Effects of cooling rates on the quality of Prochilodus lineatus (Valenciennes, 1836) sperm
Abstract
This study aims to investigate the effect of different cooling rates on the semen cryopreservation of curimba (Prochilodus lineatus). Nineteen ejaculates were obtained from adults males and cryopreserved at 15°C/min (CR15), 30°C/min (CR30) (controlled temperature inside and outside straw, speed was stable during freezing) and direct freezing in liquid nitrogen vapour (~35.6°C/min) (CRNV). The straws were thawed and seminal parameters evaluated. DNA fragmentation through the comet assay was assessed. A fresh sperm sample was not frozen and used for analyses. Data were submitted to an analysis of variance (ANOVA), and means were compared by Scott–Knott test (p < 0.05) using the R Software. Mean motility percentage was 100%, and motility duration was 39.5 ± 5.7 s for the fresh sperm (subjective analysis); 58.9 ± 8.0% and 24.5 ± 5.7 s for CR15; 64.8 ± 4.8% and 26.5 ± 7.1 s for CR30; and 50.1 ± 16% and 25.7 ± 4.7 s for CRNV, respectively. Motility percentages were higher and equal between CR15 and CR30 compared to CRNV (p < 0.05). Some sperm motion kinetics, namely average path velocity (VAP) and straight line velocity (VAS), were higher for CR30 (p < 0.05), while curvilinear velocity (VCL) and velocity progression (PRO) were lower for CRNV (p < 0.05). Straightness (STR) and wobble (WOB) were the same among treatments (p > 0.05). Sperm morphology results indicated higher means for total morphological sperm alterations in CRNV. All cooling rates caused sperm DNA fragmentation, although CR30 provided a less harmful effect. This is the first report for cryopreserved P. lineatus sperm preserved under different controlled cooling rates. The cooling rate of 30°C/min is indicated for the cryopreservation of this fish sperm as it led to the lowest detrimental spermatozoa effects.
1 INTRODUCTION
Frozen semen displays many advantages over fresh or cooled fish semen, as it allows for reproductive period flexibility and operationalization and assisted reproduction programmes for native fishes (Cabrita et al., 2011; Viveiros et al., 2010),in addition to the time in which the sperm samples can be used (Magnotti et al., 2018). Furthermore, this biotechnology can be considered an ichthyofauna recovery strategy through genetic resource cryobanking of endangered species (Asturiano, Cabrita, & Horváth, 2016).
Fish semen cryopreservation technology has expanded considerably worldwide with the use of portable nitrogen vapour cylinders, known as “dry-shippers.” Semen is packaged in straws, added to the cylinder and subjected to uncontrolled speeds between 30 and 40°C/min. Fish semen from neotropical Characiformes and Siluriformes orders has been successfully frozen using this technique (Viveiros, Taffarel, & Leal, 2014).
The heterogeneity of responses obtained from post-freezing sperm makes it difficult to standardize protocols for different fish species (Viveiros & Godinho, 2009). Studies applying controlled temperature curves have not yet been carried out for neotropical species. This factor is of extreme importance and may be one of the causes for the different results reported for the same species. In addition, according to Viveiros et al. (2014) the use of programmable freezers has allowed the use of slower speeds, more than one speed (or step) per programme and the inclusion of new steps.
The viability of the fish sperm cryopreservation is influenced by many factors including diluents, cryoprotective agents, equilibrium time, freezing rate and thawing temperatures (Ahn, Park, & Lim, 2018). The success of sperm freezing with liquid nitrogen requires cooling rates between 10°C and 60°C/min (Harvey & Carolsfeld, 1993), and cylinders exclusively containing liquid nitrogen vapour (Carolsfeld & Harvey, 1999) ensure cooling rates in this range, but do not allow for a controlled freezing curve. Freezing curves obtained with the use of programmable bio-freezers have been increasingly applied as an innovative tool for the study of several species (Fang, Blair, Zhong, Sun, & Zhou, 2016; Frankel, Theisen, Guthrie, Welch, & Woods, 2013; Silva et al., 2013).
Sperm motility is one of the most important factors when the aim is to analyse fish sperm quality and to evaluate the effect of biotechnologies, such as cryopreservation (Bobe & Labbé, 2010). During the cryopreservation process, sperm cells are exposed to physiologically inadequate external conditions, which can affect several sperm motility parameters (Butts et al., 2011; Martínez-Páramo et al., 2012).
It is important to correlate routinely evaluated sperm parameters at the moment of thawing with sperm viability and sperm DNA fragmentation evaluations, which together are able to correctly qualify an ideal sperm for fertilization. In the comet assay, sperm cells are placed on agarose gel using glass slides and, after cell discharge and DNA denaturation, alkaline electrophoresis is performed (Beirão, Cabrita, Soares, Herráez, & Dinis, 2008; Küçük, 2018; Lee & Steinert, 2003). The technique is based on different electrophoretic DNA patterns obtained from DNA fragments, where cells (DNA spermatozoa) with DNA archived in a comet-like tail structure are observed, larger when the DNA structure damage is greater (Gallego & Asturiano, 2018).
The comet assay is commonly used and has been validated successfully in several fish semen studies (Dietrich et al., 2005; Labbe, Martoriati, Devaux, & Maisse, 2001; Li, Wei, & Liu, 2008; Zilli, Schiavone, Storelli, & Vilella, 2003). Cabrita, Robles, Rebordinos, Sarasquete, and Herráez (2005) concluded that cryopreservation may lead to damage of rainbow trout and gilthead sea bream (Sparus aurata) sperm DNA, which should be taken into account when assessing freezing and thawing protocols. Diogo et al. (2018) observed that the greatest DNA fragmentation was observed for Danio rerio semen under storage conditions at −20°C/min cooling rate with storage in ultrafreezer. Nathanailides, Chanzaropoulos, Barbouti, Perdikaris, and Zhang (2011), when evaluating the DNA integrity of goldfish semen cryopreserved with different cryoprotectants by the comet assay, concluded that integrity was compromised when ethylene glycol was used and indicated that 10% or 5% egg yolk and dimethylsulphoxide provided sufficient protection, presenting significant differences compared to ethylene glycol. However, no data concerning the effect of freezing rates on Prochilodus lineatus DNA fragmentation using the comet assay are available.
The curimba P. lineatus (Valencienes, 1836) is a migratory species presenting a wide geographical distribution in South America. Well-established artificial reproduction methods and high reproduction rates are established for this species, being the highlight of several cryopreservation studies (Felizardo et al., 2010; Miliorini et al., 2011). In addition, this species has been used as a model for fish reproduction research (Paula et al., 2014; Viveiros, Nascimento, Orfão, & Isaú, 2010).
In recent years, many studies have been carried out regarding P. lineatus semen cryopreservation, and several assessments have contributed with relevant information regarding its cryopreservation and evaluation. Thus, factors such as the use of cryoprotectants and their concentrations, thawing temperatures and time, antioxidant substance use or not, semen storage time and activating solution have been determined (Carvalho et al., 2017; Di Chiacchio, Almeida, Leal, & Viveiros, 2017; Felizardo et al., 2010; Gonçalves, Nascimento, Costa, Leal, & Viveiros, 2013; Miliorini et al., 2011; Murgas, Miliorini, Freitas, & Pereira, 2007; Paula et al., 2012). Miliorini et al. (2011), for example, observed that P. lineatus cryopreserved semen samples containing 5% BTS and dimethylsulfoxide presented the lowest percentage of fractured tails and tail stumps. The authors also noted that DMSO 7.5% and methanol 5% combined with BTS provided adequate sperm cell protection after cryopreservation and thawing in this species. However, studies regarding cooling rates for this species have not been performed and are scarce even considering other South American fish species, with no consensus on several parameters that could constitute a standardized protocol. Therefore, the aim of the present study was to establish suitable cooling rates for P. lineatus sperm, through thawed semen quality assessments.
2 MATERIALS AND METHODS
2.1 Broodstock management
The biological material was obtained at the Fish Culture Station of the Minas Gerais Power Company (CEMIG), located in the city of Itutinga, Minas Gerais State, Brazil. Cryopreservation and sperm evaluations were carried out at the Multiuser Vivarium belonging to the Federal University of Lavras, located in the city of Lavras, Minas Gerais, Brazil. The sperm of 19 P. lineatus males (19 replicates; a sperm sample was considered an experimental unit) weighing 2.0 ± 0.8 kg, aged 3–4 years old, were selected during the spawning season (mean sperm volume 6.41 ± 3.17 ml, sperm concentration 31.31 ± 4.86 × 109 spermatozoa/ml). The males were transported to masonry tanks (glass aquariums) with running water at a temperature of 28–30°C for subsequent reproduction hormonal induction through the hypophysation technique.
2.2 Hypophysation technique, semen collection and evaluation
Males were selected by observing the previous release of a few drops of semen when massaged, and subsequently weighed and marked. After biometric assessments, they were treated with doses of crude carp pituitary extract (Argent Chemical Laboratories), to induce complete spermiation. The initial and final doses of the hormone were 0.5 and 5 mg/kg of fish, applied through intramuscular injections near the base of the dorsal fin at 12 hr intervals between each application (routine method currently used to induce spermiation at the Fish Culture Station). Approximately 8–10 hr after the second dose, sperm was extracted gently by abdominal massage and stored (4°C). The sperm was evaluated (scale from 0% to 100% sperm motility) immediately after collection. Briefly, 5 μl of each sample was placed on a glass slide and observed using a light microscope (OPTON TIM-2005-B, JAPAN; 400× magnification) following the addition of 40 μl of distilled water as activating agent (Martínez-Páramo et al., 2012; Murgas et al., 2007; Paula et al., 2012). Pre-activated and fresh sperm samples with motility rates of less than 80% were discarded. Osmolarity determinations were performed before freezing with the “in natura” semen of each animal (256 ± 10 mOsm/kg). The osmolarity of the cryopreservation dilution solution was also determined (261 ± 6 mOsm/kg, hyperosmotic in relation to the sperm, avoiding sperm activation). Solution osmolarities were determined using a VAPRO 5520 vapour pressure osmometer (Wescor Inc.), as suggested by Dzyuba and Cosson (2014). The effects of osmolarity and ions present in plasma are important for most fish species, as they characterize fish spermatozoa immobility in the testes. In addition, fish sperm motility is initiated after the sperm is released in aqueous media during natural reproduction or in a diluent during artificial reproduction (Alavi & Cosson, 2006). Therefore, an isosmotic solution in relation to P. lineatus semen was used for cryopreservation, guaranteeing its inactivation before thawing.
2.3 Sperm cryopreservation and thawing
Sperm samples were stored at 4°C for 48 hr and diluted in 5% BTS™ (80% glucose, 12.7% sodium citrate, 2.7% EDTA, 2.7% NaHCO3, 1.5% KCl, 0.5% gentamycin sulphate; Beltsville Thawing Solution Minitüb™) and 10% DMSO (dimethyl sulfoxide) at a 1:4 ratio as recommended by Miliorini et al. (2011) and Murgas et al. (2007). Sperm was equilibrated for 15–20 min (Viveiros, Gonçalves, Nascimento, & Leal, 2015), drawn into sealed 0.5-ml straws, in triplicate, identified and maintained in a freezing equipment (IceCube® 14S) programmed with the cooling rates adapted from Gaitán-Espitia, Martínez-Silva, Borrero, Ramírez, and Valencia (2013) as follows: a slow cooling rate of 15°C/min (CR15) and a fast cooling rate of 30°C/min (CR30) (Table 1).
| Curve | Step | Temperature (°C) | Absolute time [hh:mm:ss] | End temperature (°C) | Relative time [hh:mm:ss] | Slope (°C/min) |
|---|---|---|---|---|---|---|
| 15CR15 | 1 | 4.00 | 02:00:00 | 4.00 | 02:00:00 | – |
| 2 | −150.00 | 00:10:16 | −154.00 | 00:10:16 | −15°C | |
| 30CR30 | 1 | 4.00 | 02:00:00 | 4.00 | 02:00:00 | – |
| 2 | −150 | 00:05:08 | −154.00 | 00:05:08 | −30°C |
Concerning the CRNV, adapted from Merino et al. (2011), sperm samples were packed directly into the liquid nitrogen vapour cylinder (Taylor-Wharton, CP 300, “Dry-shipper”), which maintains a standard freezing rate of approximately 35.6°C/min) for 24 hr. The samples were then transferred to a liquid nitrogen cylinder (M.V.E. Millenium, XC 20) at −196°C for storage. Approximately 8 months later, straws were removed from the cylinder and immediately submerged in a water bath at 40°C for 5 s.
2.4 Sperm evaluation
2.4.1 Sperm motility percentage, motility duration and concentration
A 0.2 μl sperm aliquot was homogenized in 98.8 μl (1:500) (v/v) of distilled water, and 0.2 μl of this dilution was rapidly deposited in a Neubauer chamber previously focused at a 20× magnification 3 s post-activation using a camera (Basler scA780, Basler Vision Technologies) at a rate of 60 frames/s, a coverslip was applied and the images were assessed using the ImageJ software using a special plug-in developed by Wilson-Leedy and Ingermann (2007). Video recording began immediately after the focus adjustment, 3 s after motility activation. Evaluations using the CASA plug-in were recorded on video using an average of 200–500 sperm, in triplicate, at three videos per sample (triplicate activated semen), and means were calculated. The sperm examination duration using the Neubauer chamber was initiated when spermatozoa were activated and lasted until only approximately 10% of sperm cells were still moving.
The videos were captured using the AMCAP software (Basler Vision Technologies) in avi format, edited using the VIRTUALDUB-1.9.0 software and exported as a sequence of jpg format images, edited using the ImageJ software and compiled using the Computer Assisted Sperm Analysis (CASA) plug-in. The dialogue box configuration in ImageJ (Table 2) was adjusted until most sperms were covered in the generated images. Thus, different configurations were tested until reaching adequate motile sperm identification. The configuration of the dialogue box of the CASA application was performed based on the images of the tracks covered by the spermatozoa generated by the application. The microscope was connected to a video camera generating 60 images/s at 3 s post-activation (1 video second at a time).
| Input parameters | Input values |
|---|---|
| A. Minimum sperm size (pixels) | 1 |
| B. Maximum sperm size (pixels) | 150 |
| C. Minimum track length (frames) | 25 |
| D. Maximum sperm velocity between frames (pixels) | 15 |
| E. Minimum VSL for motile (µm/s) | 3 |
| F. Minimum VAP for motile (µm/s) | 20 |
| G. Minimum VCL for motile (µm/s) | 25 |
| H. Low VAP speed (µm/s) | 2 |
| I. Maximum percentage of path with zero VAP | 1 |
| J. Maximum percentage of path with low VAP | 1 |
| K. Low VAP speed 2 (µm/s) | 1 |
| L. Low VCL speed (µm/s) | 1 |
| M. High WOB (per cent VAP/VCL) | 80 |
| N. High LIN (per cent VSL/VAP) | 80 |
| O. High WOB (per cent VAP/VCL) | 50 |
| P. High LIN two (per cent VSL/VAP) | 60 |
| Q. Frame rate (frames per second) | 60 |
| R. Microns per 1,000 pixels | 258 |
| S. Print xy coordinates for all tracked sperm? | 0 |
| T. Print motion characteristics for all motile sperm? | 0 |
| U. Print median values for motion characteristics? | 0 |
The following parameters were evaluated: per cent motility, duration of sperm motility, velocity curvilinear (VCL), velocity average path (VAP), velocity straight line (VAS), straightness (STR), wobble (WOB) and progression (PRO). The sperm motility duration analysis was performed using the ImageJ software, through a complete video of sperm movement until approximately 10% of the sperm in the evaluated field were mobile (Felizardo, Murgas, Navarro, Gonçalves, & Paulino, 2011; Murgas, Miliorini, Franciscatto, & Maria, 2004; Murgas et al., 2007; Paula et al., 2012).
To determine spermatic concentrations, a 10 μl aliquot of fresh sperm of each fish was collected, diluted in 990 μl of a formaldehyde citrate solution (2.9 g sodium citrate, 4 ml formaldehyde 35% made up with distilled water to 100 ml) (Andrade et al., 2014; Felizardo et al., 2010; Paula et al., 2012; Pinheiro et al., 2016). After dilution, 10 µl of the diluted samples was mixed with 990 µl of the formaldehyde citrate solution, resulting in a final dilution of 1:104 (sperm:formaldehyde citrate). The semen was diluted again by being very concentrated, and the redilution facilitated the sperm count. After the final dilution, 10 μl of the diluted sample was transferred to the Neubauer chamber for sperm count assessments under an optical microscope (100×). Mean sperm concentration is important to identify the amount of sperm that will be frozen in each straw. The mean spermatozoa concentrations in each straw were determined as 3.91 × 109.
2.5 Morphology sperm evaluations
A 10 μl aliquot of the formaldehyde citrate/sperm solution (the same solution used for spermatic concentration determination) was collected, and spermatozoa were counted on several microscopic fields throughout the slide, in the same intercalated direction, for all samples. The microscopic fields were evaluated until 100 analysed spermatozoa in each semen sample were counted (approximately 10 fields/sample). Morphological changes were counted, at 100× magnification using a phase contrast optical microscope with episcopic illumination (Motic BA 300®). Head, midpiece and tail sperm morphological alterations were observed according to the methodology described by Miliorini et al. (2011): head – microcephaly, macrocephaly, degenerated and free normal (isolated); midpiece – degenerated, proximal droplet and; tail – fractured, strongly coiled, stump, simple bent and distal droplet. Morphological changes such as free normal head, distal/proximal droplet and simple bent tail were considered minor morphological changes and the other cited alterations considered major morphological alterations. The sperm morphology analysis was performed in both fresh and thawed sperm.
2.6 Sperm DNA fragmentation
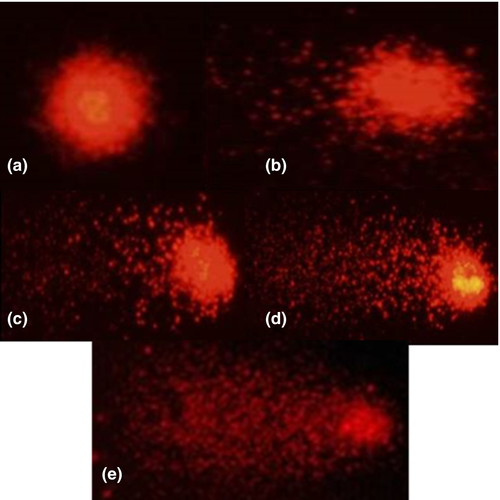
Sperm samples were subjected to a DNA fragmentation analysis using the single-cell alkaline gel Electrophoresis (comet assay) method, according to the procedures adapted from those described by Cabrita et al. (2005). Slides were prepared in advance (24 hr before analysis) applying a thin layer of normal melting point agarose (100 µl) spread across the length of the slide, eliminating excess agarose. This agarose solution was prepared by heat-dissolving 0.5% agarose powder in Milli-Q water. The slides were stored at room temperature and protected from dust and light. Low melting point agarose at the same concentration as described previously was prepared the next day for the second layer, using the same procedure. Fresh and frozen/thawed sperm were diluted in glucose 277 mM to a final concentration of 10 × 106 spermatozoa/ml. Frozen sperm was immediately prepared after thawing to prevent sperm degradation. Subsequently, 30 µl of the cell suspension was added to an Eppendorf tube containing 225 µl of low melting point agarose gel. After solidification on the previously prepared slides, a third layer of low melting point agarose gel was added to prevent material loss. Solidification was performed by exposing the slides at 4°C for 15 min. Three slides were prepared for each type of sperm and pool. The slides were placed into a Coplin jar containing a lysis solution comprising 2.5 M NaCl, 100 mM Na2–EDTA, 10 mM Tris, 1% Triton X-100 and 1% lauryl sarcosine for 1 hr at room temperature. After lysis, the slides were placed in an electrophoresis cube horizontal filled with freshly made electrophoresis solution (280 mM NaOH, 1 mM EDTA, pH > 13) for 20 min at 300 mA and 20 V to allow DNA to unravel. At the end of electrophoresis run, the slides were neutralized twice by 10 min incubation in a fresh Tris-HCl solution (0.4 M pH 7.5) and then fixed in a methanol solution for 3 min. The slides were then left to air dry and were stored protected from light and dust. For comet visualization, 40 µl of propidium iodide (0.5 µg/ml) were pipetted onto the samples, which were then covered with a coverslip. Samples were observed using an epifluorescence microscope (Nikon™ Eclipse E200) fitted with a 510–560 nm excitation filter and a 590 nm barrier filter. The nucleoids were evaluated visually, according to the methodology described by Collins et al. (1997) and characterized in “scores” or “classes” ranging from 0 to 4, according to observed damage to tail size and head diameter (damage index) (Figure 1). The nucleoids were, thus, characterized as: class 0 – no damage, presenting intact nucleoids, without a tail; class 1 – little damage, nucleoids presenting a tail size smaller than the head diameter; class 2 – intermediate damage, nucleoids presenting a tail size one time larger than the head diameter; class 3 – high damage, nucleoids presenting a tail size equivalent to twice the head diameter; class 4 – maximum damage, nucleoids presented tails over twice the head diameter.
2.7 Statistical analyses
The statistical analyses were performed using the R Development Core Team program version 2.11.0 (R Development Core Team, 2014), and the determined variables were submitted to an analysis of variance (ANOVA). Values are presented as means ± SD (standard deviation). The variables were tested for normality using the Shapiro–Wilk test, and a normal distribution was detected. The significance level for all statistical tests was set at 0.05. The means presenting differences were submitted to the Scott–Knott test.
3 RESULTS
3.1 Sperm motility percentage and duration
Sperm motility percentage and duration were higher for fresh sperm. Concerning treatments, motility percentages were higher for CR15 and CR30 (p < 0.05). Motility duration ranged between 24 and 39 s, higher for fresh sperm (p < 0.05) and the same between treatments (Table 3). Fresh sperm motility percentages did not vary (100%).
| Treatment† | Motility (%) | Motility duration (s) |
|---|---|---|
| Fresh sperm | 100 ± 0.0* | 39.5 ± 5.7a |
| CR15 | 58.9 ± 8.0a | 24.5 ± 5.7b |
| CR30 | 64.8 ± 4.8a | 26.5 ± 7.1b |
| CRNV | 50.1 ± 16.0b | 25.7 ± 4.7b |
| CV (%) | 18.3 | 20.6 |
- Abbreviation: CV, Coefficient of variation.
- a,bMeans followed by different letters in the columns differ by the Scott–Knott test (p < 0.05).
- † Treatment designations: CR15: cooling rate 15°C/min, CR30: cooling rate 30°C/min and CRNV: direct freezing in liquid nitrogen vapour.
- * All fresh semen samples showed 100% motility, indicating no variations within the treatment. Analysis of fresh semen motility was performed in the field, therefore, disconsidered in comparison with the cooling rates (different analyses).
The spermatic kinetic parameters evaluated by the CASA/ImageJ plug-in and the VCL, VAP, VSL, STR, WOB and PRO parameters are presented in Table 4. CR15 and CR30 presented higher mean values than CRNV for VCL and PRO (p < 0.05), while CR30 presented higher VAP and VSL compared to the other curves (p < 0.05).
| Curve | VCL (μm s−1) | VAP (μm s−1) | VSL (μm s−1) | STR (%) | WOB (%) | PRO (μm s−1) |
|---|---|---|---|---|---|---|
| 15CR15 | 113.5 ± 20.4a | 70.9 ± 20.9b | 57.6 ± 17.7b | 80.9 ± 1.9 | 62.6 ± 13.8 | 179.7 ± 53.5a |
| 30CR30 | 123.6 ± 22.1a | 85.9 ± 26.1a | 70.0 ± 21.7a | 81.3 ± 0.6 | 68.4 ± 12.6 | 206.4 ± 59.3a |
| NVCRNV | 94.1 ± 24.8b | 63.2 ± 17.9b | 50.1 ± 2.0b | 78.4 ± 7.4 | 67.0 ± 6.3 | 152.2 ± 40.4b |
| CV (%) | 19.2 | 29.3 | 30.5 | 5.5 | 16.9 | 27.9 |
Note
- Curvilinear velocity (VCL), average path velocity (VAP), straight line velocity (VSL), straightness (STR), wobble (WOB) and progression (PRO).
- Abbreviations: CV, Coefficient of variation.
- a,bMeans followed by different letters in the columns differ by the Scott–Knott test (p < 0.05).
3.2 Morphology sperm evaluations
Total, major and minor sperm morphological alterations observed in fresh sperm and sperm cryopreserved at different cooling rates are presented in Table 5. CRNV presented a higher major morphological change percentages. All treatments displayed higher minor morphological change percentages in relation to the controls, while the CRNV presents a higher percentage of total morphological alterations.
| Treatment | Morphological alterations (%) | ||
|---|---|---|---|
| Major | Minor | Total | |
| Fresh sperm | 11.2 ± 2.3d | 3.1 ± 1.3b | 14.4 ± 2.9c |
| Curve CR15 | 22.3 ± 3.1c | 6.8 ± 2.8a | 29.2 ± 3.7b |
| Curve CR30 | 25.0 ± 4.2b | 6.1 ± 2.0a | 31.5 ± 4.9b |
| Curve CRNV | 30.2 ± 5.3a | 5.3 ± 3.0a | 35.5 ± 4.9a |
| CV | 16.8 | 32.4 | 13.4 |
- Abbreviation: CV, Coefficient of variation.
- a,bMeans followed by different letters in the columns differ by Scott–Knott test (p < 0.05). Morphological changes (free normal head, distal/proximal droplet and simple bent tail) are considered minor alterations, while other alterations were considered major alterations.
3.3 Sperm DNA fragmentation
The DNA fragmentation results detected the comet assay are displayed in Table 6. The DI (damage index) was significantly higher in all treatments when compared to fresh sperm (p < 0.05). Not all “score” classes were observed, with control (fresh sperm) presenting a minimum level of fragmentation in class 3, while the other treatments presented all score classes. CR15 and CR30 displayed the highest DI and the highest levels of fragmentation as class 1 and 4.
| Treatment | DI | Cl 0 | Cl 1 | Cl 2 | Cl 3 | Cl 4 |
|---|---|---|---|---|---|---|
| F. sperm | 22 ± 10c | 82.2 ± 8.5a | 13.8 ± 7.3b | 3.8 ± 2.9c | 0.17 ± 0.4b | 0 ± 0c |
| Curve CR15 | 80.1 ± 11.7a | 61 ± 7.9c | 17.7 ± 5.1a | 7.6 ± 5.0b | 7.6 ± 1.8a | 6.1 ± 1.3a |
| Curve CR30 | 64.3 ± 14.6b | 68 ± 5.0b | 13.4 ± 2.3b | 8.1 ± 4.9b | 7.1 ± 2.1a | 3.3 ± 1.3b |
| Curve CRNV | 87.3 ± 21.3a | 57.3 ± 6.2c | 17.7 ± 4.0a | 11.1 ± 4.8a | 8.1 ± 2.6a | 5.7 ± 2.36a |
| CV (%) | 16.1 | 7.97 | 25.3 | 48.4 | 37.6 | 39.8 |
Note
- Different letters in the columns represent significant effects by the Scott–Knott test (p < 0.05).
- Abbreviations: Cl, Class of damage; CV, Coefficient of Variation; DI, Damage Index; F. sperm, Fresh Sperm.
4 DISCUSSION
Although changes in sperm quality are observed after cryopreservation, this is a guaranteed method for genetic material conservation (Tsai & Lin, 2012), since motility, sperm vigour and fertilization capacity can be recovered after thawing. This study indicates, for the first time, that an effect of temperature drop as a function of time is noted for P. lineatus, as greater motility for cooling rates at 15 and 30°C/min was observed when compared to the traditional cooling rate (CRNV, ~35.6°C/min). According to Harvey and Carolsfeld (1993), sperm freezing curves with liquid nitrogen require cooling rates between 10°C and 60°C/min and fast thawing. However, for most South American native fish species, ideal values within this range are still scarce, highlighting the importance of studies involving cooling rates.
Some authors have recommended methanol and egg yolk as cryoprotectants for P. lineatus, based on the subjective motility percentage of 67.79 ± 25% and motility duration of 74.26 ± 59 s using a standard freezing system applying a “dry-shipper” (Felizardo et al., 2010). Frankel et al. (2013) observed total motility of 10.06 ± 0.085% in a 40°C/min curve for post-thawed striped bass (Morone saxatilis) semen, significantly better than freezing at three slower rates (10, 15 and 20°C/min), using a computerized system. Indeed, it is possible that the use of a computerized system for motility assessments alongside a controlled cooling rate pattern may provide an explanation for the differences between previous results and those observed herein.
The influence of cooling rates on cryopreservation success is a well-known fact and has been described by several authors. Frankel et al. (2013) investigated the effect of freezing rates (10, 15, 20, and 40°C/min) on the viability of striped bass sperm (M. saxatilis) and concluded that the best rate was of 40°C/min. Yang, Norris, Winn, and Tiersch (2010) observed sensitivity regarding freezing rates, with a 5°C variation (10–15°C/min), determining a significant decrease in post-thaw motility (from 50 ± 10% to 17 ± 12%) using methanol as a cryoprotectant for Oryzias latipes sperm. The automated freezing process facilitates standardization and ensures a homogeneous and gradual fall of temperature. Furthermore, this freezing process avoids undesirable variations in the freezing curve, reducing the challenge confronted by sperm cells, such as lipid phase transitions in cell membranes (Drobnis et al., 1993), ice crystal formation (Watson & Morris, 1987) and changes to sperm mitochondria integrity (De Baulny, LeVern, Kerboeuf, & Maisse, 1997; Gandini, Lombardo, Lenzi, Spanò, & Dondero, 2006). A lower number of morphological sperm alterations and greater motility rates for controlled curves (CR15 and CR30) were observed herein.
Briefly, when the cooling rate is slower, cell damage may be associated with a longer exposure period to extremely high osmolalities. Thus, when slower cooling rates are applied, less cells could survive the osmotic stress to which they are exposed to during freezing. At higher cooling rates, the damaging factor of cell exposure to high osmolality is reduced, but, in this case, cells have less time for water and cryoprotectant transport across the membrane. However, fast cooling rates may lead to intracellular crystal formation and result in lethal cellular damage (Boryshpolets, Sochorová, Rodina, Linhart, & Dzyuba, 2017). An ideal cooling rate is slow enough to prevent intracellular ice formation and fast enough to decrease the time the cells are exposed to solution effects, factors possibly determined by the cooling rate 30°C in the present study for P. lineatus.
Bombardelli et al. (2011) used the CASA/ImageJ plug-in to evaluated fresh surubim-do-Iguaçu (Steindachneridion melanodermatum) sperm and observed 92.9 ± 6.0% motility, 169.5 ± 25.4 μm/s curvilinear velocity and 38.5 ± 1.3 µm/s straight line velocity. Carvalho, Araújo, Caneppele, and Viveiros (2011) observed 62.6–81.5 μm/s curvilinear velocity, 44.9–72.0 μm/s average path velocity and 26.6–60.0 μm/s straight line velocity for cryopreserved S. parahybae sperm. Wilson-Leedy and Ingermann (2007) observed 90 ± 6% motility, 104 ± 9 μm/s curvilinear velocity, 77 ± 15 μm/s average path velocity, 66 ± 17 μm/s straight line velocity and 74 ± 10% straightness for fresh D. rerio sperm. Viveiros et al. (2014) observed 75 ± 12% motility, 176 ± 48 μm/s curvilinear velocity, 97 ± 37 μm/s straight line velocity, 144 ± 51 μm/s average path velocity for fresh P. lineatus sperm using the Sperm Class Analyzer™ software (SCA™ 2010, Microptics, S.L. Version 5.1). The results indicate that sperm kinetic values vary according to species and type of sperm (fresh or frozen). To the best of our knowledge, the findings reported herein are the first reports of these sperm kinetics parameters for cryopreserved P. lineatus sperm through the CASA/ImageJ plug-in under different cooling rates and close to values cited for the other species.
The cryopreservation process may affect sperm morphology, leading to ultrastructural spermatozoa changes (Cabrita et al., 2010). Membrane disruption, mitochondrial function reduction, spiralization and/or axoneme rupture or adhesion can be characterized during the technique, leading to reduced motility and fertilization capacity (Marques, 2001). Moraes et al. (2004), Murgas, Silva, Mello, Santana, and Kabeya (1998) and Miliorini et al. (2011) estimated 9.54%, 40.2% and 3% of total alterations in fresh P. lineatus sperm, respectively. Galo et al. (2011) observed the degenerate tails and free head morphologies increased from 11.47% to 22.89% and from 3.70% to 40.80% in fresh and cryopreserved Brycon orbignyanus sperm, respectively. The morphological analysis of fish spermatozoa, thus, presents species-specific, genetic and environmental variations.
Sperm morphology is an important factor to be considered in the evaluation of thawed semen and varies considerably in different teleosts. However, when values exceed 50% (Miliorini et al., 2011) of sperm morphological alterations, breeding fish fertility may decrease and, thus, may not be recommended for fertilization. Herein, sperm alterations increased in frozen sperm, probably due to the toxic effect of the cryoprotectants and the freezing temperature on the sperm cell. Higher means of total and major sperm morphological alterations were found in the CRNV, which may be explained by the possible aforementioned challenges caused by uncontrolled temperature drops. However, none of the treatments exceeded 50% of sperm morphological alterations. Vasconcelos et al. (2015) did not verify significant differences between major and minor morphological changes when evaluating the effects of different cryoprotectors on P. lineatus semen freezing using the standard method (“dry-shipper + nitrogen cylinder”). However, these authors did not evaluate freezing curves and used dry-shipper (uncontrolled cooling rate) as a cryopreservation method.
The cooling rates tested herein caused sperm DNA fragmentation in P. lineatus sperm. One of the priorities in a cryopreservation protocol is to conserve spermatozoa genetic material, so that the information can be passed on to future generations (Cabrita et al., 2010). Zilli et al. observed a significant increase in per cent tail DNA' (% DNAT) from 32.7 ± 11.1 (fresh semen) to 38.2 ± 11.2 (cryopreserved semen) for Dicentrarchus labrax sperm. Other authors applying other assays (TUNEL – Terminal deoxynucleotidyl transferase-mediated dUTP-biotin end-labelling) indicate that interactions between DNA and protamines are compromised during cryopreservation (Gandini et al., 2006). In the present study, the cryopreservation protocol applying CR30 led to lower percentages of the highest DNA damage scores in P. lineatus sperm. These results demonstrate the ideal cooling rate for this species is probably 30°C/min. In addition, we can conclude that a controlled temperature drop would be a way to reduce DNA damage for this species, ensuring genetic conservation and correct embryo development. Tests on isolated sperm are not able to predict sample fertility, but the observation of several physical characteristics may determine greater potential fertility (Hafez & Hafez, 2004). Based on most of the parameters evaluated in the present study, CR30 (30°C/min) was positively evaluated compared to other treatments.
5 CONCLUSIONS
The present study suggests that the cooling rate for P. lineatus semen can be fixed at 30°C/min based on the best sperm motility, velocity average path, velocity straight line, morphology and DNA fragmentation percentages obtained at this rate. These findings indicate that cryopreserved semen can be used in artificial breeding programmes for this species, as the analysed variables are compatible with the minimum standards required to maintain post-thawed semen quality and viability.
ACKNOWLEDGEMENTS
The authors thank Flavio H. Siqueira and Manoel V. Oliveira (Estação de Piscicultura da Usina Hidrelétrica da CEMIG) for providing the broodstock and team accommodation, Gilson A. Azarias and Jailson M. Silva (Estação de Piscicultura da Usina Hidrelétrica da CEMIG) for assistance during animals manipulation, Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (150921/2014-4) and FUNDECC by the financial support of this project and CEMIG for providing fish and facilities used in this study. The authors would also like to thank the Biotério Multiusuário team at the Universidade Federal de Lavras.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare. The procedures involving animals were in accordance to the recommendation of the Ethics Committee of the Federal University of Lavras (Protocol no. 039/13).
AUTHOR CONTRIBUTIONS
Daniella: designed study, analysed data, conducting the experiment, drafted paper; Luis: orientation, designed study, drafted paper, revision; Tássia: drafted paper, conducting the experiment; Isadora: drafted paper, conducting the experiment; Rafael: designed study, analysed data; Silvana: designed study, orientation, revision.




