Decreased Plasma IL-22 Levels and Correlations with IL-22-Producing T Helper Cells in Patients with New-Onset Systemic Lupus Erythematosus
Abstract
Interleukin-22 (IL-22) and IL-22-producing T helper (Th) cells are involved in the pathogenesis of autoimmune diseases. However, the roles of IL-22 and IL-22-producing T helper cells in systemic lupus erythematosus (SLE) remain unclear. Plasma levels of IL-22 were measured in 41 patients with SLE (19 new-onset and 22 relapsing patients) and 20 healthy controls by enzyme-linked immunosorbent assay (ELISA). Meanwhile, the percentages of CD4+IFN-γ+ (Th1), CD4+IL-17+ (Th17) and CD4+IFN-γ−IL-17− IL-22+ (Th22) cells in peripheral lymphocytes were determined by flow cytometry, and plasma IL-22 autoantibodies were detected by ELISA in 19 new-onset SLE patients and 20 healthy controls. Plasma IL-22 levels in new-onset SLE patients were significantly decreased compared with relapsing SLE patients and healthy controls. After treatment with prednisone and hydroxychloroquine, the levels of plasma IL-22 in new-onset SLE patients were obviously increased but still lower than healthy controls. There was a positive correlation between plasma IL-22 levels and the percentages of Th22 cells, but not Th1 and Th17 cells. Moreover, plasma IL-22 levels as well as peripheral Th17 and Th22 cells correlated with SLE disease activity index (SLEDAI) scores and erythrocyte sedimentation rate (ESR). High frequencies of plasma IL-22 autoantibodies were detected in new-onset SLE patients. However, IL-22 levels did not correlate with IL-22 autoantibody. Decreased plasma IL-22 levels and correlation with Th22 cells may be distinct features in new-onset SLE. Moreover, IL-22 and Th22 cell correlated with SLE disease activity.
Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease characterized by multiple organ damage, and its pathogenesis still remains unclear. In addition to amounts of autoantibodies generated by excessively activated B cells, abnormal T cell subsets and related cytokines are also involved in the pathophysiology of SLE 1.
Interleukin 22 (IL-22) is a novel cytokine which belongs to IL-10 family 2. It is primarily produced by T helper (Th) cells, such as Th1, Th17 and Th22 cells which commonly named IL-22-producing Th cells 3. Th22 as a specific IL-22-producing Th cell subset can highly secret IL-22, independent of Th1 and Th17 cells, not secret IFN-γ and IL-17 4-6. Th22 cells, together with Th1 and Th17, were increased either in psoriasis skin lesions or in peripheral blood. And plasma IL-22 concentration was higher in psoriatic relative to healthy individual, which highly correlated to skin disease activity 7. Additional studies pointed to the possibility that IL-22 and IL-22-producing Th cells had also potentially implicated in the pathogenesis of Crohn's disease 8, rheumatoid arthritis (RA) 9, sjögren's syndrome 10 and immune thrombocytopenia 11. However, the roles of IL-22 and Th22 cell in the pathophysiology of SLE are unclear.
In the present study, we particularly focused on new-onset SLE patients and observed the change in plasma IL-22 levels in these patients before and after therapy. Furthermore, we first investigated the correlations between IL-22 and IL-22-producing Th cells (Th1, Th17 and Th22) to further explore the roles of IL-22 and IL-22-producing Th cells in the pathogenesis of SLE.
Materials and methods
Patients and controls
A total of 41 patients with SLE (36 women and 5 men; mean age 34 ± 12 years) were enrolled from the Department of Rheumatology at the First Affiliated Hospital of Zhejiang University, College of Medicine. All recruited patients met with 1997 revised American College of Rheumatology (ACR) classification criteria for SLE 12. Individual disease activity was assessed by SLE disease activity index (SLEDAI). Among 41 SLE patients, nineteen were new-onset (first-time diagnosis of SLE and no history of glucocorticoids and immunosuppressants use before registration). Twenty-two were relapsing patients (not first-time diagnosis of SLE and treatment with prednisone at a low dosage 5–10 mg/day and hydroxychloroquine 200 mg bid for at least 6 months before enrolment, and recent SLEDAI ≥ 5). Moreover, 19 new-onset SLE patients achieving a stable condition (SLEDAI < 5) after treatment with prednisone (0.1 mg/kg/day) and hydroxychloroquine (200 mg bid) also were observed. Clinical characteristics of patients with SLE and healthy controls are presented in Table 1. Twenty healthy volunteers (17 women and three men; mean age 30 ± 10 years) were studied as controls, and all of them did not have any rheumatologic conditions. The study was approved by the ethics committee of the First Affiliated Hospital, College of Medicine, Zhejiang University; patients and healthy volunteers were recruited after obtaining informed consent.
Enzyme-linked immunosorbent assay (ELISA)
Peripheral blood was collected in evacuated tubes containing EDTA as the anticoagulant. Plasma was obtained from all subjects through centrifugation and stored at −80 °C until tested. Plasma IL-22 concentrations were measured using a human IL-22 Quantikine ELISA Kit (R&D, Minneapolis, MN, USA) according to the manufacturer's recommendations.
To detect plasma antibodies against IL-22, 96-well flat-bottomed plates were coated with recombinant human IL-22 (R&D) at 2 μg of protein/ml in PBS, pH 7.0, and blocked with 3% HSA. Plasma was added for 2 h at 27 °C before washing and development with alkaline phosphatase-conjugated goat anti-human IgG (Sigma-Aldrich). P-Nitrophenyl phosphate substrate (Sigma-Fast) and then 3 m NaOH were added, and the absorbance was read at 405 nm.
Flow Cytometry (Intracellular cytokines measurement)
Peripheral blood samples from 19 new-onset SLE patients and 20 healthy controls were collected. Cytokine-producing cells were identified by intracellular staining. Briefly, 100 μl heparinized peripheral blood with an equal volume of RPMI-1640 medium was incubated for 4 h at 37 °C in the presence of 25 ng/ml of phorbol myristate acetate (PMA), 1 μg/ml of ionomycin and 1.7 μg/ml of monensin (all from Sigma, St. Louis, MO, USA). After incubation, cells were stained with FITC-conjugated anti-CD4. After fixation and permeabilization by Fix & Perm (Invitrogen, Carlsbad, CA, USA), the cells were further stained with APC-conjugated anti-IFN- γ, PE-Cy7-conjugated anti-IL-17A and PE-conjugated anti-IL-22. All antibodies were obtained from Biolegend (San Diego, CA, USA). Stained samples were analysed by flow cytometry using a FACSCalibur and CellQuest software (Becton Dickinson, San Jose, CA, USA).
Statistics
Results were expressed as mean ± SD. Statistical significance was determined by anova, and difference between two groups was measured by Newman–Keuls multiple comparison test (q test) unless the data were not normally distributed, in which case Kruskal–Wallis test (H test) and Nemenyi test were used. Nonparametric paired groups were tested using the Wilcoxon test. Spearman's test was used for correlation analysis. All tests were performed using spss 16.0. P value <0.05 was considered to be statistically significant.
Results
Decreased plasma IL-22 levels detected in new-onset SLE patients
The characteristics of new-onset and relapsing SLE patients were given in Table 1; there were no significant differences in age and gender distribution between new-onset and relapsing patients. Disease duration in two groups was 3.2 ± 2.0 and 36 ± 24 months, respectively. Plasma C3 levels were decreased, but SLEDAI scores and plasma CRP levels were increased in new-onset patients relative to relapsing SLE patients (P < 0.05). Instead, no significant difference was found in involved organs, erythrocyte sedimentation rate (ESR) and plasma C4 levels between two groups.
| New-onset patients (n = 19) | Relapsing patients (n = 22) | Healthy controls (n = 20) | |
|---|---|---|---|
| Age (years), mean ± SD | 33 ± 14 | 35 ± 10 | 30 ± 10 |
| Sex (F/M) | 16/3 | 20/2 | 17/3 |
| Disease duration (months) | 3.2 ± 2.0 | 36 ± 24 | NA |
| Malar or discoid rash (+/−) | 12/7 | 8/14 | NA |
| Mucosal ulcer (+/−) | 4/15 | 4/18 | NA |
| Arthritis (+/−) | 15/4 | 10/12 | NA |
| Serositis (+/−) | 6/13 | 2/20 | NA |
| Proteinurine (+/−) | 3/16 | 12/10 | NA |
| Neurological disorder (+/−) | 1/18 | 0/22 | NA |
| Leucopenia (+/−) | 15/4 | 12/10 | NA |
| Anaemia (+/−) | 5/14 | 9/13 | NA |
| Thrombocytopenia (+/−) | 9/10 | 3/19 | NA |
| ANA (+/−) | 19/0 | 22/0 | 0/20 |
| Anti-dsDNA (+/−) | 3/16 | 7/15 | 0/20 |
| Anti-Sm (+/−) | 6/13 | 5/17 | 0/20 |
| C3 (mg/dl) | 47.4 ± 23.4a | 69.2 ± 43.6 | 90.3 ± 5.5 |
| C4 (mg/dl) | 5.8 ± 2.8 | 9.0 ± 8.2 | 18.6 ± 8.2 |
| ESR (mm/h) | 30.2 ± 14.6 | 43 ± 25 | 5.0 ± 2.3 |
| CRP (mg/l) | 5.0 ± 3.8a | 2.7 ± 2.3 | 1.5 ± 0.5 |
| SLEDAI scores | 9.4 ± 3.2a | 7.7 ± 3.5 | NA |
- Proteinuria: >0.5 g/day. Leucopenia: white blood cell count <4 × 106 cells/ml. Anaemia: haemoglobin <120 g/l for man, haemoglobin <110 g/l for woman. Thrombocytopenia: platelet count <1 × 108 cells/ml. Normal range: erythrocyte sedimentation rate (ESR) 0–20 mm/h, C-reactive protein (CRP): 0–5 mg/l, C3: 75–140 mg/dl, C4: 10–40 mg/dl. NA, not applicable, +/−, with/without; SLEDAI, SLE disease activity index.
- a P < 0.05 compared with relapsing patients.
Plasma IL-22 levels were reduced in new-onset SLE patients (53.91 ± 3.94 pg/ml) compared with relapsing SLE patients (58.08 ± 4.34 pg/ml, P < 0.05) and healthy controls (72.76 ± 6.11 pg/ml, P < 0.001). Meanwhile, plasma IL-22 levels in patients with relapsing SLE were also lower than in healthy controls (P < 0.001; Fig. 1A). Interestingly, in new-onset SLE patients, significantly increased levels of plasma IL-22 could be seen after treatment with prednisone and hydroxychloroquine (63.21 ± 6.25 pg/ml, P < 0.001; Fig. 1B).

Plasma IL-22 levels correlated with the percentages of Th22 cells in new-onset SLE patients
The percentages of Th1 (CD4+IFN-γ+), Th17 (CD4+IL-17+) and Th22 (CD4+IFN-γ−IL-17−IL-22+) cells in peripheral lymphocytes were examined by flow cytometry after in vitro activation by PMA/Ionomycin in short-term cultures (Figure S1).
In 19 new-onset SLE patients, the percentages of peripheral IL-22-producing Th cells (Th1, Th17 and Th22 cells) in peripheral lymphocytes were 5.48 ± 2.41%, 1.01 ± 0.30% and 0.14 ± 0.02%, respectively. The absolute numbers of Th1, Th17 and Th22 cells were 6.83 ± 3.15, 1.29 ± 0.55 and 0.17 ± 0.15 × 106/ml, respectively. The percentages and absolute numbers of Th1, Th17 and Th22 cells in healthy controls were (5.25 ± 2.10)% [7.54 ± 3.55 × 106/ml], (0.94 ± 0.39)% [1.41 ± 0.73 × 106/ml] and (0.09 ± 0.08)% [0.12 ± 0.10 × 106/ml], respectively. There were no statistical differences between the percentages and absolute numbers of Th1, Th17 and Th22 cells in patients with new-onset SLE and healthy controls (P > 0.05). A strong correlation was found between plasma IL-22 levels and the absolute numbers of Th22 cells (r = 0.711, P = 0.001), as shown in Fig. 2. However, there were no associations between plasma IL-22 levels and the absolute numbers of Th1 cells (r = 0.246, P = 0.311) and Th17 cells (r = −0.265, P = 0.273).
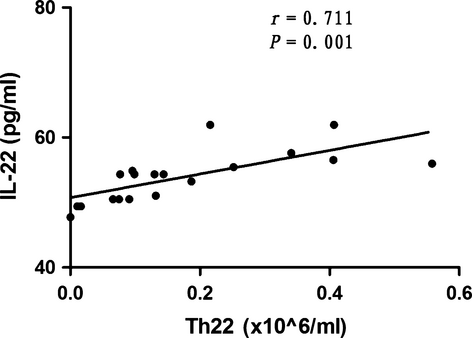
IL-22 as well as Th17 and Th22 cells, but not Th1 correlated with SLEDAI scores and ESR in new-onset SLE patients
We, respectively, analysed the correlations between plasma IL-22 concentrations and peripheral Th1, Th17 and Th22 cells percentages and clinical indicators in new-onset SLE patients (Table 2). Plasma IL-22 levels and the percentages of Th22 cells negatively correlated with SLEDAI scores (r = −0.384, P = 0.003; r = −0.557, P = 0.013, respectively) and ESR (r = −0.519, P = 0.023; r = −0.403, P = 0.032, respectively) in new-onset SLE patients. However, Th17 cells percentages had a positive correlation with SLEDAI scores (r = 0.332, P = 0.013) and ESR (r = 0.523, P = 0.032). No statistic correlation between the percentages of Th1 cells and SLEDAI scores and ESR was discovered (P = ns). And IL-22, Th1, Th17 and Th22 were not associated with disease duration, titre of antinuclear antibody (ANA), C--reactive protein (CRP), C3 and C4.
| Correlation | IL-22 (pg/ml) | Th1 (%) | Th17 (%) | Th22 (%) | ||||
|---|---|---|---|---|---|---|---|---|
| R | P value | R | P value | R | P value | R | P value | |
| Disease duration | 0.114 | ns | 0.054 | ns | −0.016 | ns | 0.055 | ns |
| SLEDAI scores | −0.386 | 0.003a | −0.436 | ns | 0.332 | 0.047 | −0.557 | 0.013a |
| Titre of ANA | −0.471 | ns | −0.232 | ns | 0.424 | ns | −0.068 | ns |
| ESR, mm/h | −0.519 | 0.023a | −0.354 | ns | 0.523 | 0.022a | −0.403 | 0.032a |
| CRP, mg/dl | −0.372 | ns | −0.243 | ns | 0.278 | ns | −0.371 | ns |
| C3, mg/dl | 0.228 | ns | 0.320 | ns | −0.285 | ns | 0.283 | ns |
| C4, mg/dl | 0.379 | ns | 0.040 | ns | −0.461 | ns | 0.295 | ns |
- SLEDAI, SLE disease activity index; ANA, ,antinuclear antibody; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; C3, complement 3; C4, complement 4; ns, not significant.
- a P < 0.05.
To clarify the correlations between clinical parameters and serum IL-22 levels, 41 SLE patients were divided into with/without nephritis, arthritis and malar rash separately. No statistic differences were observed in patients with/without nephritis (56.42 ± 4.04 versus 55.99 ± 4.99, P = 0.782), arthritis (56.00 ± 4.18 versus 56.38 ± 5.36, P = 0.801) and malar rash (57.47 ± 5.25 versus 54.89 ± 3.61, P = 0.073).
IL-22 autoantibodies detected in new-onset SLE patients
We utilized ELISA to assess whether there were autoantibodies present in plasma of patients that bind IL-22. The results indicated that high frequencies of plasma IL-22 autoantibodies were detected in new-onset SLE patients. Inversely, low or undetectable levels were found in healthy controls (Fig. 3). However, there was no statistic association between plasma IL-22 levels (pg/ml) and IL-22 autoantibodies indices (A405 nm; r = −0.100, P = 0.683).
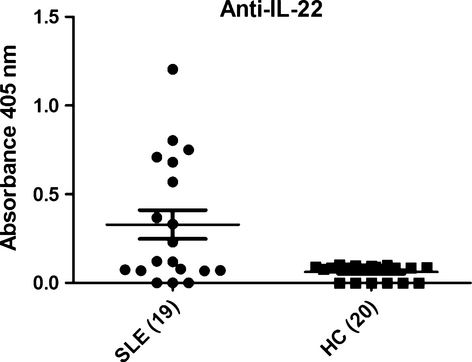
Discussion
Recently, there is emerging evidence that IL-22 is involved in the development and pathogenesis of various autoimmune diseases. Besides, under diverse tissue microenvironments, IL-22 may make an opposite contribution to disease progression 13. Considerable data indicate that IL-22 plays a beneficial role in inflammatory bowel disease by enhancing mucosa barrier function and epithelial innate immunity of intestinal tract 14. In addition, Radaeva et al. 15 have reported that IL-22 plays a protective role in T cell-mediated Marine Hepatitis. However, IL-22 is recognized as a pathogenic factor in the process of psoriasis 16, 17. Similarly, IL-22 has also been found to play a pro-inflammatory role in collagen-induced arthritis in C57BL/6 mice 18 and RA 19, 20. Thus, IL-22 has both pathogenic and protective property in autoimmune diseases.
In our study, we noticed that plasma IL-22 levels in new-onset and relapsing SLE patients were lower than those in healthy controls. Additionally, plasma IL-22 levels in new-onset patients were lower than in relapsing SLE patients. Compared with healthy controls, decreased plasma IL-22 levels were detected in patients with SLE, which consisted with two previous reports 21, 22. Maybe different sample size and individual difference account for a small difference in IL-22 levels compared with previous reports. Importantly, our study mainly focuses on new-onset lupus patients excluding glucocorticoids and immunosuppressants effect. Therefore, our results were likely to be more reliable by eliminating the confounding factor from drugs. Lower plasma IL-22 levels may be a characteristic feature of SLE. Kisand et al. 23 reported that there were low-titre autoantibodies existed in SLE patients' peripheral blood against IL-22, compared with high-titre neutralizing autoantibodies against IL-22 in patients with autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED). SLE is an autoimmune disease characterized by a variety of autoantibodies. In our study, high frequencies of IL-22 autoantibody have been detected in plasma of new-onset SLE patients compared with levels in controls. However, IL-22 levels did not statistically correlate with IL-22 autoantibody. Therefore, autoantibodies against IL-22 present in plasma of patients possibly did not influence IL-22 levels. Decreased IL-22 level is one distinct feature in the pathogenesis of SLE.
Meanwhile, plasma IL-22 levels were increased accompanied the improvement in new-onset SLE patients after treatment with prednisone and hydroxychloroquine. There were two reasons account for the augmentation of IL-22. The first was that IL-22 may do as a biomarker of improvement in lupus after therapy. The second was that prednisone and hydroxychloroquine treatment may stimulate IL-22 production by acting on lymphocytes. Ziesche et al. 24 found that dexamethasone suppresses IL-22 associated with bacterial infection in vitro and in vivo. Another study demonstrated that dexamethasone administration reduced IL-22 production in immune thrombocytopenia patients 25. In both former conditions, glucocorticoid can reduce expression of IL-22. However, McKinley et al. 26 reported that IL-22 production was not sensitive to dexamethasone treatment at any doses, and its levels were not associated with glucocorticoid treatment. To the best of our knowledge, no evidence proved that hydroxychloroquine could promote expression of IL-22. We speculated that increased plasma IL-22 levels indicated an improvement in lupus disease activity, but no association with prednisone and hydroxychloroquine themselves.
Furthermore, we found that IL-22 correlated with disease activity and ESR in new-onset lupus. In previous studies, Cheng et al. 22 found that decreased plasma IL-22 level positively correlated with disease activity in patients with SLE. Qin et al. 27 revealed that increased frequencies of IL-22-positive CD4+ T cells positively correlated with SLEDAI score in patients with SLE. In contrast, Pan et al. 21 investigated that no association was found between serum IL-22 levels and lupus disease activity. We concentrated on new-onset patients with lupus, but all recruited patients received glucocorticoids treatment in other three studies.
More importantly, we simultaneously detected plasma IL-22 levels and the frequencies of IL-22-producing Th cells in peripheral blood. Results showed that plasma IL-22 levels were positively related to Th22 (CD4+IFN-γ− IL-17− IL-22+) cells, not Th1 and Th17 cells. Accordingly, we found that Th22 cells, as well as IL-22, correlated with disease activity and ESR in new-onset lupus. Abnormal expression of peripheral Th22 cells was observed in other autoimmune diseases including psoriasis 7, systemic sclerosis 28 and RA 29. IL-22, as the most important functional cytokine of Th22 cells, exerts a pro-inflammatory role in autoimmune diseases. Therefore, Th22 cell may play an important part in lupus by producing IL-22.
Taken together, our research displayed that plasma IL-22 levels were markedly decreased in new-onset SLE compared with relapsing patients and healthy controls. Importantly, a positive association between IL-22 and specific IL-22-producing T helper cell Th22 were showed in new-onset SLE, and both of them inversely associated with SLEDAI scores and ESR. There were auto antibodies present in SLE patient's plasma that bind IL-22, but not correlate with IL-22 levels. These results advocated that decrease plasma IL-22 concentrations and correlation with Th22 percentages may be distinct features in new-onset SLE and correlate with lupus activity. Further studies are needed to elucidate how they participate in the pathogenesis of SLE.
Acknowledgment
This work was supported by grants from Medicine and Health Projects of Zhejiang Province (No. 2013KYA058). We also would like to thank healthy volunteers and patients with SLE for providing blood samples.
Conflict of interest statement
None.
Author contributions
Jin Lin and Lihuan Yue designed the research, analysed the data and edited the article; Lihuan Yue and Weiqian Chen performed the research and edited the article.




