Impact of COPD and emphysema on survival of patients with lung cancer: A meta-analysis of observational studies
Abstract
Both COPD and emphysema are associated with an increased incidence of lung cancer, but the impacts of these comorbidities on lung cancer prognosis are still unclear. Herein, we conducted a meta-analysis to clarify whether the presence of these comorbidities indicates poor survival in patients with lung cancer. A comprehensive search was conducted using PubMed, Embase, Web of Science, ASCO Abstracts and Cochrane library for articles published before 1 June 2015. Papers referenced by the obtained articles were also reviewed. Main outcomes were overall survival (OS) and disease-free survival (DFS) in patients with lung cancer. Pooled hazard ratio (HR) and 95% confidence intervals (CIs) were calculated using random-effects models. Subgroup and sensitivity analyses were also conducted. Of 58 full texts reviewed, 26 met our inclusion criteria that were derived from 21 and seven studies examining the impacts of COPD and emphysema on survival of lung cancer, respectively. Meta-analyses revealed that concomitant COPD was associated with poorer OS (HR, 1.17; 95% CI: 1.10–1.25, n = 20), which was independent of tumour staging, diagnostic criteria of COPD or location, and DFS (HR, 1.52; 95% CI: 1.04–2.23, n = 6) with high heterogeneity (I2 = 78%). The presence of emphysema in patients with lung cancer predicted worse OS (HR, 1.66; 95% CI: 1.25–2.22, n = 7), but not poorer DFS. The presence of COPD and emphysema are robust predictors of poor survival in patients with lung cancer. Early detection of these diseases should be taken into account for lung cancer surveillance and management.
Abbreviations
-
- ASCO
-
- American Society of Clinical Oncology
-
- BMI
-
- body-mass index
-
- CI
-
- confidence intervals
-
- COPD
-
- chronic obstructive pulmonary disease
-
- CT
-
- computed tomography
-
- DFS
-
- disease-free survival
-
- HR
-
- hazard ratio
-
- OS
-
- overall survival
-
- PFS
-
- progression-free survival
-
- RFS
-
- recurrence-free survival
Introduction
Lung cancer is one of the most common malignancies worldwide and harbours a poor prognosis. Comorbidities occur frequently in patients with lung cancer.1 These comorbidities are prevalent and confer significant impacts on the prognosis.2-5 The most crucial comorbidity is COPD (chronic obstructive pulmonary disease), with a prevalence of 40% to 70%.6-8 Since emphysema is a crucial constituent of COPD, one might postulate that emphysema also affects a considerable number of patients with lung cancer.
Previous studies have demonstrated that COPD and emphysema are independent risk factors for developing lung cancer.9, 10 More recently, the association between lung cancer and COPD has been verified in population-based and in vitro cell studies.11 The risk of lung cancer in patients with COPD is approximately five-fold greater than that of smokers without COPD,8, 10 independent of age and cigarette smoking.8, 12 It remains unclear whether the presence of COPD and emphysema are associated with poorer prognosis. Improving our understandings of these associations has crucial public health implications, since the incidences of COPD and lung cancer have been steadily increasing worldwide. Some studies have suggested that lung cancer patients with COPD had poorer prognosis than those without,3, 13 whilst other studies did not find differences in survival.14, 15 Arca et al.16 reported that survival time was longer in patients with COPD. Similarly, research on the prognosis of lung cancer patients with emphysema have also yielded inconsistent results.15, 17
We conducted a meta-analysis to determine whether concomitant COPD and emphysema in patients with lung cancer were associated with poorer overall survival (OS) and disease-free survival (DFS).
Methods
Search strategy
Two authors (Y.H.G. and W.J.G) searched the PubMed, Embase, Web of Science, American Society of Clinical Oncology (ASCO) Abstracts and Cochrane library without language restrictions for articles published before 1 June 2015. We identified studies by using Medical Subject Headings (MeSH) including ‘Pulmonary Disease, Chronic Obstructive’; ‘Lung Neoplasms’; ‘Pulmonary Emphysema’; ‘Survival’; ‘Prognosis’, in conjunction with the keywords including ‘lung cancer’, ‘bronchitis’, ‘COPD’, ‘chronic obstructive lung disease’, ‘emphysema’, ‘mortality’, ‘survival analysis’ and ‘outcome’. The searches excluded case-report, letter, review, editorial, comment, news, guideline and meta-analysis. Additionally, reference lists of all included studies were checked manually to include other potential eligible studies.
Eligibility criteria
The inclusion criteria were as follows: (i) comparison between lung cancer patients with COPD or emphysema and those without; (ii) outcomes were survival-related, such as OS or disease-free survival (DFS); (iii) outcomes involving human subjects published in English; and (iv) sufficient data generating relative risk estimates with 95% confidence intervals (CIs). In addition, we excluded the following studies: (i) no control patients; (ii) studies reporting lung-cancer-specific survival only; and (iii) studies reporting only postsurgical or in-hospital mortality. When studies had the same patients or reported a subgroup of another study, only the study with the most complete outcome information or a greater number of patient cohort was included.
The quality score for each study was not assigned, because no such score has been generally accepted for use in prognostic meta-analysis, especially for observational studies. Alternatively, we conducted subgroup and sensitivity analyses because of their global acceptance.
Data extraction and outcomes
Screening of potentially eligible studies was conducted independently by two authors (Y.H.G and W.J.G), and disagreement being resolved by consensus. Baseline characteristics and outcomes were extracted from the selected articles. We chose OS and DFS as endpoints for meta-analysis. Progression-free survival (PFS) or recurrence-free survival (RFS) was regarded as DFS.
Statistical analysis
The hazard ratio (HR) was used as a universal measure of prognostic value, with 1.0 or greater indicating poorer survival for the group with concomitant COPD or emphysema. HRs and 95% CIs were extracted from all articles. For those studies in which an HR and the corresponding 95% CI or standard error were not available, we calculated the estimates from survival curves as suggested by Tierney and colleagues.18 For studies that presented stratified estimates (i.e. severity of COPD or emphysema),3, 17 we combined the estimates by using random effects model and the pooled estimates were subsequently used for meta-analyses. If results of both univariate and multivariate Cox regression analyses were reported, HRs from multivariate models were selected for more accurate estimates.
Heterogeneity across studies were tested by using I2 statistic, which is a quantitative measure of inconsistency, with the suggested thresholds of 25–50% for low, 50–70% for moderate and >75% for high heterogeneity, respectively.19 A random-effect model, which considered both within-study and between-study variation, was used to represent the pooled risk estimates regardless of the magnitude of heterogeneity. Subgroup analyses were performed based on the variables including tumour staging, location, diagnostic methods of COPD and emphysema, and whether multivariate or univariate Cox regression was used. We also conducted sensitivity analysis by evaluating the influence of individual study on the overall estimate by repetitively removing any single study from the pooled literature or studies which did not report the tumour staging and treatment.
Potential publication bias was assessed by visual inspection of the funnel plot in which the logarithms of HRs were plotted against their standard errors. Begg's and Egger's test were used to estimate the severity of publication bias, with P value <0.05 being considered as statistically significant. Statistical analysis was performed by using Stata 12.0 (Stata Corp, College Station, Texas, USA) and Cochrane Collaboration Review Manager 5.1.2 (Cochrane Collaboration, Oxford, UK) software.
Results
Literature search
Figure 1 presents the flowchart for identification of relevant studies. In total, 5188 articles were screened for eligibility. After initial screening based on titles and abstracts with predefined inclusion/exclusion criteria, 58 articles were identified for full-text review, of which 26 articles were identified for meta-analysis.3, 5, 13-17, 20-38 Of these, 19 specifically reported the results on COPD,3, 5, 13, 14, 16, 20, 22-24, 27, 29, 30, 32-38 5 on emphysema,17, 21, 26, 28, 31 and 2 on both COPD and emphysema15, 25 with lung cancer prognosis.
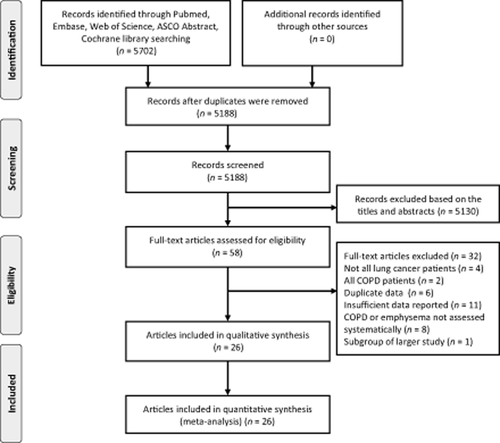
A flow chart showing the procedure for identifying the studies included in the meta-analysis. From Moher et al.51
Prognostic value of concomitant COPD on lung cancer survival
Table 1 presents the characteristics of 21 studies for concomitant COPD and lung cancer survival.3, 5, 13-16, 20, 22-25, 27, 29, 30, 32-38 These studies were published between 2001 and 2015. Of these, six studies were conducted in the United States,3, 13, 23, 25, 32, 35 seven in European countries5, 15, 16, 29, 30, 33, 34 and eight in Asian countries.14, 20, 22, 24, 27, 36-38 The sample size ranged from 114 to 18 077, resulting in a total of 60 764 participants and 11 270 cases across these studies. Seven studies included only patients undergoing surgery,13, 20, 22, 24, 27, 35, 38 and in two studies,29, 37 treatment was not reported. In defining COPD, 14 studies used spirometry,3, 14-16, 20, 22, 24, 25, 27, 33-36, 38 and the remaining seven studies used medical records or self-report data.5, 13, 23, 29, 30, 32, 37 Regarding study design, 19 were retrospective cohort studies3, 5, 13, 14, 16, 20, 22-25, 27, 29, 32-38 and two were prospective cohort studies.15, 34 Early-stage patients were exclusively enrolled in five studies,5, 13, 20, 38, 39 11 studies pooled patients with stages I–IV lung cancer14-16, 23-25, 27, 30, 33-35 and the remaining six studies had unavailable staging information.3, 22, 29, 32, 36, 37 Sixteen studies (73.7%) conducted multivariate analysis to adjust for confounding variables such age, sex, performance status, body-mass index (BMI), smoking and tumour staging.3, 5, 13-15, 20, 22, 24, 25, 27, 30, 32, 33, 36-38 Seven studies exclusively focused on outcomes of patients who underwent surgical resection.13, 20, 22, 24, 27, 34, 35, 38
| Design | No. of participants | Age, years, meana | Stage | Follow-up, months | COPD Ascertainment | Treatment strategy | Outcomes | HR estimation | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| COPD | Total | ||||||||||
| Yoshida Y,38 2015 (Japan) | R | 62 | 243 |
Non-COPD: 64.1 GOLD I: 69.5 GOLD II–III: 70.8 |
IA | Median 68.4 | Spirometry | Surgery | RFS | Reported in text | Yes |
| Jian ZH,37 2015 (Taiwan) | R | 2866 | 13 399 | NR | NR | NR | Medical record | NR | OS | Reported in text | Yes |
| Iachina M,5 2015 (Denmark) | R | 845 | 10 378 |
<67 (47.4%) >67 (52.6%) |
0–II | NR | Medical record | Surgery, chemotherapy, radiotherapy | OS | Reported in text | Yes |
| Kuo CH,20 2014 (Taiwan) | R | 59 | 181 | 63.9 | I | NR | Spirometry | Surgical Resection | OS, RFS | Reported in text | Yes |
| Lee SJ,14 2014 (South Korea) | R | 111 | 221 |
Non-COPD: 62.1 COPD: 67.3 |
I–IV | Median 84.0 | Spirometry | Surgery, chemotherapy, chemoradiation, radiation, BSC etc | OS | Reported in text | Yes |
| Zhai RH,13 2014 (United States) | R | 330 | 902 |
Non-COPD: 66.8 COPD: 67.1 |
IA–IIB | Median 41.0 | Self-reported physician-diagnosis COPD | Surgical Resection | OS, PFS | Reported in text | Yes |
| Sekine Y,24 2013 (Japan) | R | 363 | 1 461 |
Non-COPD: 62.5 GOLD I: 69.1 GOLD II: 67.6 GOLD III: 67.9 |
I–IV | NR | Spirometry | Surgical Resection | OS | Reported in text | Yes |
| Wang HM,23 2013 (United States) | R | 173 | 722 |
<65 (50.6%) >65 (49.4%) |
I–III | Median 44 | Medical record | Chemotherapy Radiotherapy | OS, DFS | Reported in text | No |
| Yamamoto S,22 2013 (Japan) | R | 104 | 319 | Median, 67 | NR | Median 28 | Spirometry | Surgical Resection | OS | Reported in text | Yes |
| Mina N,25 2012 (United states) | R | 94 | 114 |
Men: 58.7 Women: 58.0 |
Local regional distant | Median 42.5 | Spirometry or CT diagnosed COPD | Surgery, chemotherapy, chemoradiation, radiation etc | OS | Reported in text | Yes |
| Gullón JA,15 2011 (Spain) | P | 164 | 353 |
<70 (62.9%) ≥70 (37.1%) |
I–IV | 24 | Spirometry | Surgery, chemotherapy, chemoradiation, radiation, BSC etc | OS | Reported in text | Yes |
| Kondo R,27 2011 (Japan) | R | 157 | 531 |
Non-COPD: 65.9 COPD: 70.6 |
I–IV | NR | Spirometry | Surgical Resection | OS | Reported in text | Yes |
| Kiri VA,29 2010 (UK) | R | 1260 | 18 077 |
Non-COPD: 71.1 COPD: 72.9 |
NR | NR | Medical records | NR | OS | Data extrapolated | No |
| Arca JA,16 2009 (Spain) | R | 396 | 996 |
Non-COPD: 66.7 COPD: 70.0 |
I–IV | Mean 8.6 | Spirometry and/or medical records | Surgery, chemotherapy, radiotherapy, palliative treatment | OS | Data extrapolated | No |
| van de Schans SA,30 2007 (Netherlands) | R | 695 | 2 679 |
35–64 (32.0) >65 (68.0) |
I–IV | NR | Medical records | Surgery, chemotherapy, radiotherapy | OS | Reported in text | Yes |
| Birim O,33 2006 (Netherlands) | R | 464 | 766 | 64.5 (9.2) | 0–IV | NR | Spirometry | Surgery, chemotherapy, radiotherapy | OS | Reported in text | Yes |
| Dy SM,32 2006 (United States) | R | 1276 | 4 207 |
Non-COPD: 76 COPD: 76 |
NR | NR | Medical records | Surgery, chemotherapy, radiotherapy | OS | Reported in text | Yes |
| López-Encuentra A,34 2005 (Spain) | P | 1370 | 2 928 |
Non-COPD: 61.5 COPD: 67.2 |
0–IV | NR | Spirometry | Surgical Resection | OS | Data extrapolated | No |
| Tammemagi CM,3 2003 (Unites States) | R | 330 | 1 155 | NR | NR | median 27.3 | Medical record | Surgery Chemotherapy Radiotherapy | OS | Reported in text | Yes |
| Sekine Y,35 2002 (United States) | R | 78 | 166 |
Non-COPD: 63.6 COPD: 65.3 |
I–IV | NR | Spirometry | Surgical Resection | OS | Reported in text | No |
| Kurishima K,36 2001 (Japan) | R | 73 | 966 | NR | NR | NR | Spirometry | Surgery, chemotherapy, radiotherapy, palliative treatment | OS | Reported in text | Yes |
- a Unless otherwise specified, values were presented as the mean.
- BSC, best supportive care; COPD, chronic obstructive pulmonary disease; CT, computed tomography; GOLD, The Global Initiative for Chronic Obstructive Lung Disease; HR, hazard ratio; NR, not reported; OS, overall survival; P, prospective; PFS, progression-free survival; R, retrospective; RFS, recurrence-free survival.
Meta-analyses showed that concomitant COPD was associated with poorer OS compared with patients without COPD (HR, 1.17; 95% CI: 1.10–1.25), with a high heterogeneity across studies (I2 = 74%, P < 0.001) (Fig. 2a). Removal of any individual study did not alter the overall pooled HR or heterogeneity, which ranged from 1.16 (95% CI: 1.08–1.23) to 1.20 (95% CI: 1.12–1.28). In addition, exclusion of the studies which did not report treatment and staging29, 37 also did not alter the pooled estimates (HR, 1.17; 95% CI: 1.09–1.26). Six studies were included to assess the association between COPD and DFS.13, 20, 22-24, 38 COPD was associated with poorer DFS (HR, 1.52; 95% CI: 1.04–2.23), with a high heterogeneity across studies (I2 = 78%, P < 0.001) (Fig. 2b). Excluding the study by Wang HM et al.23 eliminated the heterogeneity across studies (HR, 1.19; 95% CI: 1.10–1.28; I2 = 0%, P value for heterogeneity = 0.77).
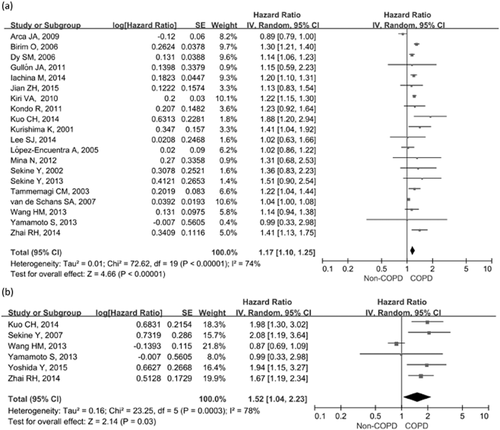
Forest plots of the associations between concomitant chronic obstructive pulmonary disease (COPD) and lung cancer survival. (a) Effect of concomitant COPD on overall survival; (b) Effect of concomitant COPD on disease-free survival.
Prognostic value of concomitant emphysema on lung cancer survival
Table 2 shows the characteristics of seven studies published between 2006 and 2014 for the presence of emphysema and lung cancer survival.15, 17, 21, 25, 26, 28, 31 Of these, two studies were conducted in the United States,17, 25 one in European countries15 and four in Asian countries.21, 26, 28, 31 The sample size ranged from 100 to 1143, resulting in a total of 2465 participants and 1068 cases across studies. Two studies included only patients who underwent surgery,28, 31 and one study did not report treatment strategy.17 Emphysema was diagnosed by computed tomography (CT) in all studies, five studies used a standard protocol for definition15, 21, 25, 26, 31 and the remaining two studies based on clinical interpretation of radiologists.17, 28 Patients with early-stage lung cancer were exclusively enrolled in three studies,17, 28, 31 and the remaining four studies pooled patients with stage I–IV lung cancer.15, 21, 25, 26 Five studies conducted multivariate analysis to adjust for confounding variables such age, sex, performance status, BMI, smoking and stage.15, 21, 25, 28, 31 Two studies specifically focused on the outcomes of patients who underwent surgical resection.28, 31
| Source | Design | No. of participants | Age, years, meana | Stage | Follow-up, months | Emphysema Ascertainment | Treatment strategy | Outcomes | HR estimation | Multivariate analysis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Emphysema | Total | ||||||||||
| Kumagai S,21 2014 (Japan) | R | 98 | 365 | Median, 69 | I–III | NR | HRCT | Surgery, chemotherapy | OS, DFS | Reported in text | Yes |
| Bishawi M,17 2013 (United States) | R | 114 | 153 |
Non-emphysema: 64 Emphysema: 68 |
I | NR | CT | NR | OS | Data extrapolated | No |
| Mina N,25 2012 (United states) | R | 86 | 114 |
Men: 58.7 Women: 58.0 |
Local regional distant | Median 42.5 | CT | Surgery, chemotherapy, chemoradiation, radiation etc | OS | Reported in text | Yes |
| Gullón JA,15 2011 (Spain) | P | 103 | 353 |
<70 (62.9%) ≥70 (37.1%) |
I–IV | NR | CT | Surgery, chemotherapy, chemoradiation, radiation, BSC etc | OS | Reported in text | Yes |
| Usui K,26 2011 (Japan) | R | 505 | 1143 |
Non-emphysema: median, 66 Emphysema: median, 70 |
I–IV | NR | CT | Surgery, chemotherapy, radiation, BSC | OS | Data extrapolated | No |
| Lee SA,28 2010 (South Korea) | R | 104 | 237 | Non-emphysema: 66.5 Emphysema: 60.4 | I–II | NR | CT | Surgical resection | OS | Reported in text | Yes |
| Ueda K,31 2006 (Japan) | R | 58 | 100 | Non-emphysema: 67.7 Emphysema: 70.4 | IA–IB | NR | CT | Surgical resection | OS | Reported in text | Yes |
- a Unless otherwise specified, values were presented as the mean.
- BSC, best supportive care; CT, computed tomography; DFS, disease-free survival; HR, hazard ratio; HRCT, high resolution computed tomography; NR, not reported; OS, overall survival; P, prospective; R, retrospective.
Pooled results showed that the presence of emphysema was associated with poorer OS than for patients without (HR, 1.66; 95% CI: 1.25–2.22). No significant heterogeneity across studies (I2 = 32%, P = 0.18) was found (Fig. 3a). Exclusion of any single study did not alter the overall pooled estimates, except for the study by Ueda et al.31 (I2 = 0%, P value for heterogeneity = 0.83), which completely eliminated the heterogeneity. Meta-analyses of two studies21, 31 did not reveal any association between emphysema and lung cancer DFS, with a pooled HR of 2.83 (95% CI: 089–8.95) and high heterogeneity (I2 = 72%, P = 0.06) (Fig. 3b).
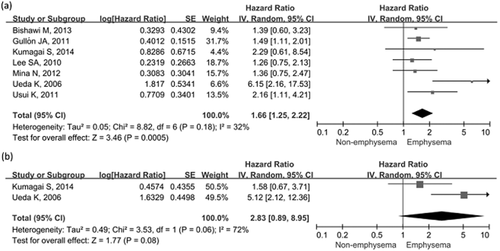
Forest plots of the associations between concomitant chronic obstructive pulmonary disease and lung cancer survival. (a) Effect of concomitant emphysema on overall survival. (b) Effect of concomitant emphysema on disease-free survival.
Subgroup analysis of the impacts of COPD and emphysema on lung cancer OS
COPD and OS
Despite the limited number of included studies, subgroup analyses focusing on patients with early-stage lung cancer (I–II) showed stronger associations between COPD and poor OS (HR, 1.42; 95% CI: 1.15–1.76; n = 4; I2 = 62%), whereas no pooled analyses specifically focused on patients with advanced stages (III–IV) could be performed due to the lack of studies included. Interestingly, a subgroup of studies which incorporated multivariate analysis showed that COPD patients had poorer OS of lung cancer (HR, 1.22; 95% CI: 1.12–1.32) than those without, whilst studies involving univariate analysis yielded no significant association between COPD and OS. Subgroup analyses according to other variables, such as the diagnostic method of COPD and study location, unanimously demonstrated that COPD patients had increased risks of poorer OS compared with the non-COPD counterparts. Results of all subgroup analyses are summarized in Table 3.
| No. of reports | HR | 95% CI | I2 | P Value for heterogeneity | |
|---|---|---|---|---|---|
| The effects of COPD on lung cancer overall survival | |||||
| Stage | |||||
| Early (0–II2B)5, 13, 20, 39a | 4 | 1.42 | 1.15–1.76 | 62% | 0.05 |
| Mixed14-16, 23-25, 27, 30, 33-35 | 11 | 1.11 | 1.00–1.24 | 77% | <0.01 |
| Unavailable3, 22, 29, 32, 36, 37 | 6 | 1.20 | 1.14–1.25 | 0% | 0.50 |
| Spirometry diagnosed COPD | |||||
| Yes3, 14-16, 20, 22, 24, 25, 27, 33-36 | 13 | 1.20 | 1.05–1.37 | 69% | <0.01 |
| No5, 13, 23, 29, 30, 32, 37 | 7 | 1.16 | 1.08–1.26 | 80% | <0.01 |
| Study location | |||||
| United States3, 13, 23, 25, 32, 35 | 6 | 1.18 | 1.11–1.25 | 0% | 0.50 |
| Europe5, 15, 16, 29, 30, 33, 34 | 7 | 1.11 | 1.01–1.23 | 89% | <0.01 |
| Asian14, 20, 22, 24, 27, 36, 37 | 7 | 1.30 | 1.12–1.50 | 0% | 0.50 |
| Multivariate analyses | |||||
| Yes3, 5, 13-15, 20, 22, 24, 25, 27, 30, 32, 33, 36, 37 | 15 | 1.22 | 1.12–1.32 | 71% | <0.01 |
| No16, 23, 29, 34, 35 | 5 | 1.08 | 0.92–1.28 | 84% | <0.01 |
| The effects of emphysema on lung cancer overall survival | |||||
| Stage | |||||
| Early (0–II2B)17, 28, 31 | 3 | 2.02 | 0.85–4.80 | 72% | 0.03 |
| Mixed15, 21, 25, 26 | 4 | 1.57 | 1.23–2.00 | 0% | 0.68 |
| Standard protocol to define emphysema by HRCT | |||||
| Yes15, 21, 25, 26, 31 | 5 | 1.92 | 1.27–2.91 | 48% | 0.10 |
| No17, 28 | 2 | 1.30 | 0.83–2.02 | 0% | 0.85 |
| Study location | |||||
| United States17, 25 | 2 | 1.37 | 0.84–2.23 | 0% | 0.97 |
| Asian21, 26, 28, 31 | 4 | 2.24 | 1.18–4.26 | 60% | 0.06 |
| Europe15 | 1 | 1.49 | 1.11–2.01 | NA | NA |
| Multivariate analyses | |||||
| Yes15, 21, 25, 28, 31 | 5 | 1.68 | 1.14–2.48 | 49% | 0.10 |
| No17, 26 | 2 | 1.82 | 1.08–3.08 | 0% | 0.42 |
- a Reference 24 and 39 reported the effect of COPD on stage IA and all pathologic stage lung cancer from the same cohort, respectively.
- CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; HRCT, high resolution computed tomography; NA, not applicable.
Emphysema and OS
As shown in Table 3, the results of subgroup analyses for the presence of emphysema in lung cancer outcomes were less consistent due to the limited number of studies available. However, the interactions among groups were not statistically significant (all P > 0.05 for interaction).
Publication bias
Visual inspection of funnel plot indicated certain asymmetry for the associations of concomitant COPD with OS (Fig. 4). However, Begg's and Egger's test did not show significant evidence of publication bias (Begg's test, P = 0.55; Egger's test, P = 0.18). We did not test the publication bias for the meta-analysis for concomitant COPD on DFS, and that for the presence of emphysema on OS and DFS due to insufficient studies available to render a valid statistical test.
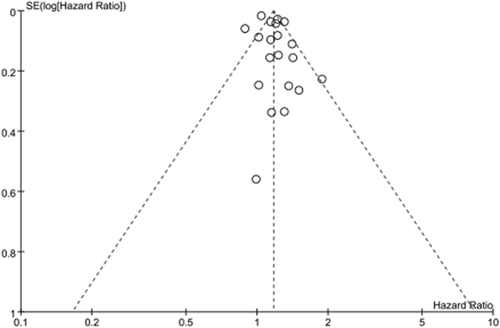
Funnel plots to assess publication bias regarding the association between concomitant chronic obstructive pulmonary disease and lung cancer overall survival.
Discussion
Our meta-analysis demonstrated that concomitant COPD in patients with lung cancer was associated with poorer OS, which was independent of the tumour staging, diagnostic method of COPD, study location and DFS. Similarly, the presence of emphysema on CT scan in patients with lung cancer was a poor prognostic indicator of OS, but not significantly impacted on DFS, probably because of the low number of studies. However, these data did not necessarily indicate a causal relationship. Notably, without evidence from randomized controlled trials, it would be inappropriate to conclude that prevention and treatment of COPD or emphysema would improve the prognosis of lung cancer. This study, however, may at least provide a solid basis for conducting further researches into this area.
With respect to COPD, our pooled results for OS were consistent across several subgroups when stratified by various studies and participants' characteristics despite the significant heterogeneity of included studies. Interestingly, if only patients with early-stage lung cancer or those who had undergone surgery were included, more pronounced associations between COPD and worse OS were demonstrated. Two possible explanations for these findings were considered. First, many comorbidities which adversely affects the outcomes of lung cancer were less common in patients who underwent surgical resection, because this would be contraindicated for surgical resection. Second, aggressive tumour growth, invasion or metastasis, but not comorbid conditions (i.e. COPD), predominantly contributed to the prognosis in lung patients with advanced stages. For COPD and DFS, the pooled results also indicated that concomitant COPD predicted poorer DFS with a high heterogeneity (I2 = 78%), which was eliminated following removal of the study by Wang HM et al.23 (P = 0.77 for heterogeneity). The primary endpoint of this study was to assess the impacts of beta-blocker intake on survival outcomes of patients treated with definitive radiotherapy, in which most patients had stage III disease compared with patients with early-stage (stage I-II) in the remaining four studies. These confounding factors might have accounted for the heterogeneity of the association between COPD and DFS. Similar to COPD, our pooled results suggested that presence of emphysema would help predict worse OS. In view that the presence of emphysema without airflow obstruction was still associated with increased risks of lung cancer and poorer prognosis, once patients were diagnosed as having emphysema (as suggested by chest CT), they should be periodically followed-up using low-dose CT for early detection of lung cancer in clinical settings.
To date, the mechanisms linking COPD or emphysema and survival remain unclear. Several possible mechanisms have been proposed. First, COPD and emphysema are characterized by local and systemic chronic inflammation, which might cause chronic mitogenesis and increase the likelihood of the conversion of endogenous DNA damage into mutations, finally leading to lung cancer pathogenesis.40, 41 Second, an imbalance between oxidants and antioxidants can lead to free radical damage of DNA,42 which was associated with poorer survival in patients with lung cancer.43 Third, both COPD and emphysema are associated with abnormal apoptosis and cell cycle regulation,44 which is a crucial mechanism implicated in the prognosis of lung cancer.45 Fourth, epigenetic modifications (i.e. aberrant DNA methylation) are fairly common in these two diseases, which play a crucial role in modulating lung cancer prognosis.46, 47 In addition, both COPD and emphysema impair lung function, which may be the factors limiting surgical interventions for lung cancer. Finally, comorbidities are prevalent in these two diseases, such as cardiovascular disease and diabetes, which also negatively affected the outcomes of concomitant lung cancer.48, 49
Several limitations should be considered when interpreting our findings. First, studies specifically addressing the effects of COPD on DFS or emphysema on OS and DFS were less common in the literature, and therefore might have been under-represented in this meta-analysis, which has complicated the measurement of publication bias. Second, we found significant heterogeneity across studies for COPD and OS, which might have resulted from the difference in study designs, the diagnostic criteria or severity of COPD, tumour staging, histologic subtypes, participants' characteristics and analytical strategies. Although high heterogeneity remained in many subgroups, the pooled HRs showed consistently negative associations across most subgroups. Third, none of the studies focused on patients with advanced (III–IV) stages or analyzed patients separately into early-stage (0-II) and advanced-stage lung cancer, which precluded the subgroup analysis in advanced-stage and restricted the derivation of accurate conclusions. Fourth, subgroup analyses investigating the gender difference in the association between these comorbidities and survival outcomes could not be performed due to the limited number of studies.13 Finally, although the design was consistent in the majority of studies, few were prospective in design. By using standardized ascertainment methods and endpoints, prospective studies are still warranted to confirm the findings of this meta-analysis and evaluate the prognostic value of COPD and emphysema on lung cancer with different histologic subtypes.
Despite these limitations, our meta-analysis has rigorously evaluated the impacts of COPD and emphysema on lung cancer survival by using all eligible studies with extensive literature searches, along with intensive subgroup analyses. Potential clinical impacts of our findings on patient management might be at least two-fold: (i) the significantly negative association between COPD or emphysema and lung cancer outcome addresses the importance of COPD and emphysema as crucial and independent variables in the development of prognostic assessment tools in patients with lung cancer; and (ii) a previous study has shown that under-diagnosis and under-treatment of COPD remained substantial in patients with lung cancer,50 therefore timely diagnosis of COPD followed by optimal treatment might improve the outcome in this population.
In conclusion, our results support the notion that concomitant COPD and emphysema herald poorer prognosis in patients with lung cancer. Further studies investigating the effect of optimal treatment for these comorbidities on prognosis of lung cancer are warranted.
Acknowledgements
GJZ has received funding from the Ministry of Major Science and Technology of Henan (201302005) and a grant from the Education Agency of Henan (122300410363). WJG received funding from the National Natural Science Foundation (No. 81400010) and 2014 Scientific Research Projects for Medical Doctors and Researchers from Overseas, Guangzhou Medical University (No. 2014C21).




