Lung function following very preterm birth in the era of ‘new’ bronchopulmonary dysplasia
Abstract
One of the most significant complications of preterm birth is bronchopulmonary dysplasia (BPD). The pathophysiology of BPD has changed in recent years as advances in neonatal care have led to increased survival of smaller, more preterm, infants who display alterations to alveolar and pulmonary microvascular development. It is becoming clear that infants with ‘new’ BPD experience lung disease that persists into later childhood, however, the oldest of these children are just now entering young adulthood and therefore the longer term pulmonary implications remain unknown. The role of lung function testing in the identification and subsequent management of patients with lung disease resulting from a neonatal classification of BPD is reviewed based on the underlying pathophysiology of the disease.
Abbreviations
-
- BPD
-
- bronchopulmonary dysplasia
-
- DLCO
-
- diffusing capacity of the lung for carbon monoxide
-
- FEF
-
- forced expiratory flow
-
- FEV1
-
- forced expiratory volume in 1 s
-
- FRC
-
- functional residual capacity
-
- FVC
-
- forced vital capacity
-
- LCI
-
- lung clearance index
-
- NO
-
- nitric oxide
-
- PMA
-
- post-menstrual age
-
- TLC
-
- total lung capacity
-
- RV
-
- residual volume
Introduction
Rates of preterm birth have increased in almost all countries over the past 20 years,1 such that approximately 15 million (11.1%) of the world's babies were delivered preterm (before 37 completed weeks of gestation) in 2010.2 Complications of preterm birth are the leading cause of child death in most high and middle-income countries3 and those who go on to survive often face a lifetime of ongoing health problems. One of the most significant complications of preterm birth and the most common form of chronic lung disease in infancy is bronchopulmonary dysplasia (BPD).4 Preterm infants generally have a significant burden of respiratory disease in the first years of life which is accentuated by lower gestational age and the coexistence of BPD,5 with increased respiratory symptoms, health-care utilization and hospital admissions consistently reported.6-8
Definition and risk factors for BPD
Many potential risk factors have been identified for the development of BPD (see Fig. 1) including lower gestational age,9 low birth weight for gestational age,10 white race,11 male gender,12 genetic factors13 and other common sequelae during the neonatal period such as intraventricular haemorrhage, necrotizing enterocolitis, patent ductus arteriosis or sepsis.14-16
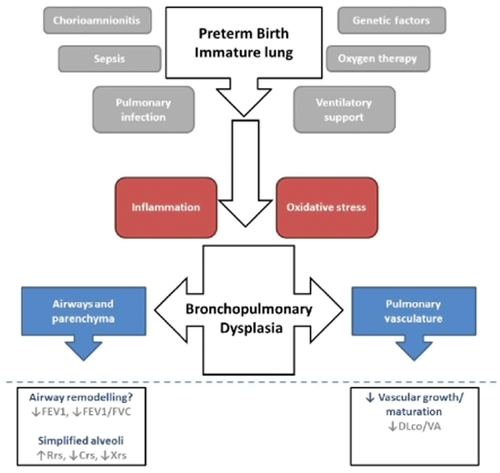
Pathophysiology of ‘new’ bronchopulmonary dysplasia (BPD). The immature lung is subjected to many insults in the neonatal period following preterm birth. Some of these insults are known to be associated with a subsequent diagnosis of BPD (grey). While the mechanisms are not entirely understood, inflammation and oxidative stress have previously been implicated in the process. Infants receiving a diagnosis of bronchopulmonary dysplasia show altered lung development (blue), the severity of which may be reflected in their lung function outcomes.
The current consensus definition determines BPD at 36 weeks post-menstrual age (PMA) in infants born less than 32 weeks gestation who required supplementary oxygen for at least 28 days. Further sub-categories are defined as mild/moderate/severe based on the amount of supplemental oxygen and method of respiratory support at 36 weeks PMA.17 The definition of BPD based on oxygen requirement has some limitations and results in a heterogeneous disease phenotype since oxygen requirement could be reflective of obstructive or central apnoea rather than gas exchange impairment alone. This is further compounded by the levels of oxygenation that are considered acceptable by different clinicians in different institutions.
Disease pathophysiology
The pathophysiology of BPD has considerably changed since it was first described as a disease characterized by severe respiratory failure following ventilation with high pressure and oxygen concentrations during infancy by Northway.18 Advances in neonatal care such as increasing use of antenatal maternal corticosteroids, widespread use of exogenous surfactant therapy and the development of less aggressive ventilation strategies has led to increased survival of smaller infants of lower gestational age such that half of live born babies under 25 weeks now survive in high-income countries.2
As a result of the earlier interruption to normal lung development and the subsequent lung injury arising from life-saving measures in the critically ill preterm neonate, the contemporary disease is characterized by disruption of the alveolar and microvascular development of the peripheral lung.19 The predominant histological findings from autopsy and lung biopsies in infants with ‘new’ BPD are a decreased number of alveoli, which are larger and more simplified in structure, as well as blunted pulmonary microvascular growth.20 Animal models of preterm birth also display fewer and larger alveoli21 and altered deposition of elastic fibres.22 In addition, animal models suggest alterations in alveolar epithelial cells, particularly thickened cytoplasmic extensions of type–I cells leading to thicker air-blood barriers for gas exchange,23, 24 and the presence of pulmonary inflammation,25 though it is unclear whether preterm birth or factors associated with the life-saving interventions are associated with such observations.
It is becoming clear that children with new BPD experience lung disease that persists into later childhood; however, it remains unknown whether these modern survivors of preterm birth experience accelerated lung function decline and what the long-term implications of lower ‘peak’ lung function is as the preterm individual ages and consequently undergoes the natural loss of lung function. This review will focus on respiratory physiology testing for the identification and subsequent management of patients with lung disease resulting from a neonatal diagnosis of BPD in the modern era, with reference to the underlying pathophysiology of the disease.
Measurement of forced flows and volumes
Measurements of forced flows and volumes have been performed from infancy through to children, adolescents and adults born preterm. In general, the results suggest that older children and adolescents born very preterm (<32 completed weeks gestation) or extremely preterm (<25 completed weeks gestation) have reduced spirometric outcomes associated with an obstructive pattern (reduced forced expiratory volume in 1 s (FEV1) and forced expiratory flow (FEF25-75) with approximately normal forced vital capacity (FVC)) that tends to be further reduced in individuals with new BPD.26-29 Interestingly, this pattern of obstructive lung function is not limited to individuals with new ‘BPD’ with studies in classical BPD reporting similar reductions in spirometry.30, 31 Data from spirometry in preschool-aged children is rare, and the clinical utility of spirometry in this age group has been questioned.32 It is suggested that the observed reductions in spirometric outcomes are the expression of an early airway remodelling processes that persist into childhood.
Assessments of forced flows and volumes in infancy are generally achieved using the raised volume rapid thoracic compression technique during sedation. Studies using this approach have reported reduced forced flows and FEV in the presence of normal FVC which did not normalize later in infancy in groups of both preterm infants without BPD33 and infants with moderate to severe BPD.34 Longitudinal data from infancy to later in life is scarce. A strong correlation between maximum flow rate at FRC (V'maxFRC) at 2 years of age and FEV1 at mid-childhood in a small group of surfactant treated children suggests persistent airflow limitation in infants with BPD.35
Airflow obstruction appears to be, at least in part, reversible with approximately one third of infants36, 37 and 20–57% of preterm children (8–11 years) showing significant responsiveness to bronchodilators, regardless of neonatal diagnosis of BPD.27, 38, 39
Considered together, these studies suggest that spirometric outcomes are sensitive to altered respiratory function in individuals born preterm and that abnormalities worsen with increasing severity of neonatal lung disease. The clinical role of bronchodilator testing in this population is not clear, but may convey additional information not evident from baseline lung function testing alone.
Measurement of respiratory system mechanics
Normal alveolarization, defined by septation originating along elastin fibres, commences after approximately 28 weeks of gestation40, 41 and proceeds rapidly such that 20–50% of the full complement of alveoli is present at term. Delivery prior to term, and therefore incomplete deposition of the parenchymal elastic network, results in less effective airway tethering and subsequent decreased airway stability, increased tendency to closure, increased airway resistance and ultimately a tendency to collapse alveolar units in the lung periphery.42, 43 As such, low compliance of the respiratory system has been consistently reported in children during the first 6 months of life using dynamic measures of compliance during tidal breathing, with outcomes appearing to normalize by 2 years of age44, 45 suggesting that increased alveolarization may take place in the first years of life. However, respiratory system compliance appears to remain low in those infants who continue to report respiratory symptoms.45 Measures of pulmonary mechanics during the first week of life in very preterm babies have not been deemed helpful in the prediction of disease severity at 28 days; however, infants with severe BPD do have lower lung compliance and increased pulmonary resistance compared with those with mild BPD at 28 days of age.46
Given the structural changes of the lung parenchyma described by histological samples in BPD and the relative ease of techniques such as the forced oscillation technique, it is perhaps surprising that very few studies have assessed respiratory mechanics in older children. From the limited studies available, we can conclude that increased respiratory system resistance and decreased respiratory system reactance (a measure of peripheral lung elasticity) is evident in 3 to 8-year olds who were born preterm, with increased abnormalities in those who received a BPD diagnosis during infancy.28, 47, 48 Further studies to examine the changes in respiratory system mechanics, with particular focus on the peripheral lung as employed by the oscillatory techniques may be particularly beneficial at understanding lung development following preterm birth.
Measurement of lung volumes and ventilation homogeneity
Morphological changes in the lungs of infants and children with new BPD, such as larger airspaces, intuitively highlight the possibility of functional consequences on lung volumes and ventilation homogeneity. However, data examining these outcomes in infants born preterm remain controversial. Several studies of prematurely born infants with new BPD show that they have lower end-expiratory lung volumes (FRC) and increased ventilation inhomogeneity using inert gas washout techniques.49-51 In contrast, others conclude no clear trend in changes of FRC/kg or measures of ventilation homogeneity such as the lung clearance index (LCI) in preterm infants, regardless of BPD severity, compared to healthy controls.52-54 Numerous factors likely contribute to these different findings including the inert gas used for the washout (oxygen, helium or sulphur hexafluoride), whether the infants are sedated and the breathing pattern (particularly dead space/tidal volume).55 Thus, further studies are required to properly elucidate the utility of inert gas washout in infants with BPD.
To our knowledge, the EPICure study is the only study to report LCI values outside of infancy. This group of extremely preterm (<25 weeks gestation) children had mildly reduced gas mixing efficiency (elevated LCI), though the large extent of overlap with the control population should be noted indicating that LCI may not be the best indicator of lung disease in this population.56
Lung volumes have also been measured by plethysmography in this population, which may prove more insightful given the potential to measure trapped gas in addition to the lung volume components that are communicable via the airway opening. Such measurements have demonstrated that, although total lung capacity (TLC) is in the normal range, residual volume (RV) and RV/TLC are elevated by 1 year of age in infants with BPD.37 These findings tracked into the second year of life34 and beyond, with the majority of studies in school-aged children with new BPD also reporting normal TLC and increased RV,47, 56, 57 which was more abnormal with increasing neonatal disease severity. Such findings align with the observations from spirometry and measures of respiratory system mechanics. Together, they suggest that obstructive airway disease with air trapping occurs within the first year of life and persists in children with new BPD.
Measurement of pulmonary gas exchange
Inefficient gas exchange during the neonatal period is a common consequence of very preterm delivery, with functional gas exchange dependent on sufficient development of the alveolar-capillary membrane at birth. The distal lung undergoes substantial increases (approximately 800%) in alveolar-capillary membrane per unit surface area from 22 to 32 weeks of gestation,58 and therefore infants born prior to 32 weeks gestation are at particular risk of inefficient gas exchange during the neonatal period. It also stands to reason that the clinical evaluation of pulmonary gas exchange may be a useful measure to evaluate the long-term consequences of impaired alveolar septal and vascular development associated with preterm birth.
The diffusing capacity of the lung for carbon monoxide (DLco) is the most commonly applied measure of alveolar-capillary gas transfer in this population and has generally shown lower diffusing capacity in children and adolescents who were born very preterm. The DLCO is often expressed as the carbon monoxide diffusing capacity per unit of alveolar volume (DLCO/VA or KCO) to more directly reflect the quality of gas transfer across the alveolar-capillary membrane, though it may be important to report both when examining the clinical aetiology of altered diffusing capacity in this group.
To date, only one group have developed a technique to sufficiently measure DLCO in infants.59 Thirty-nine infants with new BPD (mean gestational age ∼26 weeks) were compared to term matched healthy controls at approximately 11 months of age and demonstrated lower DLCO and DLCO/VA, despite an unchanged VA.60 These findings are corroborated in school-aged children, with several studies reporting reduced DLCO and DLCO/VA at 7–11 years in those born extremely preterm with a neonatal diagnosis of BPD in the surfactant era.56, 57, 61, 62 Such findings suggest that infants and children with BPD have a reduced membrane surface area available for gas exchange (DM), alveolar capillary volume (ƟCO.VC) or both, which supports the notion that the histopathological findings of ‘new’ BPD during infancy persist into later childhood.
The partitioning of the two components of CO transfer, expressed as the equation 1/DLCO = 1/DMCO + 1/(ƟCO.VC), may provide some further insights into the mechanisms behind reduced DLCO in children born preterm. Such partitioning would allow estimation of the diffusing capacity of the alveolar-capillary membrane for CO (DMCO), which is affected by the total alveolar surface area and the thickness of the alveolar-capillary membrane, in addition to the pulmonary capillary blood volume (Vc) using duplicate measurements of the DLCO with high and low oxygen concentrations.63 While theoretically promising, this test is yet to become a standard tool in clinical practice, largely due to methodological issues associated with the inaccuracies of estimating ƟCO and the two separate breath holding periods with high and low oxygen which introduce a number of potential problems.64 These problems may be negated by the use of nitric oxide (NO) since the diffusing capacity of the lung for NO (DLNO) is much less influenced by changes in the Vc and therefore more accurately reflects the properties of the alveolar-capillary membrane than the DLCO.65
Increasing length of oxygen exposure, but not gestational age or birth weight, has been shown to correlate with reductions in DLCO/VA,62 suggesting that neonatal factors may contribute to later gas exchange function rather than preterm birth per se. However, it must be considered that lower gestational age is generally associated with increased exposure to supplemental oxygen.
Conclusions
Premature birth interrupts normal in utero lung development and results in an early transition to a relatively hyperoxic atmospheric environment, along with many other stressors, that play a role in subsequent lung dysfunction (see Fig. 1 for summary). Our current knowledge indicates that alterations in alveolar and pulmonary vascular development are the prominent pathophysiology and as such, a combination of lung function tests to assess these particular aspects of respiratory function provides the most insight when monitoring the long-term sequelae of preterm delivery.
The significance of reduced airway function early in life cannot be understated since lung function in infancy is suggested to track through life.66 It is therefore possible that many children born preterm will be at increased risk of developing chronic obstructive pulmonary disease in adulthood and will benefit from regular assessment of lung function through life.




