Increases in peripheral SIRT1: A new biological characteristic of asthma
Abstract
Background and objective
Silent information regulator 1 (SIRT1) is a class III histone deacetylase that exerts both anti-inflammatory and anti-aging effects. However, no data are available regarding SIRT1 expression in patients with asthma. Here, we studied SIRT1 levels in the serum of patients with asthma and analysed the distribution of SIRT1 in both the serum and the lungs in an asthmatic mouse model to determine its clinical significance.
Methods
Serum SIRT1 levels, total immunoglobulin E (IgE) levels and peripheral blood eosinophil percentages as well as pulmonary function were quantified in 97 patients with asthma and 118 healthy volunteers. BALB/c mice were sensitized and challenged using ovalbumin (OVA) to produce the asthmatic model, and SIRT1 levels in both the serum and the lung tissues were subsequently measured.
Results
The serum SIRT1 levels were significantly elevated in the patients with asthma compared with the controls. Serum SIRT1 levels positively correlated with total IgE levels and negatively correlated with pulmonary function. In the OVA-sensitized and challenged mice, an increased serum SIRT1 level was confirmed, whereas decreased SIRT1 expression was observed in the lung tissues.
Conclusions
These data indicate that lung SIRT1 expression decreased while serum SIRT1 increased in the setting of asthma. Serum SIRT1 levels correlate positively with both IgE levels and negatively with pulmonary function, suggesting that increased peripheral SIRT1 levels represent a new biological characteristic of asthma. Increased serum SIRT1 may be an auxiliary index for the diagnosis of asthma and elevating lung SIRT1 levels may be a new strategy for asthma therapy.
Abbreviations
-
- AHR
-
- airway hyperresponsiveness
-
- BALF
-
- bronchoalveolar lavage fluid
-
- COPD
-
- chronic obstructive pulmonary diseases
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- FEV1
-
- forced expiratory volume in one second
-
- FVC
-
- forced vital capacity
-
- IgE
-
- immunoglobulin E
-
- IL-13
-
- interleukin 13
-
- IL-4
-
- interleukin 4
-
- IL-5
-
- interleukin 5
-
- IL-6
-
- interleukin 6
-
- OVA
-
- ovalbumin
-
- PCR
-
- polymerase chain reaction
-
- SIRT1
-
- silent information regulator 1
Introduction
Asthma is a chronic inflammatory disorder that affects 300 million people worldwide.1 Exacerbations, poor symptom control and decreased quality of life remain significant problems in spite of the progress made in asthma pharmacotherapy.
SIRT1, also known as sirtuin 1, belongs to the class III histone deacetylase family. Seven SIRT family members (SIRT1–SIRT7) are found in mammals; however, only SIRT1 is reportedly associated with asthma.2 All SIRT members have conserved sequences that are expressed in organisms ranging from bacteria to humans.3 SIRT1 deacetylates histones and a variety of non-histone proteins,4-6 and therefore plays a role in several key pathways such as energy metabolism, cell differentiation, cell aging, tumour suppression and anti-inflammation.7, 8 Recently, several studies suggest that SIRT1 protein is involved in the pathogenesis of asthma. For example, resveratrol, a natural SIRT1 activator, was found to suppress allergic-induced airway hyperresponsiveness (AHR), eosinophilia and mucus hypersecretion in asthmatic mice.9, 10 Similarly, SRT1720, a synthetic SIRT1 activator, has also been shown to suppress both inflammatory cell infiltration and cytokine production in ovalbumin (OVA)-induced asthmatic mice.11
Although a number of studies have suggested that SIRT1 may play a role in inflammation in animal models,12, 13 SIRT1 protein expression has not been investigated in the setting of human asthma. We hypothesized that SIRT1 expression levels vary in patients with asthma. To test this hypothesis, we quantified serum SIRT1 in patients with asthma patients and in non-allergic control subjects, and analysed the relationships between serum SIRT1 levels and other clinical parameters such as total immunoglobulin E (IgE) and pulmonary function. To confirm the SIRT1 expression profiles in both the serum and the lung tissues, we produced a mouse model of asthma via OVA immunization and detected SIRT1 expression in both the serum and the lung tissues of asthmatic mice.
Methods
Study design and clinical subjects
Ninety-seven patients with asthma, who were referred to the Affiliated Hospital of Guangdong Medical College (Zhanjiang, China) from January to December 2013, were included in our study. The diagnosis of asthma was based on the Global Initiative for Asthma guidelines.14 The exclusion criteria included the following: oral corticosteroid use or the occurrence of a respiratory tract infection within 4 weeks of enrolment in the study, as well as any chronic cardiopulmonary disease other than asthma (including chronic obstructive pulmonary disease (COPD)), or pregnancy. A total of 161 healthy volunteers were recruited; 118 age-matched and sex-matched subjects among them were included in the study's control group (Table 1).
| Characteristics | Asthma (n = 97) | Controls (n = 118) | Healthy participants (n = 161) | P-value |
|---|---|---|---|---|
| Age (year) | 43.78 ± 0.96 | 41.36 ± 0.84 | 14∼60 | NS |
| Serum IgE (IU/mL) | 500.3 ± 77.27 | 140.5 ± 24.44 | <0.0001 | |
| Eosinophils in WBC (%) | 4.5 ± 0.35 | 2.3 ± 0.17 | <0.0001 | |
| FEV1 (% of predicted) | 71.58 ± 2.32 | 92.24 ± 1.23 | <0.0001 | |
| FEV1/FVC (%) | 70.09 ± 1.54 | 92.03 ± 1.06 | <0.0001 | |
| Sex (male/female) | 43/54 | 54/64 | 78/85 | NS |
| Smoking status, n (%) | NS | |||
| Current | 20 (20.62) | 34 (28.81) | 35 (21.74) | |
| Former | 1 (0.01) | 5 (4.24) | 5 (3.11) | |
| Never | 76 (78.36) | 79 (66.95) | 121 (75.16) |
- Data are presented either as the means ± SEMs or as n (%).
- FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SEM, standard error of the means.
This study was approved by the ethics committee of our institution (Affiliated Hospital of Guangdong Medical College, Zhanjiang, China), and written informed consent was provided by each of the participants.
Pulmonary function tests
Spirometry was measured using standard spirometric techniques (POS Instrumentation, Inc., Louisville, USA) at least 6 h after each patient's most recent treatment with albuterol, according to the American Thoracic Society guidelines.14
Measurement of serum SIRT1 and total IgE levels
Human: Venous blood samples were obtained, and serum samples were subsequently prepared. The serum SIRT1 concentrations were measured using a SIRT1 enzyme-linked immunosorbent assay (ELISA) kit (USCN Life Science Inc. Wuhan, China) specific for SIRT1 of human or mouse according to the manufacturer's instructions. The minimum detection limit of the SIRT1 assay was 0.055 ng/mL. The total serum IgE levels and the percentages of peripheral blood eosinophils were each determined using a routine blood test in each patient and control subject.
Mice: 0.5 mL blood was collected from the abdomen of each mouse, and serum samples were prepared. Serum SIRT1 was measured using an ELISA kit as described above.
Mice sensitization and the airway challenge
All animal experimental processed in this study were approved by the Institutional Animal Care and Use Committee of the Guangdong Medical School. Twelve 8-week-old female BALB/c mice (Vital River, Peking, China) were divided into an experimental group and a control group (n = 6 each).11 Mice were sensitized and challenged as reported.15 All mice were sacrificed at day 28, and the appropriate samples were subsequently obtained.
Bronchoalveolar lavage fluid collection, cell counting and cytokines measurement
Forty-eight hours following the last challenge, the mice were sacrificed with 50 mg/kg of pentobarbital (Sigma P3761). Tracheotomies were performed, and 0.5 mL of pre-chilled phosphate buffer saline was instilled into the lungs; bronchoalveolar lavage fluid (BALF) was subsequently collected. The BALF was centrifuged, and the pellets were re-suspended in Hanks. To evaluate the differential cell count in BALF, we prepared BALF cells by cytospin. To determine the cell differentials, the smears were stained with a Diff-Quik solution (Propbs Scientific Ltd. China). Cells were counted under a microscope. Cells of four random sights were counted in each sample. The mean of the values from the four random sights was used for each cell count. The BALF supernatants were also collected; the levels of the cytokines interleukin 4 (IL-4), IL-5 and IL-13 in supernatants were measured via ELISA.
Lung tissue histopathology
Following BALF collection, the right lungs were removed and fixed in 4% formalin, processed and embedded in paraffin, and cut into three microns thick sections. The treated sections were stained with SIRT1 antibodies (Rabbit anti-mouse; 2028s, Cell Signaling Technology; 1:100 diluted) by streptavidin-peroxidase technique, and the nuclei were stained by hematoxylin.
Ribonucleic acid isolation and real-time reverse-transcriptase polymerase chain reaction analysis
The mouse left lungs were removed and washed with saline. The tissues were lysed using TRIzol reagent (Invitrogen); the total ribonucleic acid was purified according to the manufacturer's instructions. The primer sequences for SIRT1 were as follows: forward, 5′-TTCCAGCCGTCTCTGTGTCA-3′ and reverse, 5′-ATTCCTGCAACCTGCTCCAA-3′.11 The primer sequences for GAPDH were as follows: forward, 5′-TGAAGCAGGCATCTGAGGG-3′ and reverse, 5′-CGAAGGTGGAAGAGTGGGAG-3′.16
Statistical analysis
The Kolmogorov–Smirnov test was performed to determine the normality of distribution. Comparisons between two visits were analysed with the paired t-test. The interrelationships between different parameters were determined using the Pearson correlation. Data were presented either as the means ± standard error of the means or as n (%), unless otherwise stated. The analyses and graphs were completed using GraphPad Prism 6.0 software (http://www.graphpad.com/scientific-software/prism/). Statistical significance was set at a P-value < 0.05.
Results
Patient characteristics
The clinical features and laboratory findings of the patients enrolled in this study are summarized in Table 1. The mean age of the patients with asthma was 43.78 years. There was no significant difference in gender composition between the patients with asthma and the controls or healthy participants. In regard to factors associated with asthma, there were significant differences between asthmatic patients and control subjects; for example, the asthmatic patients exhibited increased total serum IgE levels and percentages of peripheral blood eosinophils (500.3 ± 77.27 IU/mL and 4.586 ± 0.35%, respectively, P < 0.0001). The forced expiratory volume in 1 s (FEV1) and FEV1/forced vital capacity values of patients with asthma patients were also lower than that of control subjects (both P < 0.0001). There were no significant differences in smoking habits between the patients with asthma and the control subjects.
Serum SIRT1 in both the healthy individuals and the asthmatic patients
Among the 22 healthy teenagers we examined, the serum levels of SIRT1 were significantly higher than those of the adults. Among the 20∼60-year-old adults, there were no significant differences in the serum SIRT1 levels between different age groups (Fig. 1). Additionally, the serum SIRT1 levels were significantly higher in the patients with asthma patients than in the control subjects (2.819 ± 0.158 ng/mL vs 1.026 ± 0.022 ng/mL, respectively, P < 0.0001) (Fig. 2a). In the patients with asthma, the serum SIRT1 levels were not affected by smoking (Fig. 2b).
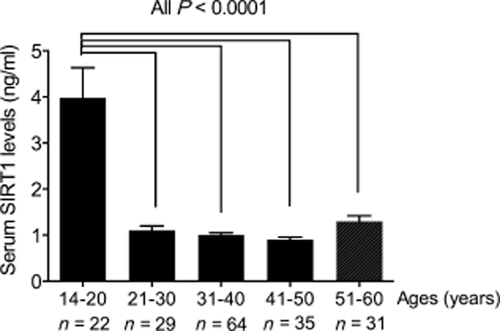
Silent information regulator 1 (SIRT1) levels in healthy subjects of different ages. A bar diagram showing the differences in serum SIRT1 concentrations among subjects of different ages. Serum SIRT1 concentrations in teenagers are significantly higher than those in other age groups (3.98 ± 0.14 ng/mL vs 1.11 ± 0.02 ng/mL (21∼30 group), 1.01 ± 0.01 ng/mL (31∼40 group), 0.93 ± 0.01 ng/mL (41∼50 group), 1.30 ± 0.02 ng/mL (51∼60 group), respectively; All P < 0.0001).
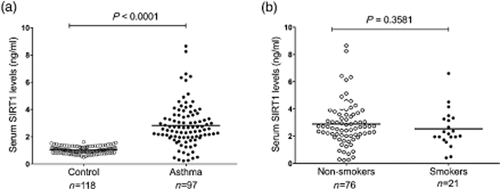
The silent information regulator 1 (SIRT1) levels in the serum of the patients with asthma and the healthy controls. (a) Serum SIRT1 concentration analysed using enzyme-linked immunosorbent assay. The mean serum SIRT1 levels were higher in the patients with asthma than in the normal controls (P < 0.0001); (b) In the patients with asthma, the serum SIRT1 concentrations were not significantly different between the smokers and the non-smokers (P = 0.3581). ○ Non-smokers; ● Smokers.
The correlation between serum SIRT1 and other clinical parameters
The total serum IgE levels and the predicted pulmonary function parameter of FEV1/FVC%, two characteristics closely associated with asthma,17-19 were measured. In the patients with asthma, the Spearman's rank correlation analysis demonstrated that the serum SIRT1 levels correlated positively with the total IgE levels (r = 0.267, P = 0.008) (Fig. 3a; Table 2). The SIRT1 levels were also correlated negatively with pulmonary function (r = −0.29, P = 0.004) (Fig. 3b; Table 2). However, we did not observe a significant correlation between serum SIRT1 levels and other clinical parameters such as the levels of peripheral blood eosinophilia, gender or smoking habits (Table 2).
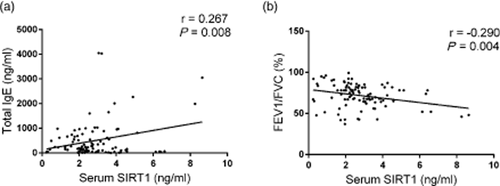
The correlation between the serum silent information regulator 1 (SIRT1) levels and the clinical markers in 97 patients with asthma. (a) The Spearman's rank correlation analysis demonstrated that the serum SIRT1 levels correlated positively with total serum immunoglobulin (Ig) E levels (r = 0.267, P = 0.008); (b) Serum SIRT1 levels correlated negatively with the ratio of FEV1/FVC (r = −0.29, P = 0.004).
| Characteristics | Serum SIRT1 | |
|---|---|---|
| r | P-value | |
| Serum IgE (IU/mL) | 0.267 | 0.008* |
| Blood eosinophils (%) | 0.022 | 0.831 |
| FEV1/FVC (%) | −0.29 | 0.004* |
| Sex | 0.024 | 0.815 |
| Smoking status | −0.09 | 0.358 |
- *P < 0.05.
- FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Cytokines expression and SIRT1 distribution in the OVA-sensitized and challenged mice
In the BALF of the OVA-sensitized and challenged mice, the numbers of eosinophils and lymphocytes increased; the expression levels of IL-4, IL-5 and IL-13 also increased significantly compared with those of the control group (Fig. 4a,b). We found that SIRT1 levels also increased in the serum of the OVA-sensitized and challenged mice (6.112 ± 0.751 ng/mL vs 2.459 ± 0.221 ng/mL, respectively, P < 0.01) (Fig. 4c), which was consistent with our findings in the human samples. However, both the immunohistochemistry and quantitative polymerase chain reaction (PCR) results demonstrated a decrease in the expression of SIRT1 in the lung tissues of the OVA-sensitized and challenged mice compared with those of the control group (Fig. 5).
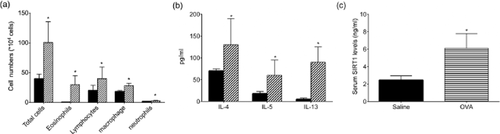
The characteristics of the ovalbumin (OVA)-sensitized and challenged mice model. (a) Cell counting indicated that the total numbers of cells, eosinophils and lymphocytes in the bronchoalveolar lavage fluid (BALF) were significantly higher in the OVA-sensitized and challenged mice than in the control subjects (n = 6 each); (b) The enzyme-linked immunosorbent assay (ELISA) results showed that interleukin (IL)-4, IL-5 and IL-13 increased in the BALF of the OVA-sensitized and challenged mice (n = 6 each group); (c) The ELISA results suggested that serum SIRT1 increased in the OVA-sensitized and challenged mice compared with those of the saline controls (n = 6 each). *P < 0.05. ■ Saline;  OVA.
OVA.
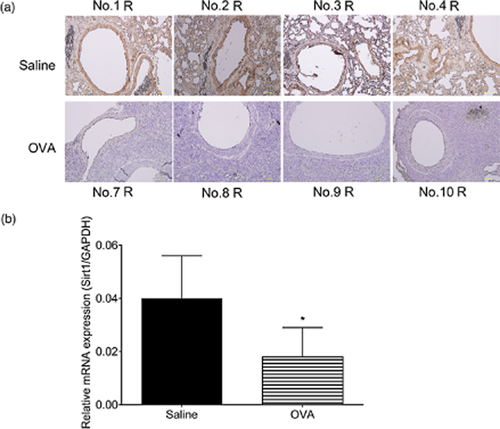
Silent information regulator 1 (SIRT1) expression in the lungs of the ovalbumin (OVA)-sensitized and challenged mice. (a) The immunohistochemistry results indicated that eosinophils, lymphocytes, macrophage, neutrophils infiltration and SIRT1 expression decreased in the OVA-sensitized and challenged mice (down) compared with that in the saline controls (up). Animal numbers shown in the picture above or below, ‘R’ stands for specimen origin from the right lung; (b) The real time polymerase chain reaction (PCR) results indicated that SIRT1 expression was lower in the OVA-sensitized and challenged mice than in the saline controls. *P < 0.05.
Discussion
To the best of our knowledge, the present study was the first to demonstrate that peripheral SIRT1 levels increased in both patients with asthma and mice, and provided direct evidence linking SIRT1 to asthma. Additionally, our data demonstrated that serum SIRT1 levels remained elevated in healthy adolescents but decreased significantly in adults. However, the serum SIRT1 levels were much higher among patients with asthma than among healthy control subjects of the same age group. To identify the source of the SIRT1 in the peripheral circulation of patients with asthma, we established a murine model of asthma and observed that although SIRT1 increased in the serum, its expression level in the lungs decreased.
SIRT1 is associated with aging and inflammation
Aging is an important contributing factor to the pathogenesis of many chronic inflammatory diseases.20, 21 In this study, serum SIRT1 levels remained elevated among healthy adolescents but decreased significantly among adults. We have not collected serum from individuals older than 60; however, in a recent study, Rahul Kumar et al. tested serum SIRT1 levels in young adults among post-graduate students and subjects between 65 and 75 years of age; they found that the serum SIRT1 levels of the elderly subjects were much lower than those of the younger subjects.22 SIRT1 is an anti-aging factor and has been proven to delay aging.23 SIRT1 expression decreases with increasing age; therefore, the protective effect it exerts on the tissue also decreases. Asthma is common among all age groups and has emerged as a significant problem for elderly people. Morphologic and physiologic abnormalities have been observed in aging human lungs, including decreased mucociliary function, air space dilatation, elastic recoil loss and elastin fibre loss, diminished diffusing capacity and evidence of low-grade inflammation of the respiratory tract.24 These changes may superimpose their effects upon those induced by asthma-related chronic inflammation of the airways, making aging a harmful factor in the evolution of airway inflammation.
SIRT1 is an effective deacetylase that contributes to the maintenance of normal lung function. SIRT1 was recently found to inhibit pro-inflammatory mediator release via Nuclear Factor Kappa-light-chain-enhancer of activated B cells in the setting of sustained inflammation in aging lungs.25 Transgenic mice with modest SIRT1 over expression that were receiving a high-fat diet demonstrated reduced levels of pro-inflammatory cytokines such as IL-6 and tumour necrosis factor-alpha.12 Conversely, myeloid cell-specific SIRT1 knockout mice demonstrated increased cytokine section when challenged with lipopolysaccharide.13 The SIRT1 activator, resveratrol, which exerts potent anti-inflammatory effects in cell culture (A549 lung epithelial cells),26 was also found to suppress asthmatic reactions;9 another SIRT1 activator, SRT1720, was found to exert similar effects in an OVA-induced asthmatic mouse model.11 These results suggest that SIRT1 may exert both protective effects and potent anti-inflammatory effects in mouse models of allergic airway diseases.
Serum SIRT1 correlated with IgE levels and pulmonary function and may serve as a diagnostic marker and therapeutic target for asthma
In our study, serum SIRT1 levels correlated positively with serum IgE levels and negatively with pulmonary function. The interaction between IgE and antigens causes a series of immunological reactions in asthma. Cross-linked IgE binds to mast cells via the high-affinity IgE receptor and triggers the subsequent transcription of cytokines, the synthesis of prostaglandins and leukotrienes and the release of preformed vasoactive mediators. These mediators rapidly induce mucosal oedema, mucous production and smooth muscle constriction, and result in the production of asthmatic inflammatory infiltrates and in bronchial hyperresponsiveness.27 Serum IgE levels increase in patients with severe asthma compared with those in patients with non-severely asthma, independent of the levels of allergic sensitization, which is considered an important asthma biomarker.28, 29 Additionally, a recent study suggested that the intake of resveratrol was associated with higher FVC levels;30 the authors of this study explained the mechanism by hypothesizing that resveratrol mediated SIRT1 activation. SIRT1 is helpful in sustaining normal lung function. When SIRT1 expression decreased, the ability of the lungs to resist damage reduced. High-serum SIRT1 may have an adverse effect on pulmonary function in the setting of asthma. Serum SIRT1 correlated with both IgE levels and pulmonary function, suggesting that serum SIRT1 possibly correlate with asthma severity.
SIRT1 decreasing in lungs aggravates the inflammation of asthma
In the present study, we demonstrated that serum SIRT1 levels increased in patients with asthma patients. In the OVA-sensitized and challenged mice, SIRT1 increased in the serum but decreased in the lung tissues coupled with eosinophils, lymphocytes, macrophage and neutrophils infiltration. We speculate that the high-serum SIRT1 levels were due to airway inflammation and the subsequent release of SIRT1 from inflammation cells. Ichikawa et al. reported that SIRT1 decreased in the lung homogenates of asthmatic mice,11 which is consistent with our findings. SIRT1 is a multifunctional molecule and is involved in a variety of pathways. The loss of SIRT1 may lead to multiple molecular pathway disorders and weaken the anti-inflammatory functions of various types of cells. A recent study demonstrated that lung SIRT1 levels are decreased in other lung inflammatory diseases such as COPD and emphysema.31, 32 As an anti-aging and anti-inflammatory factor, lung SIRT1 decreases may be responsible for the uncontrolled airway inflammation of asthma.
In summary, our findings indicated that serum SIRT1 varies with age. The change may be the result of changes in protein expression or the leakage of SIRT1 from the lung tissues. The anti-aging and anti-inflammatory roles of SIRT1 have been proven in other studies; decreased SIRT1 expression may result in adverse events in asthmatic lungs. Perspective studies will be necessary to determine whether the serum levels of SIRT1 decline in patients with asthma following an appropriate treatment. Although the exact function of SIRT1 in the setting of asthma remains unclear, our study indicates that high levels of serum SIRT1 are a biological characteristic of asthma; modulating SIRT1 with activators may be an attractive therapeutic strategy in the treatment of asthma.
Acknowledgements
We thank Dr Huahao Shen (Second Affiliated Hospital, Institute of Respiratory Disease, Zhejiang University School of Medicine, Department of Respiratory and Critical Care Medicine) for support in the conceptual discussions related to this project. This project was supported by grants from the Natural Science Foundation of China (No. 81400023) and the Research Funds for the Doctoral Program of Guangdong Medical College (No. XB1329 and No. XB1326).