Why are people with asthma susceptible to pneumonia? A review of factors related to upper airway bacteria
ABSTRACT
Asthma and pneumonia are common respiratory conditions globally, affecting individuals of all ages. Streptococcus pneumoniae is the predominant bacterial cause of pneumonia, with nasopharyngeal carriage an important step towards invasive and pulmonary disease. Vaccines provide individual protection, and also prevent nasopharyngeal carriage, providing herd immunity. Asthma is associated with an increased risk of pneumonia, but there is limited information on the underlying mechanism of this predisposition. Both asthma and its treatment may conceivably alter propensity to, and density of, carriage through an altered epithelial microenvironment driven by disease-related inflammation or treatment-related immunomodulation, for example with inhaled corticosteroids. The relative importance of these factors could impact the efficacy of vaccines in this vulnerable patient population. In this review, we summarize the evidence for an increased risk of pneumonia in asthma, and discuss factors affecting nasopharyngeal carriage in the context of current guidelines for pneumococcal vaccination.
INTRODUCTION
Pneumonia is a common condition and disproportionately affects people at the extremes of age, the immunocompromised and those with chronic respiratory conditions.1, 2 Asthma predisposes to pneumonia, but the magnitude and mechanism of effect are not fully elucidated.
Pneumonia remains a leading cause of morbidity and mortality worldwide, with the burden of disease being greatest in low- and middle-income countries (LMIC).3 It accounts for more deaths in children under the age of 5 than any other illness. The most common cause of bacterial pneumonia is Streptococcus pneumoniae, resulting in approximately 40 000 hospitalizations and 70 000 primary care consultations annually in the United Kingdom.4 S. pneumoniae is commonly found in the nasopharynx, the source of primary spread5 and usually the initial step towards infection.6 Carriage is most prevalent in children7, 8 and declines with increasing age,9 probably due to acquired immunity from exposure. A high carriage density in the nasopharynx is associated with subsequent development of pneumonia.10 Nasopharyngeal carriage is a dynamic process with changing prevalence, density and serotype of S. pneumoniae. Replacement with a more virulent and resistant serotype may lead to pneumonia.11
Preventing carriage is therefore an important step in controlling pneumococcal transmission,6 and reducing the incidence of respiratory tract conditions (sinusitis, otitis media and pneumonia) as well as invasive disease (septicaemia and meningitis).12 The perturbation of the airway microbiome may lead to pneumococcal pneumonia without dense nasal colonization.13, 14 This pathway to disease requires further study.
Pneumococcal vaccination is part of the childhood immunization programme in around half of World Health Organization member states.15, 16 Vaccination reduces hospitalizations due to pneumonia in children and adults,17 and the risk of invasive pneumococcal disease.18, 19 Two types of vaccines are available: pneumococcal polysaccharide vaccine (PPV) and pneumococcal (polysaccharide) conjugate vaccine (PCV). PPV provides serotype-specific immunity, targeting capsular polysaccharides (CPS) and induces short-term immune response by stimulating a subset of B cells producing immunoglobulin (Ig) G2 subclass antibodies.20, 21 PCV comprise of a polysaccharide in conjunction with a protein, stimulate a T cell-dependent serotype-specific immune response and stimulate memory B cells.22, 23 The 23-valent PPV has been available since 1983 and the 7-valent PCV (PCV-7) was licenced in 200023 followed by 10- and 13-valent vaccines in 2009 and 2010, respectively.24, 25 Vaccination programmes may potentially lead to serotype replacement by more virulent strains.17
Asthma affects people worldwide across all age groups and is associated with significant morbidity. People with asthma are at increased risk of pneumonia.26 This review will consider the evidence that this increased risk is driven by: (i) a greater prevalence of carriage of S. pneumoniae, (ii) a disordered immune response from exposure to the bacteria, (iii) impaired bacterial clearance and (iv) a suboptimal response to vaccination. It will consider the direct effects of asthma and indirect effects of asthma treatments. Inhaled corticosteroids (ICS), for example, increase the risk of pneumonia in COPD27 and this has led to a change in treatment guidelines.28
PNEUMOCOCCAL CARRIAGE IS MORE COMMON IN PEOPLE WITH ASTHMA
Early life pneumococcal carriage and subsequent asthma
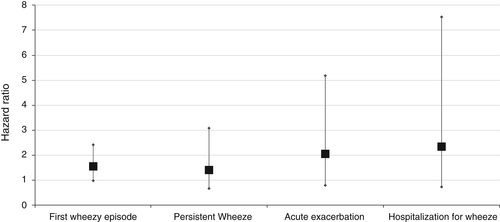
The Copenhagen Prospective Study on Asthma in Childhood (COPSAC) is a prospective, longitudinal birth-cohort of infants born to mothers with current or previous asthma. A total of 321 asymptomatic infants had hypopharyngeal swabs collected at 1 and 12 months, and bacteria were isolated using microbiological culture. The infants found to be colonized with S. pneumoniae had an increased risk of a first wheezy episode, developing persistent wheeze or asthma, and hospitalization for wheeze (Fig. 1) during follow-up. Early nasopharyngeal carriage was also associated with increased blood eosinophil count, higher total serum IgE and airway reversibility.29
The prevalence of asthma overall at 5 years of age was 14%, and 33% of those were initially colonized with S. pneumoniae. Blood eosinophil count and total IgE were significantly higher in colonized children at the age of 4 years.29 This study is strongly suggestive that early life nasopharyngeal carriage of S. pneumoniae is associated with subsequent asthma.
Pneumococcal carriage and current asthma
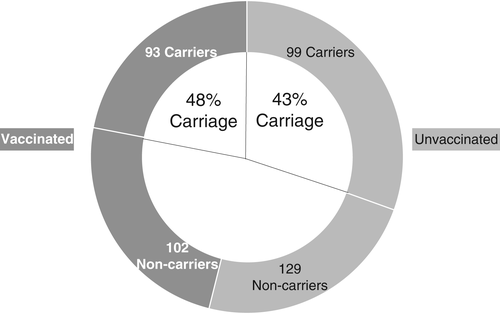
People with asthma have an increased prevalence of S. pneumoniae carriage in observational studies.30-32 For example, a cross-sectional study from Italy investigated S. pneumoniae carriage in children and adolescents with asthma by collecting oropharyngeal swabs at one time point. These samples were analysed using polymerase chain reaction (PCR) for S. pneumoniae genes.32 The observed carriage rate of 45% amongst the 423 children tested is similar to other studies of younger children.33 There was no association between previous PCV-7 and non-pharyngeal carriage, although time since vaccination was not studied32 Parental smoking, recent use of systemic corticosteroids and the severity of asthma did not affect carriage rates. Results are described in Figure 2.
There also appears to be a higher prevalence of pneumococcal carriage in adults with asthma. An observational study of army recruits compared S. pneumoniae carriage in individuals with (n = 224) or without (n = 668) asthma using swabs taken in the first 2 weeks of service. The rate of carriage confirmed by microbiological culture was twice as high in those with self-reported physician-diagnosed asthma.34 Just under half of those with asthma were on medication for the condition (107, 48%), and this factor did not influence the carriage rates observed in this study.34
Having an asthma exacerbation in the previous 12 months is associated with an increased risk of pneumococcal carriage. A cross-sectional population-based study of over 1000 adolescents in Brazil found a nasopharyngeal carriage rate of 19% in the 37 participants who had experienced an acute exacerbation of asthma in the previous 12 months (OR = 2.89 vs healthy controls).30 Hospitalization increases the risk of pneumococcal carriage,35 so a proportion of this effect may be explained by the exposure of individuals to a healthcare setting. However, only a small minority of individuals are admitted for more than 48 h with an asthma exacerbation, so this factor seems unlikely to be a major driver. Consistent with this are results from a recent cross-sectional study that assessed rates of carriage of S. pneumoniae amongst children with chronic conditions attending paediatric outpatients and healthy children attending routine ‘well-child’ assessments.36 Nasopharyngeal swabs were used to collect samples and processed by multiplex PCR. The prevalence of carriage in 394 healthy children was 8.6%, and 13.5% in 260 children with asthma. Children with asthma had a higher carriage than children with diseases that could also result in hospitalizations such as congenital heart disease (9.3% carriage), solid organ tumours (6.7%) and nephrotic syndrome (8%).
In summary, pneumococcal carriage appears more common in people with asthma, especially in individuals with a recent asthma exacerbation.
PATHOPHYSIOLOGICAL FACTORS AFFECTING CARRIAGE RATES IN ASTHMA
In this section, we discuss aspects of the immune system that mediate airway inflammation in asthma and may influence pneumococcal carriage and clearance in the nasopharynx.
Airway inflammation
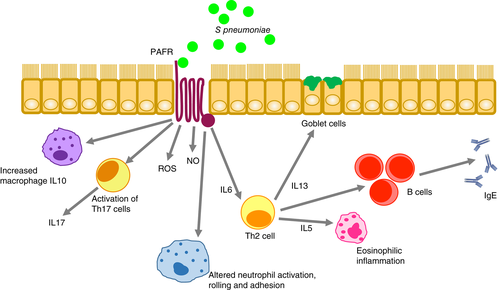
The hallmark of asthma is chronic airway inflammation mediated by innate and adaptive mechanisms.37 This causes differential damage throughout the airway epithelium38: surface epithelial thickening, metaplasia, goblet cell hyperplasia, increased mucus secretion with altered density and hypertrophy of smooth muscles can be present.39 Chronic inflammation secondary to eosinophils is seen in the nose and plays a part in development of nasal polyps.40 This inflammation is associated with higher pneumococcal carriage due to increased sites for bacterial attachment41 through expression of platelet-activating factor receptor (PAFR).42 Pneumococcus binds to PAFR through phosphorylcholine in its cell wall.43 PAFR is upregulated during viral infections and secondary to other inflammatory stimuli such as asthma.44 It is itself an important pro-inflammatory mediator which contributes to asthma pathogenesis by activating immune cells, causes chemotaxis of eosinophils, increases vascular permeability, mucus production and bronchial constriction (Fig. 3).45-47 PAFR antagonists represent potential therapies in acute and chronic asthma.48 in vitro studies show that mice lacking PAFR are relatively protected against pneumococcal pneumonia.49 In another murine model, mice were sensitized by intraperitoneal injection of ovalbumin, and subsequently their sinuses were dosed with S. pneumoniae with concomitant administration of ovalbumin to induce local allergic inflammation. Infection with S. pneumoniae was increased in response to the allergic inflammation induced by ovalbumin.50 In summary, the types of airway inflammation seen in asthma appear to be associated with increased bacterial adherence and carriage, and progression to infection.
CPS-specific and pneumococcal protein antibody responses
Impaired antibody responses may account for increased rates of carriage and risk of invasive pneumococcal disease in asthma.
Pneumococcal CPS IgG is usually protective against nasopharyngeal carriage. In a human infection model with pneumococcus, CPS IgG-mediated bacterial agglutination in the nasopharynx was observed in adults vaccinated with PCV and conferred protection against carriage acquisition.51 PCV reduces serotype-specific nasopharyngeal carriage, probably resulting in herd immunity and protection from disease.52, 53
In a small cross-sectional study, levels of serotype-specific CPS antibodies were lower in children and adults with asthma compared with healthy volunteers.54 There was no difference in nasopharyngeal carriage between the two groups (half of each group had been vaccinated) and their community exposure to S. pneumoniae appeared equivalent. The median number of positive vaccine serotype-specific antibodies in the PCV-7 cohort was also lower in asthma when compared to healthy volunteers (5 vs 7, respectively).54 In addition, there was an inverse relationship between the number of seropositive antibodies and a Th2 immune profile in response to house dust mite stimulation of peripheral blood mononuclear cells.54 A similar investigation compared levels of serum IgG to anti-pneumococcal surface protein in people with and without asthma. On this occasion, anti-pneumococcal surface protein C (Psp C) levels were inversely correlated with Th2 immune profile. Psp C is an adhesin to polymeric Ig receptor (PIgR): This receptor helps bacterial adhesion to the nasopharyngeal epithelium, initiates carriage and leads to invasive disease.55 Antibodies against pneumococcal surface proteins may also have a protective role against bacterial nasopharyngeal carriage and subsequent disease.56 Hales et al. identified lower levels of IgG1 titres to Psp C and Haemophilus influenzae antigens P4 and P6 in children with asthma.57 Taken together, all these results suggest an impaired immune response in people with asthma which may predispose to an increased risk of pneumococcal disease.
Cytokine IL17
Asthma is a syndrome with multiple sub-phenotypes and underlying mechanisms. Advances in our understanding of this heterogeneous condition have led to more tailored use of therapies such as monoclonal antibodies which are effective in patients showing Th2 cell-mediated inflammation. However, many asthmatic patients have at least some inflammation secondary to Th17 responses. This pathway appears more important in individuals who are older, often obese and non-allergic.37 Th17 cells produce cytokines such as interleukin (IL) 17 and IL22.58 In mouse models of asthma, IL17 contributes to airway remodelling by promoting fibroblast proliferation59 and opposing the anti-inflammatory features of T regulatory cells.60 It can also lead to direct contraction of smooth muscle leading to bronchial hyperresponsiveness.61
Although IL17 is an important pro-inflammatory cytokine which promotes airway remodelling in asthma, it facilitates timely clearance of pneumococcal carriage. IL17 secretion leads to neutrophil and macrophage recruitment and activation which in turn facilitate clearance of the bacteria.62, 63 Th17 responses have been associated with acquired immunity to carriage in mice models and control of duration and density of pneumococcal carriage.63
Allergic inflammation involving Th2 cytokines is associated with reduced host antibacterial activity including diminished IL17 and beta defensin.64 This is also seen in other allergic conditions such as atopic eczema, and associated with multiple and often persistent skin infections.65 A murine model studying the effect of allergic inflammation on innate immunity found increased viable bacteria in the lungs of sensitized mice after bacterial exposure along with reduced antimicrobial activity.65 Reduced immune responses in allergic airway inflammation may lead to an increased risk of pneumonia. Exogenous IL17 improves host defences against S. pneumoniae in mouse models, and reduces pulmonary eosinophil recruitment and bronchial hyper reactivity.66
The generalization that Th17-driven asthma has better protection against pneumococcal infection, and allergic asthma predisposes to infections appears a useful construct. However, it is evident that there is a considerable overlap in these processes within individuals over time37 and in the context of their differential response to corticosteroids.67 These findings emphasize that it is difficult to make general statements regarding individuals with a clinical label of asthma without better understanding what kind(s) of asthma they have.
Therapy with corticosteroids
ICS are associated with an increased risk of pneumonia in patients with COPD.27 They are the mainstay of treatment for asthma and could increase risk by altering nasal carriage or predisposing to infection if carriage is present.
In a large retrospective analysis of the manufacturer's trial data, use of budesonide was not associated with increased risk of pneumonia.68 This study analysed double-blind placebo-controlled trials as primary data set, including studies of both ICS, and ICS with long-acting beta-agonist (LABA) as the active intervention. The inclusion of LABA is likely to reduce infection risk.69 Pneumonia as an adverse event (AE) was observed in 0.5% of the budesonide cohort and 1.2% of the placebo group (95% CI: 0.36–0.76; P < 0.001), and was recorded as a serious AE (SAE) in 0.15% of the budesonide group and 0.13% (95% CI: 0.53–3.12; P = 0.58) in the primary set (only 22 occurrences in almost 15 000 individuals). In a secondary data set of 70 placebo or active comparator-controlled trials, pneumonia as an AE occurred in 0.70% and as an SAE in 0.17%.
In contrast to controlled trial findings, a real-world case–control study in the UK reported an increased risk of pneumonia and lower respiratory tract infection (LRTI) in asthma with any ICS (OR: 1.96, 95% CI: 1.15–1.33) and further increased if on high-dose ICS (OR: 2.04, 95% CI: 1.59–2.64).70 Patients with a diagnosis of pneumonia or LRTI in the preceding 3 years were identified from a primary care database and matched by age and gender to controls from the same geographical area. ICS dose and type were identified from prescriptions in the preceding 90 days.71 Adjustments were made for lung function and asthma severity along with oral steroid use. Of the commonly used asthma treatments, fluticasone was associated with the greatest risk of pneumonia (OR: 1.64; 95% CI: 1.50–1.79; P < 0.0.001), and budesonide a more modest risk (OR: 1.20; 95% CI: 1.06–1.35; P < 0.003).70
An observational study from Brazil reported an increase in nasopharyngeal carriage of S. pneumoniae in children with asthma taking ICS72 compared with age-matched controls. The children were on treatment with ICS for at least 30 days (n = 96) and the controls had no ICS for at least 90 days (n = 96); rates of pneumococcal vaccination were similar in both groups. The pneumococcal carriage was higher in the ICS group (27%) as compared to the controls (8%) (95% CI: 1.72–8.18; P = 0.001). A high dose and an increased duration of ICS was associated with increased prevalence of pneumococcal carriage (31.7% for 400–800 μg and >6 months treatment vs 19.4% for <100–300 μg and <6 months). It is unclear whether higher dose ICS was causative or simply a marker of more severe asthma.
When considering the effect of ICS on pneumococcal carriage and infection, it should be remembered that ICS are interacting with a broader airways’ microbiome. Pneumococcal carriage is linked to airway microbiome diversity in healthy adults.73 The diverse airway microbiome in asthma with predominance of proteobacteria may influence the nasopharyngeal carriage acquisition73 and certainly appears to influence corticosteroid responsiveness.74
Pneumococcal biofilms are structured cellular aggregates seen during nasopharyngeal colonization and diseases such as sinusitis.75 Functionally, biofilms promote antimicrobial resistance and the structural clustering of cells in close proximity facilitates horizontal transfer of genetic material.76, 77 This helps the bacteria adapt to the local environment—a well-established factor in pneumococcal virulence. Environmental factors are the major determinants of biofilm formation.78 Both ICS and the excipients in inhaled powders may affect biofilm formation in people with asthma.79 Genetic transformation and bacterial resistance may explain the increased pneumococcal virulence and given the prevalence of ICS use and the variety of potential mechanisms of action, the discrepancy between findings from highly selected population in trials with their restricted ecology of care and observations from practice warrants further study.
PNEUMOCOCCAL VACCINATION IN ASTHMA
Guidelines for pneumococcal vaccination in asthma differ between adult and paediatric patient populations and between countries, and this can create confusion in clinical practice.80 The current United Kingdom guidelines recommend vaccinating patients with chronic respiratory disease including asthma using the PPV.2 The Centres for Disease Control and Prevention (CDC) recommends vaccination according to disease severity in adults, with PPV for mild asthma and PCV for severe disease.81 Epidemiological evidence suggests less than half of the eligible adults with asthma receive vaccination.82
People with asthma have an altered pneumococcal antibody response
Children with asthma who have been recurrently exposed to bacteria such as S. pneumoniae have low antibody levels, including those with recurrent clinical infection.83 In a study by Rose et al., putatively protective levels were seen in between 20% and 54% of young children depending on serotype. Vaccination of these children with PCV was apparently more effective than PPV in raising antibody titres to putatively protective levels.83 A further study by this group investigating sequential immunization with PCV then PPV showed that around 80% children did not have an initial protective level of 0.35 μL/mL.84
Similarly, a Korean study measured IgG levels to six serotypes before and after PPV in children aged 2–14 years. They also found that children with asthma had a lower baseline antibody level, although the fold increase post-vaccination was similar to that seen in healthy children.85
It is uncertain if people with asthma are less likely to gain clinical benefit from pneumococcal vaccination
It is unclear if this apparent blunted antibody response to vaccination results in reduced clinical benefit. Modelling suggests the ‘number needed to vaccinate’ to prevent one case of invasive pneumococcal disease is comparable to other high-risk conditions.86 However, the limited available observational evidence does not confirm or refute this. A retrospective observational study examined pneumonia episodes in over 2700 patients with asthma before and after pneumococcal vaccination and compared this to age-, gender- and region-matched controls with no airway disease. There was no significant difference in rates of hospitalization for pneumococcal pneumonia pre- or post-vaccination compared with the control group.87 However, the patient population had a mean age of over 50 years, so was perhaps not typical of asthma patients, and the infrequent events gave rise to wide CI around estimates.
The paucity of evidence around the clinical effect of vaccination in people with asthma suggests that further research is needed. As a ‘real-world’ trial is unlikely to happen given the size of the study required, experimental challenge in humans appears the most appropriate way forward.
SUMMARY
Nasopharyngeal carriage is the first step towards invasive pneumococcal disease,6 and inflammatory conditions lead to an increase in both the rate and density of S. pneumoniae nasal carriage.10 Damaged airway mucosa and use of ICS in asthma together may increase carriage, biofilm formation and carriage density leading to a higher risk of pneumonia in asthma.88
CPS are an important defence mechanism of S. pneumoniae, and low antibody levels are seen at baseline with a reduced response after vaccination in asthma. There is little information about the impact and responses of PCV and PPV in clinical practice.
There are cost implications in checking baseline antibody levels and vaccinating everyone with asthma, particularly as effectiveness is uncertain. Better characterization of asthma phenotypes may help in understanding the pattern of immune responses and target both testing and vaccination.
Nasopharyngeal carriage, disease from S. pneumoniae and low immune responses require further investigation in asthma, as do pneumococcal virulence factors such as pneumolysin which have the ability to cause necroptosis of immune cells and may increase the risk of disease and carriage. Large prospective observational and vaccine intervention studies can address these gaps in our knowledge. However, these are costly, time-consuming and often challenging to deliver. Controlled human challenge models provide a more suitable option to study immune mechanisms in response to bacterial colonization, and eliminate the need to translate data from animal models into human trials whilst requiring a smaller and more intensely studied cohort. Experimental human pneumococcal challenge has been shown to be well tolerated and informative in people without asthma.89 This approach could yield important pathological insight into the interaction between asthma, its treatment and pneumococcus.
Acknowledgements
Professor Daniela Ferreira and Dr Jamie Rylance both contributed to the review by providing feedback on the structure and content.
Abbreviations
-
- AE
-
- adverse event
-
- COPD
-
- chronic obstructive pulmonary disease
-
- CPS
-
- capsular polysaccharide
-
- ICS
-
- inhaled corticosteroid
-
- LABA
-
- long-acting beta-agonist
-
- LRTI
-
- lower respiratory tract infection
-
- OR
-
- odds ratio
-
- PAFR
-
- platelet-activating factor receptor
-
- PCV
-
- pneumococcal conjugate vaccine
-
- PCV-7
-
- 7-valent PCV
-
- PPV
-
- pneumococcal polysaccharide vaccine
-
- Psp C
-
- pneumococcal surface protein C
-
- SAE
-
- serious AE