, it would be correct, for example, to use CD56 PE with CD14 FITC or CD19 APC with CD3 APC-Cy7. In each case, the first marker is weakly expressed, but it is present on a completely different subset of cells from the second highly expressed marker.
A series of novel reagents have been designed to offer superior quality with regard to sensitivity and consistency of results on flow cytometers. Amongst these fluorochromes are the brilliant violet dyes including BV421, BV510, BV605, BV650, BV711, BV786 and V500, which can provide excellent population resolution, especially for dim populations, and could be very useful in multicolour flow cytometric applications. These reagents are violet-excitable dyes with significantly improved brightness over other dyes offered for the violet laser. In addition, they are often brighter than reagents for the blue and red lasers, making them one of the brightest dyes on the market. As with some conventional organic dyes, brilliant violet dyes are compatible with standard buffers used in surface and intracellular staining protocols, making the reagents easy to incorporate into current workflows. These reagents also demonstrate compatibility in paraformaldehyde-based fixatives. However, like for all fluorochromes, care should be taken when combining these antibodies in a multicolour application. For example, if detecting a dim marker in the BV605 detector, and a bright marker in the V500/BV510 detector, V500 might be a better choice due to the fact that BV510 has more spillover into the BV605 detector than V500. In other words, V500 could be used for highly expressed antigens when spillover into neighbouring channels may be of greater concern than brightness.
Recommendations with regard to choosing fluorochrome combinations
Antigen expression levels and detection channels
With respect to antigen expression levels, it is imperative to understand that a weakly expressed antigen works best with a bright dye, while a strongly expressed antigen works with all dyes. Also noteworthy is the fact that a weakly expressed antigen works best on a channel that does not receive significant fluorescence spillover from other channels, while a strongly expressed antigen works best on a channel that does not contribute to the signal on other channels (see Table 1).
Excluding and co-expressed antigens
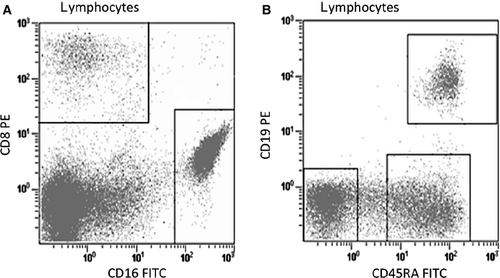
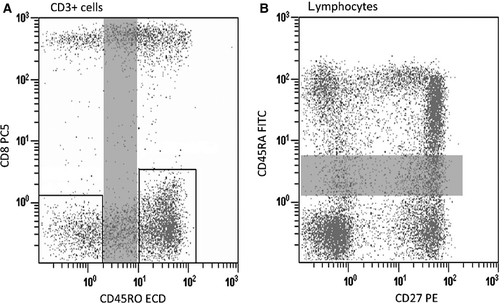
Distortion through crosstalk and subsequent loss of sensitivity is only relevant when looking at co-expressed antigens, but not for patterns with excluding antigens. Therefore, allow spillover between excluding antigens, that is, between markers that are not co-expressed on the same population of cells (see Fig. 2A). And, avoid spillover between non-exclusively co-expressed markers; however, a considerable amount of crosstalk may be tolerated for co-expressed antigens when co-expression is discrete and strong (Fig. 2B). Similarly, allow crosstalk between excluding antigens and avoid crosstalk between modulated co-expressed antigens (see Fig. 3A, B).
The tandem conjugates
One of the key problems of tandem dyes is that they produce fluorescence spillover in the channel of the parent fluorochrome (for instance, PE parent population for PE-CY5 and PE-CY7 subpopulations; APC parent population for APC-CY5.5 and APC-CY7 subpopulations). The fluorescence of certain tandems in the parent fluorochrome channel is mostly due to dye degradation, formation of highly reactive aggregates, as well as handling and storage processes 20, 21, 25. To minimize tandem degradation, antibodies and stained cells should be kept in the fridge and protected from light 54. Crosstalk from subpopulation to parent has no impact on sensitivity, while the adverse case might affect sensitivity. Therefore, it is advisable to allow spillover from subpopulation markers to parent markers and to avoid vice versa.
Panel construction and dye–dye interactions
A major problem in the multicolour flow cytometry is the development of optimized staining panels 39. Because compensation generated with singly stained cells does not take into consideration dye–dye interactions or dye–cell interactions, which might affect the fluorochrome spectrum of a given dye 2, it is imperative to check the full set of antibodies to be used in a panel against single colour data to ensure that the mixed antibodies demonstrate equivalent staining characteristics compared to cells stained with single colour 19. Ideally, the fluorescence intensity of the major subsets (e.g. CD3) is first identified. This is followed by the subsequent addition of the remaining antibodies – in the sequential order that will be used for gating purposes during analysis – one at a time to evaluate the effect of each added antibody to the overall staining pattern 26. If a decrease in sensitivity is noticed for a given marker, it might be due to a couple of reasons: dye–dye interaction, antibody dilution in the presence of other antibodies that are highly concentrated or insufficient compensation. A possible solution for the first two reasons could be to perform separate incubations for the antibody that showed reduced sensitivity and the other antibodies. Pairwise plots of all the possible combinations could be viewed to determine whether there are compensation problems.
Far-red channels and drug influence
Because of inescapable measurement errors in the fluorescence of cells that have low or no fluorescence in the far-red channels (APC, Alexa Fluor 680 and APC-Cy7) 2, 18, weak positive cells should not be chosen for these channels. Markers that stain brightly with a good separation of positive and negative cell populations should be used in the far-red channels 42. Particularly important, the APC detector seems to receive significant fluorescence spillover from more detectors than the other detectors 18, 48, leading to greater magnitude of errors in the APC signal 21. Therefore, APC (even though one of the brightest fluorochromes) is less suitable in multicolour flow cytometry analysis.
Another consideration in the selection of fluorochrome-conjugated antibody combinations is the presence of fluorescent properties in certain drugs. In vivo chemotherapy, at least in the case of anthracyclines, has been reported to affect the staining of specimens and might necessitate a change in the choice of fluorochromes used 55, 56.
According to lasers
Finally, the selection of fluorochrome may also rely on the number and type of laser excitation available on the flow cytometer. Virtually, all flow cytometers are equipped with a blue laser (488 nm), and in general, at least three fluorescent parameters can be measured using this excitation wavelength. More sophisticated instruments are designed to measure up to five fluorescent parameters simultaneously from the 488-nm laser. In addition to the blue (488-nm) laser, some instruments are also configured with the red (633-nm) and violet (405-nm) lasers. A multiple laser instrument can offer the advantage of minimizing spectral overlap by choosing less fluorochrome-conjugated antibodies per laser. For example, the combination of CD3 FITC, CD19 APC and CD8 pacific blue, which are, respectively, in the blue, red and violet lasers, does not require any compensation.
Data acquisition and analysis
The threshold number of events to be collected per sample depends on the scientific question to be addressed, for instance, whether one is interested in analysing total T cells or just a subpopulation of T cells. Of course, the total number of cells that are in a subpopulation gate can be very few depending on the number of co-expression markers required.
A fundamental strategy in the analysis of flow cytometric data is the grouping of cell events into distinct subsets on the basis of similarities in the light scattering and fluorescence 57. This process is usually accomplished by a visual inspection of plots and a manual gating of cell events into specific cell population for the examination of parameter expression. In general, singlets are first defined by having a similar height and area measurement in the forward scatter (see Fig. 4). Next, a gate is set around the CD3+ cells – in the case of lymphocytes – and these cells are back-traced in the forward scatter area versus side scatter area histogram to facilitate the localization of the lymphocytes. Thereafter, a gate is set around the lymphocyte. This is followed by the gating of the lineage markers for the lymphocyte subset (e.g. CD3, CD8) and subsequently by the separate analysis of each lineage for various parameters (CD28, CD45RO, etc.). Clearly, the process of manual analysis of multiparameter data is highly dependent on how the researcher determines the cut-off between positive and negative populations. It is therefore subjective and potentially error prone.
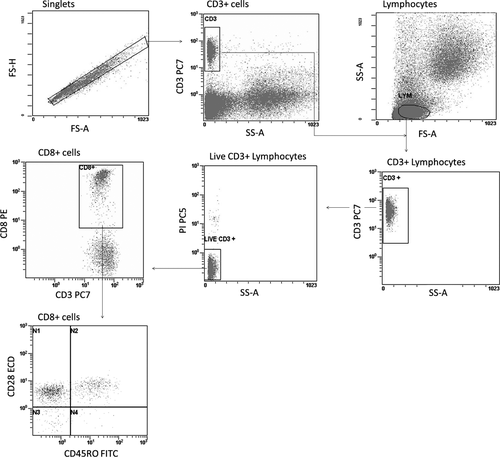
Automated computer-driven analysis techniques, including the supervised and unsupervised learning techniques, have been proposed in an effort to address the problems faced in gating and interpretation of flow cytometric data 57-62. In supervised learning, the variables under investigation are split into two categories: explanatory variables (e.g. measurement of events) and one or more dependent variables (e.g. cell type). The goal here is to achieve an association between the explanatory variables and the dependent variable. Once this association is known, the algorithm can predict the dependent variable for any event of unknown label 63. For supervised learning, typically the values of the dependent variable must be known for a sufficiently large part of the data set. On the other hand, in unsupervised learning situations, there is no dependent variable; all the variables are treated in the same way 63. The purpose is to identify the events that are in the same cluster. Clustering refers to the procedure of gating similar cell populations in a sample. Many methods for clustering have been developed, which fall into two general categories: heuristic-based algorithms and model-based cluster analyses. In heuristic algorithms, clustering is obtained by using search trees. However, instead of generating all possible solution branches, it optimizes a certain target function that is more likely to produce outcomes than others 64. In contrast to heuristic methods, model-based clustering methods make inferences based on probabilistic assumptions about the data distribution. Gaussian or modified Gaussian mixture models use the expectation-maximization algorithm to find the parameters of the distributions that are fitted to the data; then, Bayesian information criterion or some variants to determine the optimum number of clusters. On the other hand, the new-generation algorithms such as misty mountain are based on spatial exploration of the histograms. A complete description of the techniques implemented in automated gating as well as the associated drawbacks is beyond the scope of this publication.
Recommendations with regard to data acquisition and analysis
Data acquisition
Generally, flow cytometric data are plotted with a threshold set on the forward scatter to minimize the acquisition of noise and other unwanted events that are relatively huge compared to the population of interest. It should be ensured that the population of cells is within the threshold; otherwise, the voltage gains should be increased for the forward scatter to get the cells in scale. Noteworthily, this is done only during data acquisition and by using the forward versus side scatter histogram.
Gates and regions
The approach to analyse parameters is to draw gates or regions around the subset of interest and compare the relative intensity of events between a control and a test sample. Quadrant method – by drawing a threshold based on unstained cells – is commonly used for specifying gates; however, the distinction between weak positive and negative cells or overlapping antigen-positive and antigen-negative cell populations cannot be optimally delineated using this method. For this purpose, data are collected for cells stained with FMO control stain sets and gated in the same way as cells stained with the full stain set. The background fluorescence for each subset in the display is demonstrated by the upper boundary of the subset in the FMO control.
Data analysis
In flow cytometry, MFI is often used to abbreviate mean or median fluorescence intensity. Of course, for normally distributed data, mean is equal to median. However, in reality, flow cytometry data are rarely normal, and the more the data skew, the more the mean shifts in the direction of skew and becomes less representative of the data being analysed. Besides, if any part of the distribution lies off-scale at either end of the axes, it will be impossible to get a true mean value. In fact, the use of median is generally considered a much better statistical approach in that median is less influenced by skew or outlier events. Noteworthily, although median gives better information about the central tendency of the population, if more than half of the population is off-scale, median will not give the central tendency of the entire population.
Conclusions
Recent advances have made multicolour flow cytometry more accessible. This advanced flow cytometric procedure has been a real revolution, by significantly improving our ability to resolve important subsets of cells within a complex population. However, this process comes with a price, as this advanced technology has its own set of drawbacks that users must be aware of and must overcome in order to obtain interpretable data. To insure that data have been properly acquired, researchers must have an understanding of the entire process, including the instrumentation of the flow cytometer. Finally, it is crucial to publish the gating strategies applied for data analysis as well as the type of controls used to enable readers to fully evaluate results pertaining to the flow cytometric technology.
Acknowledgment
The study was supported by a scientific grant from the ‘Wetenschappelijk Fonds Willy Gepts’ from the Universitair Ziekenhuis Brussel.




