Effects of different concentrations of zinc oxide nanoparticles on the quality of ram cauda epididymal spermatozoa during storage at 4°C
Abstract
Zinc oxide nanoparticles (ZnO-NPs) are known for their antioxidant effects. In this study, ZnO-NPs were synthesized using aqueous extract of Maclura pomifera fruit; then, their effects on the quality of ram cauda epididymal spermatozoa were evaluated during storage at 4°C. ZnO-NPs formation was characterized by ultraviolet–visible spectroscopy (UV–vis), energy dispersive spectroscopy (EDX), field emission scanning electron microscopes (FE-SEM) and Fourier transform infrared (FTIR) spectroscopy. Cytotoxicity responses were investigated by 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT). Cauda epididymal spermatozoa were obtained from testicles collected from abattoir(s). The sperm samples were pooled. The samples were diluted by extender and supplemented with different concentrations of ZnO-NPs (0, 1, 5 and 25 µg/ml) and cooled to 4°C for 72 hr. Total motility, viability, DNA and membrane integrity, morphological abnormality, malondialdehyde (MDA) and antioxidant activities: superoxide dismutase (SOD) and total antioxidant capacity (TAC) of cooled diluted samples were evaluated. Addition of 1 μg/ml of ZnO-NPs increased sperm viability under normal conditions (p < .05). Extender supplementation with 1 μg/ml of ZnO-NPs improved sperm total motility, viability, DNA and membrane integrity during storage periods (p < .05). Moreover, using 1 μg/ml of ZnO-NPs improved (p < .05) MDA, TAC and SOD activities after 72 hr compared to other treatments. In conclusion, there were some beneficial effects of ZnO-NPs supplementation in ram epididymal sperm extender during oxidative stress conditions and cooled storage.
1 INTRODUCTION
Nanotechnology is a multidisciplinary science concerning generating new materials at the nano-scale size, which can be integrated into different utilizations (Samak et al., 2020). Nanotechnology is an important branch in the major fields of physics, medicine, chemistry and biotechnology (Bhumi & Savithramma, 2014; Moradi et al., 2021a; Soltani & Darbemamieh, 2021b). Nanobiotechnology shows the merging of nanotechnology and biotechnology to develop green, synthetic and eco-friendly technology for the synthesis of nanomaterials (Buazar et al., 2016; Sobha et al., 2010). The nanoparticles synthesized through the green method are found to be non-toxic, cost-effective and biodegradable in nature (Darroudi et al., 2014). This eco-friendly synthesis method decreases the use of hazardous substances as the process utilizes natural materials including fruits, roots, flower and leaves extracts and microorganisms like bacteria, algae and fungi (Navale et al., 2015). The green synthesis of zinc oxide nanoparticles (ZnO-NPs) is very useful because the biomolecules available in the herbal extracts act as efficient capping agents thereby playing a vital and versatile role in nanoparticles synthesis (Ajitha et al., 2016). Recently, different nanoparticles are synthesized by green methods such as Se, Ag, ZnO, CuO and others that are integrated into different biological activities (Collenburg et al., 2017; Mohamed et al., 2020; Soltani & Darbemamieh, 2021a).
An important trace mineral as a cofactor in wide range of enzymes playing a significant role in cellular functions is zinc (Cortese et al., 2008; Prasad, 2009). In commercial and biomedical applications, zinc nanoparticles have gained more attention as antibacterial, anti-inflammatory, anticancer, anti-diabetic agents and zinc ions release agents (Jiang et al., 2018; Osmond & Mccall, 2010). The Zn-NPs also play an important role in DNA replication, DNA repair, cell division and protection against oxidative stress (Roy et al., 2013). Also, zinc is required for conception and embryo implantation, and it participates in sperm capacitation and acrosome reaction (Eggert-Kruse et al., 2002). Deficiency of zinc can impair spermatogenesis and reduce serum testosterone levels (Wong et al., 2002). Also, zinc helps stabilization of polymeric macromolecules including DNA, RNA and protein (Kotdawala et al., 2012).
Cold storage of semen is applied to decrease metabolism and maintain sperm viability over an extended period of time (Perumal et al., 2013). Semen quality during this extended storage deteriorated. One reason of this reduction is due to the action of the reactive oxygen species (ROS) produced by the cellular components of semen, such as superoxide anion radical (O2−) and hydrogen peroxide (H2O2) (Perumal et al., 2011a,b). The low toxicity and high bioavailability of nanoparticles makes them an effective means for preserving sperm (Orzołek et al., 2021).
Moreover, some researchers showed that Zn is a protecting antioxidant because of its capacity to bring malondialdehyde (MDA) levels to approach their normal levels (Dani & Dhawan, 2005; Malekirad et al., 2010). Also, Raajshreer and Durairaj (2017) stated that synthesized ZnO-NPs displayed antioxidant activity via scavenging of free radicals and chemical reduction activities. It has been showed that addition of ZnO-NPs to the semen extender in humans can reduce any detrimental effects via scavenging free radicals formed during the freeze–thawing process and by stabilizing sperm DNA (Prasad, 2009).
Since there is poor knowledge about the synthesis of green nanoparticles using extracts of Maclura pomifera fruit, the present study contributes to medical and pharmaceutical researches by synthesis of green Maclura pomifera nanoparticles with their specific properties. Also, there is little information investigating the effects of green synthesized ZnO-NPs as a pool of antioxidants on quality of liquid preserved epididymal spermatozoa of rams. Therefore, the aim of this study was to synthesize ZnO-NPs by employing eco-friendly approach in terms of use of aqueous extracts of Maclura pomifera fruit. In addition, the study was conducted to determine the effects of different concentrations of ZnO-NPs on fresh ram epididymal sperm under normal and oxidative stress conditions, as well as after cooled storage.
2 MATERIALS AND METHODS
2.1 Chemicals
Chemicals were obtained from Sigma–Aldrich chemicals if otherwise is indicated.
2.2 Preparation of the extract
To prepare extract, fruits of Maclura pomifera were thoroughly washed with tap water followed by distilled water to remove any contamination. The fruits were chopped into thin slices and dried in the shade for a week at room temperature. Then, the dried fruits were powdered and 20 g of powder was mixed with 200 ml deionized water and heated at 80°C using a heater-stirrer for 30 min. Then, the solution was centrifuged and the supernatant was separated and passed through Whatman's paper. Extract was stored for further uses.
2.3 Green synthesis of ZNO-NPS
As depicted in Figure 1, ZnO-NPs were prepared by a green synthesis approach via fruit extract of Maclura pomifera. ZnO-NPs using 0.1 M aqueous solution of zinc nitrate were prepared in 50 ml of distilled water under constant stirring at room temperature. The resultant aqueous Zn (NO3)2 solution was added drop-wise to the 50 ml of fruit extract at 90°C. Stirring continued for 7 hr. Then, the powder, which had a beige colour, was calcined in a muffle furnace (CWF, 1300, Carbolite, Derbyshire, UK) at 500°C for 2 hr. A white powder containing ZnO-NPs was obtained.

2.4 Characterization of ZNO-NPS
The synthesized nanoparticles were investigated by different techniques. The morphological and elemental analyses of ZnO-NPs were examined by field emission scanning electron microscopes (FE-SEM) coupled with energy-dispersive X-ray spectroscopy (EDX) (LMU TESCAN BRNO-Mira3). For FE-SEM study, dry powder samples were sprayed over the carbon tape and coated with gold. For the ultraviolet–visible spectroscopy (UV–vis) analysis, 1 ml of the suspension was collected from the purified sample at the end of the reaction and was sonicated at 1790 g for 15 min. The UV–Vis spectrum was recorded in the wavelength of 200–800 nm. The Fourier transform infrared (FTIR) spectra were recorded in the range of 4000–400 cm−1 (FTIR Bruker Alpha). The zeta-potential was investigated using electrophoretic mobility of nanoparticles in U-type tube at 25°C using a zetasizer (Horiba- SZ-100-Z model). Additionally, SEM images were imported into ImageJ® 1.48v software (National Institute of Health, USA) to study the particles size.
2.5 Experimental design
Experiment 1 was conducted to determine whether biologically synthesized ZnO-NPs (0, 1, 5 and 25 μM) could protect fresh ram spermatozoa collected from epididymides against H2O2 induced oxidative stress. Viability (by 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay) was measured on fresh ram epididymal spermatozoa after 2 hr of incubation at 37°C.
Experiment 2 was designed to evaluate whether biologically synthesized ZnO-NPs could improve the quality of ram spermatozoa collected from epididymides during liquid preservation. During liquid preservation at 4°C for 3 days, sperm motility, viability, morphological abnormality, membrane and DNA integrity were determined in the 0, 1, 5 and 25 μM of ZnO-NPs treatment groups. Also, superoxide dismutase (SOD) activity, MDA content and total antioxidant capacity were measured on liquid preserved epididymal spermatozoa after 72 hr of incubation at 4°C.
2.6 Testicles
Testicles (with epididymides) of 24 adult rams (1–5 years), of Sanjabi breeds, collected in slaughterhouses, were used in the present study. Each pair of testicles (with respective epididymides) was separated and stored at 4°C (ice cold water in thermic box). Epididymal sperms were collected from the caudae epididymides just 2 hr after slaughter. In the laboratory, the testes were rinsed twice with normal saline and were then trimmed to remove the extra testicular tissue and washed 10 times with saline containing 0.1% streptomycin sulphate. Connective tissue covering the caudae epididymis was removed by dissection with care to avoid rupturing blood vessels or the epididymal duct. Epididymis were separated, cleaned (alcohol 70%), underwent an incision technique and immersed in 5.0 ml of Tris buffer (Tris 297.58 mM, citric acid 96.32 mM and fructose 86.66 mM: pH 6.8) for sperm migration for 10 min. Collected spermatozoa were washed once by centrifugation with Tris buffer at 448 g for 5 min. The pellet obtained was suspended in Tris buffer and evaluated for motility (Almeida et al., 2012).
2.7 Extender
Tris-based extender (Tris 297.58 mM, citric acid 96.32 mM, fructose 86.66 mM and 6% of glycerol egg yolk of 15% (v/v): pH 6.8) was used as the base extender.
2.8 Liquid preservation of ram sperms
The one-step dilution procedure was used for sperm liquid preservation. The sperm suspension was divided equally and added to the diluent at room temperature to create a 30 × 106 sperm/ml sperm dilution. The extended sperm was placed in a 35°C water jacketed tube and cooled to 4°C. Samples were stored at this temperature until 72 hr.
2.9 MTT assay
The MTT assay is a colorimetric assay for assessing cell metabolic activity. Mitochondrial enzymes are capable of reducing tetrazolium to insoluble formazan (Kime et al., 2001). In experiment 1, to evaluate sperm viability, MTT assay was performed according to a method described by Mohammadi and Soltani (2021). The MTT solution was prepared as a 5 mg/ml stock solution in Dulbecco's phosphate-buffered saline (DPBS), filtered and kept for no more than two weeks in the dark at 4°C. Next, 10 μl of the MTT stock solution was warmed at 37°C and added to the sperm suspension in an Eppendorf tube containing 100 µl of diluted sperm. The treatment groups were as follows: (1) the group receiving no supplement was considered as control; (2) Control +1 µg/ml of ZnO-NPs as T1; (3) Control +5 µg/ml of ZnO-NPs as T2; (4) Control +25 µg/ml of ZnO-NPs as T3; (5) oxidative stress (S) was induced with 50 μM hydrogen peroxide (de Castro et al., 2016); (6) (S) group +1 µg/ml of ZnO-NPs (S+T1); (7) (S) group +5 µg/ml of ZnO-NPs (S+T2); and (8) (S) group +25 µg/ml of ZnO-NPs (S+T3). The content of ZnO-NPs was defined according to our study (not reported). After 2 hr from incubation, the MTT reduction rates were determined through an ELISA reader (BioTek (Power Wave XS2)) at a wavelength of 570 nm. In order to calculate number of viable cells, standard curve prepared. Samples were prepared by combining aliquots of viable and freeze-killed sperm in ratios 10:0, 8:2, 6:4, 4:6, 2:8 and 0:10 (v/v). Then, the MTT test was then applied to determine sperm viability in each sample. Next, MTT reduction rates at 37°C were measured immediately and after 2-hr incubation. The relationship between the MTT reduction rate and sperm viability was expressed as a regression equation.
2.10 Sperm total motility
Sperm motility was investigated by visual estimation under a phase-contrast microscope at ×400 magnifications, as described by Zhu et al. (2017). Briefly, a 10-μl drop of sperm suspension was placed on a pre-warmed (37°C) clean glass slide, and covered with a coverslip. Sperm motility was determined by calculating the percentage of sperm displaying progressive movement after monitoring five various fields.
2.11 Assessment of sperm viability
Viability of sperm was investigated on cooled storage sperm using the eosin-nigrosin stain (Björndahl et al., 2003). One drop of sperm sample and two drops of the stain were placed on a warm slide to prepare the sperm smear. Viability was then determined by counting 200 sperms using bright-field microscopy (400×). Sperms with white or light pink heads were considered to be alive, while those with red or dark pink heads were regarded dead.
2.12 Sperm morphology
Morphological evaluation of spermatozoa was performed by Hancock's solution method (Schaffer & Holzmann, 2000). Hancock's solution contained of 62.5 ml formalin, 150 ml buffer solution, 150 ml sodium saline solution and 500 ml double-distilled water. Three drops of sperm samples were mixed with 1 ml of Hancock's solution; one drop of this mixture was put on a microscope slide and mounted. The percentage of abnormal sperms was recorded by counting 200 spermatozoa under phase-contrast microscopy at 1000×.
2.13 Hypo-osmotic swelling test
Hypo-osmotic swelling test (HOST) was used to determine the functional integrity of sperm plasma membrane as the procedure described previously (Vasquez et al., 2013). For this, 10 µl of each sperm sample was added in 150 µl of the HOST medium (100 mOsm/kg, 57.6 mM fructose and 19.2 mM sodium citrate citrate). After 60 min at 37°C, a drop of incubated samples was placed on a microscope slide, cover-slipped and immediately evaluated at 400× magnification for calculation of HOST reacted spermatozoa. Spermatozoa having visible coiled tail were determined as HOST reacted spermatozoa. A total of 200 spermatozoa were counted to determine percentage of HOST reacted spermatozoa.
2.14 DNA integrity
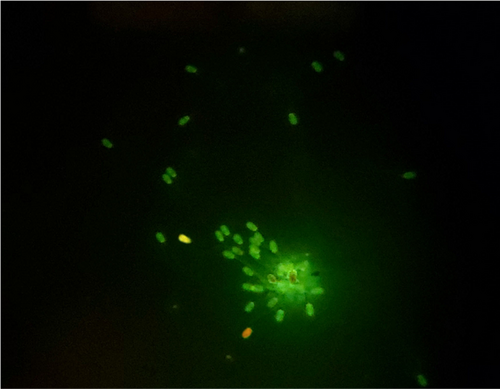
Sperm DNA integrity was assessed using Acridine orange (AO) staining following the method described in a former study (Moradi et al., 2021b). Briefly, smears from each of treated and untreated groups were smeared on a glass slide, and air-dried. The smears were fixed in a daily-prepared methanol-glacial acetic acid (Carnoy's solution; 3/1 v/v) overnight. Smears were washed with distilled water and air-dried. Then, we stained with AO staining solution (1% AO diluted in distilled water) for 5 min. After staining, slides were rinsed with deionized water and air-dried at room temperature and cover-slipped. Two hundred sperms were counted under the fluorescent microscope. The heads of the sperms with normal DNA integrity (double-stranded) emitted green fluorescence, while those with denatured or single stranded DNA exhibited yellow, orange or red fluorescence. The stained slide was investigated within 1 hr after staining.
2.15 Antioxidant indices
Malondialdehyde kit (Purchased from TPR INNOVATIVE), superoxide dismutase kit (Purchased from TPR INNOVATIVE) and total antioxidant capacity kit (Purchased from TPR INNOVATIVE) were used to detect various antioxidant indicators of liquid preserved ram epididymal spermatozoa. Referred to the kit instructions for specific operation methods.
2.16 Statistical analysis
The study was replicated six times. Results were expressed as the mean ± SD. A completely randomized factorial design was employed to carry out experiment 1 on cells viability. In experiment 2, means were analysed by one-way analysis of variance (ANOVA), followed by the Duncan's test to distinguish significant differences in all the parameters among all groups using the SPSS software (Version 16.0; SPSS, Chicago, IL). Differences with values of p < .05 were considered to be statistically significant.
3 RESULTS
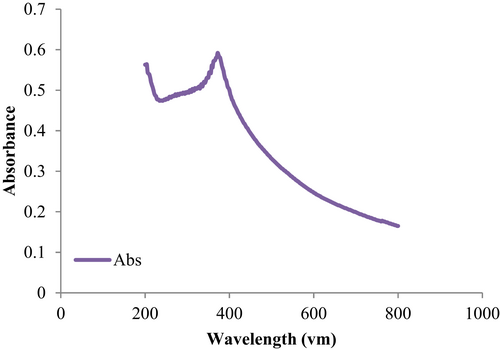
Figure 2 shows the UV–visible absorption spectrum of ZnO-NPs. UV–visible spectrum was determined in a quartz cuvette using Shimadzu UV spectrophotometer by sample analysing in the range of 200–800 nm. A peak at 386 nm present on UV-vis spectra of ZnO-NPs shows the complete synthesis of ZnO-NPs.
The size of the nanoparticles synthesized by Maclura pomifera extract is shown in Figure 3. The SEM images of ZnO-NPs were almost spherically in shape. The particle size was found to be approximately 64.01 ± 35.44 nm.

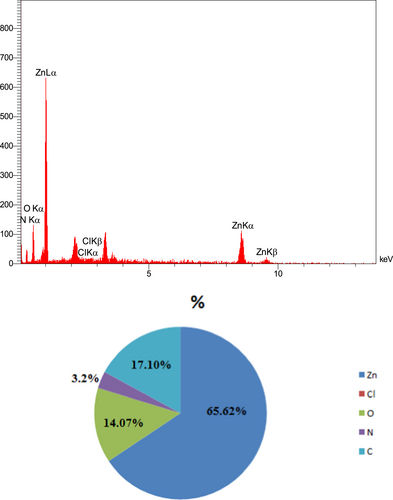
EDX was used to analyse the chemically elemental composition of ZnO-NPs (Figure 4). Five signals can be observed from EDX analysis: a strong signal from Zn atom (65.62%) along with those from C atom (17.10%), O atom (14.07%), N atom (3.2%) and Cl atom (0.01%). No visible peaks were monitored for other elements.
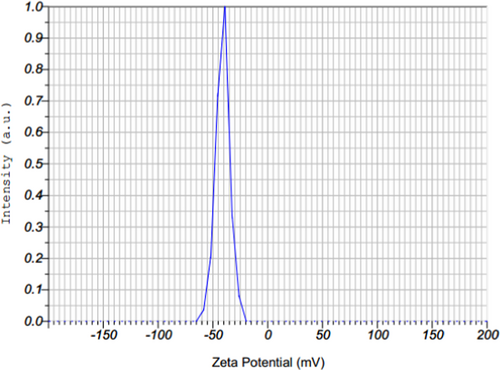
A zeta-potential study was performed to indicate the stability and surface charge of the created nanoparticles and showed the presence of a distinct peak at −41.1 mV, which indicates that the synthesized ZnO-NPs possess good stability (Figure 5). This means that the capping molecules available in the surface of green synthesized ZnO-NPs are mainly comprised of negatively charged groups and also responsible for the moderate stability of the NPs.
FTIR analysis was used to recognize functional groups present in the herbal extract that contribute to the mechanism of bonding with ZnO-NPs. A comparison between the FTIR spectrum of the fruit extract and that of the synthesized ZnO-NPs is represented in Figure 6. Therefore, the FTIR spectrum of the fruit extract showed different peaks at 3414, 2933, 1620, 1391, 1056, 777 and 619 cm−1. The peaks at 3414 (O–H), 1620 (N–H), and 1056 (C–O) or 777–1391 (RCOO) cm−1 are related to flavonoids, alkaloids and phenolic compounds (Figure 6a).
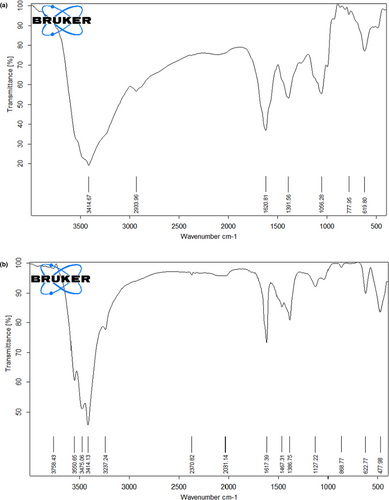
Figure 6b shows the FTIR spectra of ZnO-NPs synthesized by the green approach displayed a peak at 477 cm−1, which is corresponding to the hexagonal ZnO symmetric bending vibration and a peak at 868 cm−1 due to weak vibration of ZnO. The wide peaks present at 1127 cm−1 and 3414 cm−1 reflect the presence of C–OH and OH stretching vibrations, respectively. Other smaller bond vibration peaks including 2370, 1617 and 1467 cm−1 are due to the presence of primary and secondary amines that are characteristics of proteins/enzymes and C–O stretching regions of phenolic and polysaccharides groups.
Figure 7 shows the cell viability in different groups of fresh ram epididymal sperms under normal and oxidative stress conditions. Under normal condition, viability significantly was preserved in 1 and 5 μg/ml of ZnO-NPs in compared to the 25 μg/ml of ZnO-NPs and control group (p < .05; Figure 7). Under oxidative stress conditions, sperm viability was improved in groups treated with the varying concentrations of ZnO-NPs in compared to the oxidative stress group.
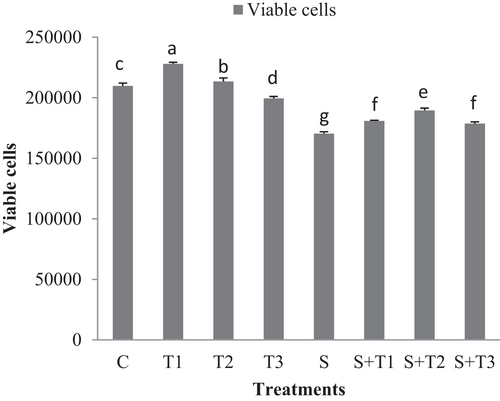
The percentage of total motility, viability and plasma membrane integrity were higher in the 1 µg/ml of ZnO-NPs compared to the 5 µg/ml and 25 µg/ml of ZnO-NPs and control group at 24, 48 and 72 hr of storage (p < .05; Tables 1–3).
| Time (hr) | ZnO-NPs (µg/ml) | |||
|---|---|---|---|---|
| C | 1 µg/ml | 5 µg/ml | 25 µg/ml | |
| 24 | 64.08 ± 0.66b | 70.79 ± 0.99a | 63.75 ± 0.92b | 58.48 ± 0.57c |
| 48 | 58.60 ± 2.04b | 64.83 ± 0.86a | 59.22 ± 0.80b | 52.56 ± 1.83c |
| 72 | 53.76 ± 2.45b | 60.08 ± 0.52a | 54.71 ± 0.90b | 48.81 ± 1.33c |
Note
- Different superscripts within the same row demonstrate significant differences (p < .05), whereas the same superscripts do not demonstrate significant differences (p > .05).
| Time (hr) | ZnO-NPs (µg/ml) | |||
|---|---|---|---|---|
| C | 1 µg/ml | 5 µg/ml | 25 µg/ml | |
| 24 | 68.64 ± 1.29b | 72.65 ± 2.96a | 66.68 ± 2.29b | 60.84 ± 1.28c |
| 48 | 61.68 ± 0.87b | 67.65 ± 1.32a | 62.57 ± 1.13b | 57.25 ± 2.26c |
| 72 | 57.51 ± 0.77b | 63.22 ± 1.06a | 57.14 ± 1.91b | 52.94 ± 0.93c |
Note
- Different superscripts within the same row show significant differences (p < .05), whereas the same superscripts do not show significant differences (p > .05).
| Time (hr) | ZnO-NPs (µg/ml) | |||
|---|---|---|---|---|
| C | 1 µg/ml | 5 µg/ml | 25 µg/ml | |
| 24 | 61.90 ± 1.80b | 65.44 ± 1.14a | 61.03 ± 1.22b | 56.62 ± 0.61c |
| 48 | 56.64 ± 1.11b | 61.13 ± 1.27a | 57.48 ± 0.60b | 52.60 ± 1.43c |
| 72 | 52.35 ± 0.92b | 57.87 ± 0.45a | 53.00 ± 0.89b | 48.72 ± 0.5c |
Note
- Different superscripts within the same row show significant differences (p < .05), whereas the same superscripts do not show significant differences (p > .05).
Sperm DNA integrity was higher in 1 µg/ml of ZnO-NPs group compared to the other treatment groups at 24 hr (p < .05; Table 4). And this trend continued until the 72 hr (p < .05; Table 4; Figure 8).
| Time (hr) | ZnO-NPs (µg/mL) | |||
|---|---|---|---|---|
| C | 1 µg/ml | 5 µg/ml | 25 µg/ml | |
| 24 | 79.54 ± 2.63b | 88.12 ± 1.66a | 82.42 ± 1.85b | 78.67 ± 1.94b |
| 48 | 78.51 ± 0.70c | 87.82 ± 1.17a | 81.30 ± 0.57b | 76.66 ± 1.86c |
| 72 | 77.53 ± 1.21c | 86.03 ± 1.27a | 80.13 ± 0.99b | 75.97 ± 0.96c |
Note
- Different superscripts within the same row show significant differences (p < .05), whereas the same superscripts do not show significant differences (p > .05).
At 24 hr, the percentage of abnormal spermatozoa was significantly higher in the 25 µg/ml of ZnO-NPs compared to the other treatment groups (p < .05; Table 5). At 48, the 1 μg/ml ZnO-NPs had a lower percentage of abnormal spermatozoa compared to the other treatment groups (p < .05). At 72 hr, a significant difference was also found between the 1 μg/ml ZnO-NPs and the 25 μg/ml ZnO-NPs group (p < .05; Table 5).
| Time (hr) | ZnO-NPs (µg/ml) | |||
|---|---|---|---|---|
| C | 1 µg/ml | 5 µg/ml | 25 µg/ml | |
| 24 | 22.15 ± 1.24a | 20.54 ± 0.93a | 22.25 ± 1.01a | 24.67 ± 2.03b |
| 48 | 24.50 ± 1.46b | 22.80 ± 0.85a | 24.55 ± 1.03b | 26.06 ± 0.60b |
| 72 | 26.34 ± 1.01ab | 24.90 ± 0.76a | 25.62 ± 0.85ab | 27.61 ± 1.86b |
Note
- Different superscripts within the same row show significant differences (p < .05), whereas the same superscripts do not show significant differences (p > .05).
After 72 hr of storage, the TAC level in 1 µg/ml of ZnO-NPs group was significantly higher than that of the control group (p < .05; Table 6), while the TAC level in 25 µg/ml of ZnO-NPs was significantly lower than the control group. The content of MDA was lower in 1 µg/ml of ZnO-NPs group than that of the other treatment groups (p < .05; Table 6). When the sperms were stored for 72 hr, the SOD activity of 1 µg/ml of ZnO-NPs group was higher than that of the control group (p < .05; Table 6), while the SOD activity in 25 µg/ml of ZnO-NPs was significantly lower than the control group (p < .05; Table 6).
| Parameters | ZnO-NPs (µg/ml) | |||
|---|---|---|---|---|
| C | 1 µg/ml | 5 µg/ml | 25 µg/ml | |
| SOD | 46.86 ± 2.21b | 57.00 ± 0.64a | 49.22 ± 2.03b | 39.90 ± 2.03c |
| TAC | 12.10 ± 0.16b | 14.28 ± 0.45a | 12.02 ± 0.04b | 9.89 ± 0.29c |
| MDA | 2.17 ± 0.03c | 1.77 ± 0.01a | 2.01 ± 0.02b | 2.04 ± 0.04d |
Note
- Different superscripts within the same row show significant differences (p < .05), whereas the same superscripts do not show significant differences (p > .05).
4 DISCUSSION
To investigate spermotoxicity of ZnO-NPs, various concentrations of ZnO-NPs (1, 5 and 25 µg/ml) were provided and added to sperms for 2 hr under normal and induced oxidative stress condition. In this study, the rate of cell death was measured by MTT assay. According to our results, when cells were treated with higher concentrations of ZnO-NPs (25 µg/ml), their toxicity was increased. Also, addition of different concentrations of ZnO-NPs to ram sperm extender decreased the toxicity of H2O2 compared to non-treated oxidative stress condition. Similar to our study, Barkhordari et al. (2013) showed that ZnO-NPs are toxic for human spermatozoa at high concentrations. Also, in a study carried out by Halo et al. (2021) negative dose-dependent effect of ZnO nanoparticles on spermatozoa motility and viability parameters was observed. Apoptosis can be induced by ZnO-NPs via the intrinsic mitochondrial pathway (Kao et al., 2012). In vitro treatment with ZnO-NPs has been demonstrated to decrease mitochondrial membrane potential and conversely increase Bax/Bcl2 ratio (Sensi et al., 2003). The results of Pinho et al's study showed that higher concentrations of ZnO-NPs cause intracellular ROS levels, DNA damage, cytoskeleton and nucleoskeleton dynamics alterations and, ultimately, cell death (Pinho et al., 2020). However, the exact mechanism of cytotoxicity has not yet been clarified. Observations suggest that surface reactivity is a major contributor to the generation of ROS in cells exposed to ZnO-NPs (Nel et al., 2009; Sharma et al., 2012).
Our results revealed that adding low concentration of ZnO-NPs could improve the liquid preserved of ram epididymal spermatozoa quality. This improvement can be illustrated by their antioxidative properties. Therefore, the high MDA level and the low TAC and SOD levels were considered in control group, indicating that ZnO-NPs can protect sperm from oxidative stress during low temperature storage that reflected on spermatozoa quality parameters including total motility, vitality, DNA and plasma membrane integrity, and morphological abnormality. Isaac et al. have suggested that the addition of different concentrations of ZnO-NPs to cryopreserved normozoospermic men samples did not improved the total motility and viability in frozen–thawed samples compared to control, but, ZnO-NPs significantly decreased MDA and sperm chromatin damage (Isaac et al., 2017). They concluded that the protective effect on sperm chromatin during freeze–thaw process seems to be due to the formation of a protective layer of ZnO-NPs around the spermatozoa, which can prevent lipid peroxidation at membrane level (Isaac et al., 2017). As well, Liu et al. (2016) showed an increase in cell cycle alterations and DNA damage. Cell cycle proteins are more expressed and cell numbers are greater in the S-phase and lower in the G1 and G2 phases. The application of an antioxidant decreased these alterations, indicating that oxidative stress contributes to ZnO-NPs cell cycle arrest. Zinc effects on sperm motility by controlling energy utilization in the ATP system, which participates in muscle contraction and the regulation of phospholipid energy reserves (Orzołek et al., 2021). Also, our findings are in accordance with report of Shahin et al. (2020), who reported that ZnO-NPs improved the post-thawing quality of dromedary camel epididymal spermatozoa. In the study of Ashtari et al., (2021), the best protective effect of ZnO-NPs was observed after addition of 100 ppm to diluted normal human semen samples improving percentage of motility and viability of cryopreserved human spermatozoa (Ashtari et al., 2021). Similar to our finding, Jahanbin et al. (2021) reported that treatment with Zn-NPS improved the plasma membrane integrity and mitochondrial activity, simultaneously reducing the MDA level in sperm of bovine exposed to freezing/thawing processes. In contrast, Orzołek et al. (2021) reported that turkey ejaculates supplemented with Zn-NPs and preserved for 48 hr showed a few reduced sperm parameters, including total and progressive motility and mitochondrial membrane potential. Maybe sperm quality was reduced in the study of Orzołek et al due to using a high concentration of Zn-NPs. In our study, we observed adverse effects of ZnO-NPs on sperm parameters. Zhandi et al. (2020) showed that the addition of ZnO to semen diluents has increased the freezability of rooster spermatozoa. The optimal ZnO concentration was 0.77–1.27 µg/ml, whereas higher concentrations compromised post-thaw sperm quality indices. The cytotoxicity of ZnO-NPs on the male reproductive system depends on cell type, concentration and time of exposure (Barkhordari et al., 2013; Han et al., 2016; Liu et al., 2016). However, the structural characteristics of ZnO-NPs, for example, surface area and size, as the variables of analysis were not investigated.
In conclusion, the obtained results from the present study revealed that aqueous extract of Maclura pomifera fruit had several natural bioactive compounds in which those could be effectively utilized in the fabrication of ZnO-NPs without using other chemical reducing agents. Addition of low concentrations of ZnO-NPs can improve the outcome of liquid preserved ram epididymal spermatozoa, particularly, with respect to total motility, viability, and membrane and DNA integrity at different incubation periods. We recommend the inclusion of ZnO-NPs to diluents to improve the liquid preserved of ram epididymal spermatozoa. Further studies are essential to confirm that it does not hamper the cryopreservation of ram spermatozoa. Additionally, more research needs to be done to determine whether ZnO-NPs supplementation affects fertilization and embryo development during IVF or ICSI.
ACKNOWLEDGEMENT
The authors are thankful to the staff of research unit of Razi University (Kermanshah, Iran) for emotional support.
AUTHORS’ CONTRIBUTION
Leila Soltani, Sarah Samereh and Tayebeh Mohammadi performed methodology, formal analysis and investigation, and wrote original draft preparation; Leila Soltani and Tayebeh Mohammadi reviewed and edited; Leila Soltani, Sarah Samereh and Tayebeh Mohammadi contributed to funding.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding upon reasonable request.




