Cost-effectiveness of endobronchial valve treatment in patients with severe emphysema compared to standard medical care
ABSTRACT
Background and objective
Bronchoscopic lung volume reduction using endobronchial valves (EBV) is an effective new treatment option for severe emphysema patients without interlobar collateral ventilation. The objective of this study was to perform an economic evaluation including the costs and cost-effectiveness of EBV treatment compared with standard medical care (SoC) from the hospital perspective in the short term and long term.
Methods
For the short-term evaluation, incremental cost-effectiveness ratios (ICER) were calculated based on the 6-month end point data from the STELVIO randomized trial. For the long-term evaluation, a Markov simulation model was constructed based on STELVIO and literature. The clinical outcome data were quality-adjusted life-years (QALY) based on the EuroQol5-Dimensions (EQ5D) questionnaire, the 6-min walking distance (6MWD) and the St George’s Respiratory Questionnaire (SGRQ).
Results
The mean difference between the EBV group and controls was €16 721/patient. In the short-term (6 months), costs per additional QALY was €205 129, the ICER for 6MWD was €160 and for SGRQ was €1241. In the long term, the resulting cost-effectiveness ratios indicate additional costs of €39 000 per QALY gained with a 5-year time horizon and €21 500 per QALY gained at 10 years. In comparison, historical costs per additional QALY 1 year after the coil treatment are €738 400, 5 years after lung volume reduction surgery are €48 415 and 15 years after double-lung transplantation are €29 410.
Conclusion
The positive clinical effects of EBV treatment are associated with increased costs compared with SoC. Our results suggest that the EBV treatment has a favourable cost-effectiveness profile, also when compared with other treatment modalities for this patient group.
Abbreviations
-
- 6MWD
-
- 6-min walking distance
-
- EBV
-
- endobronchial valve
-
- EQ5D
-
- EuroQol5-Dimensions
-
- GOLD
-
- Global Initiative for Chronic Obstructive Lung Disease
-
- ICER
-
- incremental cost-effectiveness ratio
-
- LVRS
-
- lung volume reduction surgery
-
- LY
-
- life-years
-
- MID
-
- minimal important difference
-
- QALY
-
- quality-adjusted life-year
-
- RCT
-
- randomized controlled trial
-
- SGRQ
-
- St George’s Respiratory Questionnaire
-
- SoC
-
- standard medical care
INTRODUCTION
The randomized-controlled STELVIO trial performed in our hospital showed that endobronchial valve (EBV) treatment significantly improved pulmonary function, exercise capacity, physical activity and quality of life after 6 months in COPD patients characterized by emphysema and the proven absence of interlobar collateral ventilation compared with standard of care.1, 2 Furthermore, these improvements were still present after 1-year follow-up.3 Afterwards, two larger randomized-controlled multicentre trials using the same technique in both homogeneous and heterogeneous emphysema confirmed our findings.4, 5 Therefore, the EBV treatment is an important asset to the treatment options for this patient group who have hardly any treatment options left. Only for a few of these patients, invasive surgical procedures such as lung volume reduction surgery (LVRS) and lung transplantation remain. Recently, the EBV treatment was included for the first time in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines.6
Although showing significant efficacy, and being important for the quality of life of our patients, it is also important to perform an economic evaluation of this new treatment. Such an evaluation can provide justification for the costs of the treatment by relating it to the clinical benefit and allows comparison with other treatment modalities in support of policy decisions regarding reimbursement. Until now, only one study investigated the cost-effectiveness of the EBV treatment in a study population from Germany and concluded that the EBV treatment is cost-effective in the German healthcare system.7
The aim of our study was to perform a cost-effectiveness analysis in the short term (6 months) and long term (using a simulation model up to 10 years) of EBV treatment compared with standard medical care (SoC) in patients with severe emphysema in the Dutch healthcare system.
METHODS
Study population and design
Sixty-eight patients participated in the STELVIO trial1 (trial registry number: NTR2876), which was a randomized-controlled trial comparing the EBV treatment (n = 34) with SoC (n = 34). After 6 months, the SoC group also received the treatment. We recently published the 1-year follow-up results.3 The study was approved by the local ethics committee and all patients provided informed consent. The STELVIO trial was supported by a grant from the Netherlands Organization for Health Research and Development (ZonMw-171101008) and the current economical evaluation was part of the original trial. The cost-effectiveness was evaluated separately for the short term (up to 6 months follow-up) and long term (10 years follow-up).
Outcome measures
Health-related quality of life was measured by the EuroQol5-Dimensions (EQ5D) questionnaire.8 Utility values were based on the Dutch tariffs for the corresponding EQ5D scores.9 Furthermore, health-related quality of life was measured by the St George’s Respiratory Questionnaire (SGRQ)10 and an established mapping algorithm was used to predict EQ5D utility scores.11 Exercise capacity was measured using the 6-min walk distance test (6MWT), performed according to the American Thoracic Society guidelines.12
Short-term economic evaluation
The short-term evaluation was performed using data from the randomized patients in the STELVIO trial up to 6 months follow-up. Costs were calculated from a hospital perspective and only direct medical costs were included. Data regarding treatment (number of catheters and Zephyr EBV; PulmonX Inc., Redwood City, CA, USA) and clinical events up to 6 months follow-up (exacerbations, pneumonia and re-bronchoscopy with or without valve replacement) were used to calculate the costs per patient. In the STELVIO trial, the median hospital stay was 1 day (range: 1–13). However, due to clinical experience regarding the pneumothorax risk on general, the routine hospital stay for all patients currently is 5 days; therefore, the costs of 5 days hospital admission were used in our evaluation. The prices were derived from the Dutch health insurance perspective (price level 2016). The unit costs and prices are depicted in euros (Table 1). The short-term evaluation was performed using SPSS (version 23; IBM-Corp., New York, NY, USA). Incremental cost-effectiveness ratios (ICER) were calculated by dividing the difference in costs between the EBV group and SoC group by the difference in clinical improvements after 6 months. A bootstrap analysis with 5000 replications was performed to evaluate uncertainty surrounding the ICER.13 This method, performed using R-project software (version 3.2.0; R-project, Vienna, Austria), implies replication of data sets from the original study data, simulating repetition of the study.
| Unit costs (€)† | Resource use (€) | Resource use (€) | |||
|---|---|---|---|---|---|
| EBV group (n = 34) | Control group (n = 34) | ||||
| n | n | ||||
| Treatment | |||||
| Bronchoscopy with EBV treatment including 5 days hospital stay | 3676.61 | 34 | 125 005 | NA | |
| Chartis catheter | 750 | 34 | 25 500 | NA | |
| EBV catheter | 200 | 47 | 9400 | NA | |
| Zephyr EBV | 1900 | 152 | 288 800 | NA | |
| Complications | |||||
| COPD exacerbation without hospitalization | 246.57 | 15 | 3699 | 17 | 4192 |
| COPD exacerbation with hospitalization | |||||
| Hospitalization due to COPD exacerbation 1–4 days | 3676.61 | 1 | 3677 | 1 | 3677 |
| Hospitalization due to COPD exacerbation 5–14 days | 6026.48 | 2 | 12 053 | 1 | 6026 |
| Hospitalization due to COPD exacerbation 14–28 days | 13 746.44 | 1 | 13 746 | 0 | 0 |
| Pneumonia with hospitalization | |||||
| Hospitalization due to pneumonia 1–5 days | 3699.46 | 1 | 3699 | 1 | 3699 |
| Hospitalization due to pneumonia 6–28 days | 6318.71 | 2 | 12 637 | 0 | 0 |
| Pneumothorax with or without drainage | 2642.10 | 6 | 15 853 | 0 | 0 |
| Re-bronchoscopy with valve removal | 3676.61 | 4 | 14 706 | 0 | 0 |
| Re-bronchoscopy with valve removal with pneumothorax | 7685.27 | 3 | 23 056 | 0 | 0 |
| Re-bronchoscopy with valve replacement (average price) | 10 716.61 | 5 | 53 583 | 0 | 0 |
| Total | 605 414 | 17 594 | |||
- † Unit costs for the year 2016.
- EBV, endobronchial valve; €, Euro.
Long-term economic evaluation
For the long-term cost-effectiveness evaluation, a Markov simulation model was constructed using Excel2010 (Microsoft Corp., Redmond, WA, USA). Transition probabilities derived from the STELVIO trial and subsequent follow-up and mortality data from the literature14 were used to model the monthly transition of patients (Fig. 1). Because of the detailed information for the first 6 months of the study, the time scale in the simulation model was in months. Table 2 shows the transition probabilities, costs and utilities (all fixed parameters). As prognosis in our patient group is closely linked to their GOLD stage, we distinguished three GOLD stages in our model, that is, GOLD II, III and IV. Transitions between the GOLD stages were modelled according to the short-term data from the randomized controlled trial (RCT) up to 6 months. After that, no transitions between GOLD stages were modelled and patients could only leave their GOLD stage by dying. Mortality estimates for these transitions were taken from the literature.14 In addition, background mortality due to other causes was included based on age-specific data from Statistics Netherlands.15 For each month that a simulated patient was in a certain state, costs corresponding to this state were calculated using half-cycle correction. Costs of dying and costs of transition between GOLD stages were set to zero. The total costs per month were summed up to present total costs of EBV treatment and usual care for the duration of the time horizon of the model. Summation was done with and without discounting, that is, with and without reflecting depreciated value of future costs and effects. The net effect of discounting is that early costs and effects receive more weight in the summation than late costs and effects. Common annual discount rates in economic evaluations are 3–5%, and we applied annual discount rates of 4%.
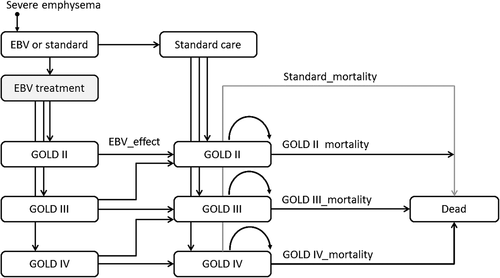
| Parameter | Untreated | EBV | Source |
|---|---|---|---|
| Mortality | |||
| GOLD II | 0.06/year | 0.06/year | Afonso et al.14 |
| GOLD III | 0.11/year | 0.11/year | Afonso et al.14 |
| GOLD IV | 0.25/year | 0.25/year | Afonso et al.14 |
| Costs | |||
| 0–6 months | |||
| Treatment | €0 | €13.197 | STELVIO trial |
| Events | €86/month | €506/month | STELVIO trial |
| Re-bronchoscopy | €0 | €263/month | STELVIO trial |
| 6–12 months | |||
| Re-bronchoscopy | €0 | €90/month | STELVIO trial |
| >12 months | |||
| Re-bronchoscopy | €0 | €67/month | STELVIO trial |
| Utility scores | |||
| SGRQ 0 month | 0.52 | 0.53 | STELVIO trial |
| SGRQ 1 month | 0.55 | 0.71 | STELVIO trial |
| SGRQ 6 months | 0.55 | 0.74 | STELVIO trial |
| SGRQ 12 months | 0.58 | 0.68 | STELVIO trial + extrapolated |
| EQ5D 0 month | 0.66 | 0.63 | STELVIO trial |
| EQ5D 1 month | 0.72 | 0.75 | STELVIO trial |
| EQ5D 6 months | 0.67 | 0.75 | STELVIO trial |
| EQ5D 12 months | 0.66 | 0.77 | STELVIO trial + extrapolated |
- EBV, endobronchial valve; EQ5D, EuroQol5-Dimensions; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SGRQ, St George’s Respiratory Questionnaire; €, Euro.
The model was populated with 100 patients in each group and distributed across the GOLD stages in accordance with the RCT population. The average patient age at the start of the Markov model simulation was set at 50 years. In view of the limited availability of long-term estimates of patient survival and the known poor overall survival of this patient group,14 the time horizon of the long-term evaluation was restricted to 10 years.
RESULTS
Study population
Patient characteristics, the CONSORT study flow chart and clinical outcomes are described previously in detail.1 Briefly, at 6 months, the EBV treatment group had a significant increase compared with the SoC group of 191 mL in forced expiratory volume in 1 s (FEV1), 106 m in 6-min walking distance (6MWD) and −14.7 points in SGRQ.
Short-term evaluation
The resource use of both groups is shown in Table 1. In the EBV group, per patient on average 1 Chartis catheter, 1.4 EBV catheters and 4.5 EBV were used. The mean average cost of the EBV treatment in the STELVIO trial was €13 197. At 6 months follow-up, the mean cost difference between the EBV group and SoC group per patient was €16 721 (95% CI: €16 675–16 766 after bootstrapping). In the randomized 68 patients, the mean difference in change in EQ5D utility score between groups at 6 months was 0.12 (95% CI: 0.01–0.24, P = 0.04) and for SGRQ utility score was 0.16 (95% CI: 0.07–0.24, P < 0.01) (Table 2). Unfortunately, there was a significant difference between groups at baseline in EQ5D utility score (control group: 0.66 and EBV group: 0.63). As a consequence, the ICER was not reliable for this outcome (−€1 941 250). After bootstrapping and adjusting for the 1-year time period, the ICER per additional quality-adjusted life-year (QALY) was €205 129 (95% CI: €203 547–206 709) using the SGRQ.
We also calculated the ICER for the change in 6MWD and for the change in SGRQ total score (Fig. 2). The ICER for the 6MWD at 6 months follow-up was €160.46 (95% CI: €159.66–161.27), meaning that 1-m improvement in 6MWD costs €160 extra in comparison with SoC. The ICER for SGRQ was €1240.71 (95% CI: €1253.99–1227.42), meaning that 1-point improvement in SGRQ total score costs €1241 extra.
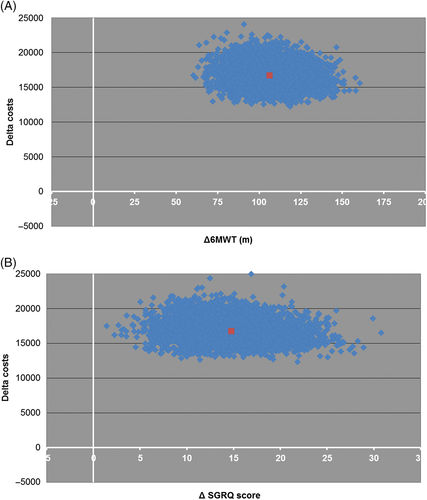
Long-term evaluation
During the first 6 months, changes in GOLD stages reflecting improvements in lung function were modelled, leading to increased number of patients in GOLD III and GOLD II and a decreased number of patients in GOLD IV in the EBV group. After 5 years, the number of deaths in the EBV group was around 50 versus 60 in the control group. This difference remained more or less stable until the end of the modelled time horizon. Changes in quality of life measured with the EQ5D questionnaire and the SGRQ result in an additional advantage of EBV over control over the entire remaining duration of the modelled time horizon.
The cumulative differences in costs, life-years and QALY are summarized in Table 3, with and without discounting. The resulting cost-effectiveness ratios indicate that higher costs of EBV treatment are partially compensated by lower mortality and better quality of life, requiring additional costs of around €71 500 per life-year gained and around €39 000 per QALY gained with a 5-year time horizon. For a 10-year time horizon, the additional costs of the EBV treatment are €29 000 per life-year gained and around €21 500 per QALY gained. The impact of discounting on costs was small as the majority of costs occurred during the first year. Due to the effect of discounting on life-years, the incremental cost-effectiveness was negatively influenced.
| Undiscounted | Discounted | |||
|---|---|---|---|---|
| Untreated | Valve treatment | Untreated | Valve treatment | |
| 5 years | ||||
| Cost | €49 416 | €2 020 968 | €49 416 | €2 001 520 |
| Life-years | 330 | 358 | 309 | 334 |
| EQ5D-QALY | 220 | 272 | 206 | 253 |
| SGRQ-QALY | 190 | 240 | 178 | 223 |
| ICER-Cost/LY | €71 512 | €79 100 | ||
| ICER-Cost/EQ5D-QALY | €38 525 | €41 870 | ||
| ICER-Cost/SGRQ-QALY | €39 638 | €42 775 | ||
| 10 years | ||||
| Cost | €49 416 | €2 171 464 | €49 416 | €2 116 914 |
| Life-years | 472 | 545 | 418 | 477 |
| EQ5D-QALY | 314 | 416 | 278 | 363 |
| SGRQ-QALY | 272 | 367 | 241 | 321 |
| ICER-Cost/LY | €29 046 | €34 883 | ||
| ICER-Cost/EQ5D-QALY | €20 848 | €24 255 | ||
| ICER-Cost/SGRQ-QALY | €22 375 | €25 827 | ||
- EQ5D, EuroQol5-Dimensions; ICER, incremental cost-effectiveness ratio; LY, life-years; QALY, quality-adjusted life-year; SGRQ, St George’s Respiratory Questionnaire; €, Euro.
DISCUSSION
We investigated the cost-effectiveness for the EBV treatment in severe emphysema patients with proven absence of collateral ventilation using data from a randomized-controlled trial.1 For the first 6 months, the incremental costs per QALY gained was €205 129 and the ICER for the 6MWD was €160 and for the SGRQ total score was €1241. Consequently, reaching the minimal important difference (MID) in 6MWD of 26 m would cost €4160 extra in comparison with SoC and reaching the MID of 7 points in SGRQ total score would cost €8687 extra. In the long term, the cost-effectiveness ratios were much more favourable, as higher costs of EBV treatment were partially compensated by lower mortality and better quality of life. The additional costs per life-year gained were around €71 500 and per QALY gained were around €39 000 with a 5-year time horizon. With a 10-year horizon, the incremental costs were around €29 000 per life-year gained and around €21 500 per QALY gained.
The other study investigating the cost-effectiveness of the EBV treatment in Germany found an ICER of €46 322 per QALY gained over 5 years and an ICER of €25 142 per QALY over 10 years.7 We found comparable values despite the differences in modelling structure and input variables (5 years: €39 638, 10 years: €22 375 using the same SGRQ). For example, there is a large difference in the price of the EBV treatment. In the German population, the treatment price per patient was €9851 compared with €13 197 in our population. This could be explained by the higher average number of valves used in our trial, the inclusions of the (Chartis) catheters in the price evaluation and the difference in costs of hospital admissions between countries. Unfortunately, no short-term economic evaluation is reported in the German study. Regarding the generalizability of our results in a Dutch population from an international perspective, it is positive that similar results were observed in a German population. However, even though Germany and The Netherlands are similar countries with respect to population, unit prices differed between these countries which will influence the actual cost-effectiveness ratio. Therefore, the generalizability of our cost-effectiveness ratios should be evaluated taking the country’s healthcare system and differences in unit prices into account.
To put the cost of the EBV treatment in perspective, we compared the costs-effectiveness analyses of other treatments for advanced emphysema patients such as lung volume reduction coil treatment, LVRS and lung transplantation. Another bronchoscopic lung volume reduction treatment option is the treatment with coils.16, 17 The REVOLENS trial investigated the lung volume reduction coil treatment and reported an ICER of $782 598 (approximately €738 400) per additional QALY, calculated over a 1-year period.18 This is much higher than our calculated ICER of €205 129 using the same SGRQ at 1 year. This could be explained by the higher cost difference of the coil treatment versus the EBV treatment ($47 908/€45 071 vs €16 721) and the less distinct efficacy improvements of the coil treatment. Therefore, the relative cost-effectiveness is in favour of EBV treatment. However, there is a large group of patients who cannot be treated with EBV but could benefit from the coil treatment. For LVRS, one study in Canada found a cost-effectiveness ratio of €90 611 per QALY gained ($133 900, Canadian Dollars) compared with SoC over a 2-year time horizon. Furthermore, the NETT trial investigating LVRS reported a cost-effectiveness of LVRS versus medical therapy of €125 521 per QALY gained ($140 000, American Dollars) at 5 years and €48 415 per QALY gained ($48 000, American Dollars) at 10 years.19 Therefore, the cost-effectiveness of LVRS compared with EBV treatment also seems in favour of EBV treatment. For lung transplantation, a study in the UK reported costs-effectiveness of €43 413 ($48 241) for single lung, €29 410 ($32 803) for double lung per QALY gained over a 15-year time period.20 Therefore, the cost-effectiveness of EBV treatment also seems to be superior to lung transplantation. However, lung transplantation is only an option for a small group of patients.
There is a large difference between the costs of the treatment in terms of QALY in the short term compared with the long term. Unfortunately, until now, no efficacy and safety results in the long term are published, but we believe that potentially the patient will benefit on average from the treatment at least until 5-year follow-up. The mortality risk of this severe patient group is very high (Table 2 14) and the positive clinical effects support the risk/benefit ratio and additional costs of this treatment. Furthermore, long-term follow-up data on survival of successfully treated patients with EBV confirm this assumption.21 However, this study did not include an SoC group, and therefore, more research is needed to gain insight in the potential improved survival after the EBV treatment. Therefore, the costs related to treatment should be allocated over the total duration of the time that patients benefit. Unfortunately, statistical modelling is necessary for this evaluation as no 5-year follow-up data are available and also it is not ethical to have a control group for this long time period. However, modelling will always be a simplification of the real-world situation. The short-term economic evaluation was based on actual measured data, which is a strength of this analysis.
A limitation of this trial is that the follow-up of the control group was only 6 months before these patients received the EBV treatment. A longer control group follow-up for the cost-effectiveness evaluation would have been better as a QALY is calculated per year and to avoid the use of estimates based on clinical experience.
Another limitation could be that we only included the indirect costs and in the long term only included the EBV-related complication costs like revision bronchoscopies. We did not have information on, for example, the number of exacerbations in the long term in a control group or EBV group, and therefore, we did not include this in the model. Similarly, we did not have data on healthcare use outside of our hospital, such as general practitioner visits. However, we expect that including these costs would probably be in favour of the EBV treatment and that by excluding them we do not overestimate the real difference.
In conclusion, our results suggest that the EBV treatment has a favourable cost-effectiveness profile, also when compared with other treatment modalities for this patient group. We believe that the favourable cost-benefit profile would justify this treatment for this severe patient group with hardly any other treatment options left.
Disclosure statement
D.-J.S. is a physician advisor to PulmonX Inc., USA, an investigator for trials sponsored by PulmonX Inc. and received lecture and travel fees for case support, educational and scientific sessions. PulmonX Inc. had no involvement of any kind in this trial.




