M/XDR-TB treatment perspective: How to avoid mountains of pills via digital technologies
Abstract
See Reply
Multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant TB (XDR-TB) are life-threatening illnesses associated with poor treatment adherence, catastrophic cost of treatment as well as high morbidity and mortality. According to the 2017 World Health Organization (WHO) global report,1 treatment success rates for XDR-TB are now alarmingly similar to TB in the pre-antibiotic era in upper-middle and high-income countries, such as Thailand 14%, Germany 18% and Estonia 12%.
As Dheda et al. highlight, the latest clinical guidelines for shorter treatment of drug-resistant TB and introduction of new (bedaquiline and delamanid) and repurposed (meropenem, linezolid and so on) medicines remain controversial2 because of different drug-resistance patterns in Mycobacterium tuberculosis seen across the world.
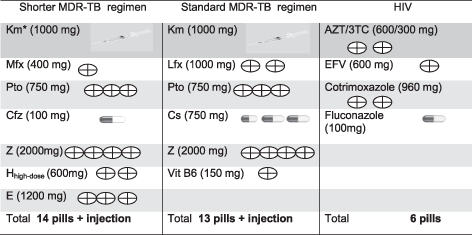
To this discussion it is important to add that the pill burden (Table 1) is enormously high for patients treated from multidrug and extensively drug-resistant TB (M/XDR-TB) and co-morbidities, such as human immunodeficiency virus (HIV). The number of pills in a daily pile reaches 20 apart from additional injection of antibiotics. Almost 2 years long, high pill burden regimen decreases patient’s adherence, quality of life and complicates managing of the treatment for the health care staff. This is particularly worrying given global efforts to improve access to molecular diagnostics3 and as new agents have limited benefits for those M/XDR-TB patients who cannot tolerate or complete conventional regimens.
| Daily pill burden in intensive phase |
|---|
|
- 3TC, lamivudine; ART, anti-retroviral therapy; AZT, zidovudine; Cfz, clofazimine; E, ethambutol; EFV, efavirenz; Hhigh-dose, high-dose isoniazid; Km, kanamycin; Lfx, levofloxacin; MDR-TB, multidrug-resistant tuberculosis; Mfx, moxifloxacin; Pto, prothionamide; TB, tuberculosis; Vit B6, pyridoxine hydrochloride; Z, pyrazinamide.
One daily pill with fixed drug combination could alleviate these issues but the huge variation in global resistance patterns make the development of fixed drug combinations for MDR-TB treatment seem an unachievable goal. Novel approaches offer hope of a solution: in 2015 the US Food and Drug Administration (FDA) agency approved the first three-dimensional (3D) printed product—antiepileptic levetiracetam. Furthermore, a polypill (12 mm in diameter) containing five active compartments has recently been printed for cardiovascular diseases. Computer-guided drug fabrication could enable the mixing of multiple anti-TB drugs in modified release preparations with minimal negative interactions.
There are also potential solutions to improve adherence: in November 2017 the FDA approved an innovative preparation of the antiphychotic aripiprazole which contains a digital ingestion tracking system allowing adherence monitoring through a web-based portal. Digital medication monitoring is a safe and cost-effective method of identifying non-adherence in real time.4
The length of the regimen should be also personalized: at present, rifampicin-resistant TB and M/XDR-TB treatment regimens are of equal length which ignores resistance profiles, the severity of disease, clinical response and HIV status. To catalyse the paradigm shift envisaged within the End TB Strategy we propose a focus on biomarkers that can predict treatment duration to allow a move away from empirical treatment lengths. In addition, drug plasma concentrations can be measured enabling individualized dosing that prevents toxicity.
Truly personalized low pill burden and shorter regimens for M/XDR-TB patients are achievable. The individualized drug formulation based on bacterial drug-resistance pattern could be 3D printed in easy-to-swallow combinations with inbuilt adherence monitoring. Further research in this field is urgently required to confine ‘mountains of pills’ to the past.




