Effects of meiotic inhibitors and gonadotrophins on porcine oocytes in vitro maturation, fertilization and development
Contents
This study evaluated the effect of three reversible meiotic inhibitors (MINs) and their interaction with gonadotrophins (Gns) on the meiotic maturation and developmental competence of porcine oocytes. In experiment 1, the oocytes were matured for 22 hr in the presence or absence of dbcAMP (1 mM), cycloheximide (7 μM) or cilostamide (20 μM) with or without Gns, and for an additional 22 hr in the absence of MINs and Gns. At 22 hr of maturation, regardless of the presence of Gns, a higher proportion (p < .001) of oocytes cultured in the presence of MINs were effectively arrested at the germinal vesicle stage compared with the oocytes cultured without MINs. At 44 hr of maturation, the proportion of oocytes that reached MII was higher (p < .05) in groups with Gns compared with groups without Gns. In experiment 2, oocytes that were matured as in experiment 1 were inseminated and cultured for 7 days to evaluate fertilization parameters and blastocyst formation. Only oocytes from the dbcAMP + Gns group had higher (p < .05) efficiency of fertilization compared with the other treatment groups. The presence of dbcAMP during maturation also increased (p < .05) blastocyst formation and efficiency of blastocyst formation in both the presence and absence of Gns. These results indicate that the interaction of Gns with the tested MINs improved meiotic progression. In addition, regardless of supplementation with Gns, the presence of dbcAMP during the first maturation period increased and even doubled the capacity of oocytes to develop to the blastocyst stage.
1 INTRODUCTION
There is considerable and rapidly increasing interest in the production of large quantities of matured porcine oocytes and embryos through in vitro maturation (IVM) and fertilization (IVF) for basic scientific research and for many emerging reproductive technologies. As a consequence, significant developments have been made in IVM-IVF and embryo culture (IVC) systems by modifying the culture media and techniques used. However, despite major efforts, several problems remain unresolved, including a high incidence of polyspermy, a low rate of development and the low quality of blastocysts resulting from IVM–IVF (Gil et al., 2010; Grupen, 2014).
One of the critical aspects of in vitro porcine embryo production is the intrinsic quality of the oocytes. Most porcine IVM-IVF systems utilize immature oocytes recovered from the ovaries of slaughtered prepubertal gilts, which results in a mixture of oocytes at different growth phases and developmental stages. Moreover, it has been demonstrated that mammalian oocytes can resume meiosis spontaneously when they are removed from the follicle and cultured in vitro (Pincus & Enzmann, 1935), resulting in oocytes with asynchronous nuclear and cytoplasmic maturation. This is extremely important because adequate oocyte developmental competence requires synchronization between nuclear and cytoplasmic maturation. The heterogeneity of intrafollicular oocytes together with nuclear-cytoplasmic asynchrony has been considered largely responsible for the differences in developmental competence among individual in vitro matured porcine oocytes (Abeydeera, 2002).
Synchronization techniques based on the use of reversible meiotic inhibitors (MINs) in a “pre-maturation culture” or during the first 12–20 hr of in vitro maturation culture have been reported for the oocytes from different species, including humans (Lange Consiglio, Arrighi, & Cremonesi, 2010; Leal, Mamo, Fair, & Lonergan, 2012; Nogueira et al., 2006). The purpose of this inhibition is to temporarily block meiotic progression during maturation; the block is then removed to allow the oocytes to mature under in vitro conditions. In swine, specific MINs such as the protein synthesis inhibitor cycloheximide (Le Beux, Richard, & Sirard, 2003; Ye, Campbell, Craigon, & Luck, 2005; Ye, Flint, Campbell, & Luck, 2002), dibutyryl cyclic adenosine monophosphate (dbcAMP; Funahashi, Cantley, & Day, 1997), the cell cycle-dependent kinase inhibitor roscovitine (Romar & Funahashi, 2006) and the phosphodiesterase inhibitors such as cilostamide (Dieci et al., 2013; Laforest, Pouliot, Gueguen, & Richard, 2005; Sasseville, Cote, Guillemette, & Richard, 2006) have resulted in fully reversible inhibition of meiosis.
In addition, to mimic in vivo conditions, in which meiotic resumption and subsequent ovulation are controlled by gonadotrophins (Gns), porcine IVM media are supplemented with pituitary FSH and LH or non-pituitary gonadotrophins (equine and human chorionic gonadotrophin—eCG, and human chorionic gonadotrophin—hCG). Although the precise mechanism of meiotic resumption by gonadotrophins is not fully understood, it is widely assumed that porcine oocytes need to be exposed to gonadotrophins for the resumption and progression of meiosis. As Funahashi and Day (1993) first demonstrated that the removal of hormone supplements from the maturation medium at 20 hr after the start of culture improved the ability of porcine oocytes to form the male pronucleus, most laboratories supplement IVM media with Gns in the primary phase (the first 20 hr of maturation) but not in the secondary phase (the last 20 hr of maturation).
Given the important role of Gns and MINs during oocyte maturation, most meiotic inhibitor studies add Gns to the maturation medium to resume meiosis. Yet, the use of a Gns-free medium is crucial for understanding the potential of MINs in IVM. Thus, the aim of this study was to assess the effect of three MINs (dbcAMP, cycloheximide and cilostamide) and their interactions with Gns on the meiotic maturation and developmental competence of porcine oocytes.
2 MATERIAL AND METHODS
All experimental procedures used in this study were performed in accordance with the 2010/63/EU EEC Directive for animal experiments and were reviewed and approved by the Ethical Committee for Experimentation with Animals of the University of Murcia, Spain.
2.1 Culture media
Unless otherwise stated, all chemicals used in this study were purchased from Sigma-Aldrich Co. (Alcobendas, Madrid, Spain).
The media used were essentially the same as those previously described by Gil et al. (2003). Dulbecco's phosphate-buffered saline medium (mDPBS) was used for the collection of the cumulus–oocyte complexes, protein-free tissue culture medium 199 (TCM199) was used for oocyte maturation, modified Tris-buffered medium (mTBM) supplemented with 1 mM caffeine and 0.2% BSA was used for fertilization, and North Carolina State University 23 (NCSU 23) containing 0.4% BSA was used for embryo culture.
2.2 Oocyte recovery and maturation
Prepubertal gilt ovaries were collected from a local slaughterhouse and transported to the laboratory at 35°C in 0.9% (w/v) NaCl with 70 μg/ml of kanamycin. The ovaries were rinsed in a NaCl solution, and medium-sized follicles (3–6 mm in diameter) were punctured using a single sterile scalpel blade. Oocytes with a compact cumulus mass and a dark, evenly granulated cytoplasm (COCs) were washed, and 70–75 oocytes were transferred into each well of a four-well multidish (Nunc, Roskilde, Denmark) containing 500 μl of maturation medium supplemented with 10 IU/ml eCG (Folligon; Intervet International B.V., Boxmeer, the Netherlands) and 10 IU/ml hCG (VeterinCorion; Divasa Farmavic, S.A., Barcelona, Spain) for 22 hr. The oocytes were then incubated for another 20–22 hr in the maturation medium without hormones. Oocyte maturation was carried out under mineral oil at 39°C in a humidified atmosphere of 5% CO2.
2.3 In vitro fertilization
After maturation, the COCs were denuded with 0.1% hyaluronidase in maturation medium by vortexing for 2 min at 1,660 revolutions/min and were washed in fertilization medium. After washing, 30–40 denuded oocytes were placed into 50 μl of the same medium in a 35 × 10 mm Petri dish (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ, USA) under mineral oil and held at 39°C in an atmosphere of 5% CO2 for approximately 30 min prior to the addition of spermatozoa. For each replicate, one pool of semen from two straws was thawed in a circulating water bath at 37°C for 20 s and washed three times by centrifugation at 1900 × g for 3 min in mDPBS. The resulting pellet was resuspended in fertilization medium, and after the appropriate dilution, 50 μl of this sperm suspension was added to 50 μl of the fertilization medium containing the oocytes such that each oocyte was exposed to 1,000 spermatozoa. The gametes were co-incubated at 39°C in a humidified atmosphere of 5% CO2 for approximately 5 hr.
2.4 Embryo culture
The presumptive zygotes were removed from the fertilization medium and transferred to a four-well multidish (30 zygotes per well), with each well containing 500 μl of embryo culture medium under mineral oil. Presumptive zygotes were cultured for the first 2 days in glucose-free NCSU-23 supplemented with 0.33 mM pyruvate and 4.5 mM lactate and then in fresh NCSU-23 medium containing 5.5 mM glucose until day 7 (day 0 = day of fertilization). Embryo culture was carried out under mineral oil at 39°C in a humidified atmosphere of 5% CO2.
2.5 Assessment of maturation
To evaluate maturation, the oocytes were mounted on slides, fixed in a solution of acetic acid:ethanol (1:3) for 48–72 hr at room temperature, stained with 1% lacmoid in 45% (v:v) acetic acid and examined under a phase-contrast microscope at magnification ×400. The nuclear maturation levels of the oocytes were classified as (i) the germinal vesicle (GV) stage, in which the chromatin is enclosed in a nuclear membrane and is either filamentous or highly condensed (ii) the intermediate stage, in which the nuclear membrane of the oocytes has disappeared and the chromatin is condensed or organized in metaphase I; or (iii) the mature stage, in which the chromosomes are organized in metaphase with an extruded first polar body (metaphase II, MII). The maturation rate was expressed as the ratio of the number of MII-stage oocytes to the total number of oocytes cultured.
2.6 Assessment of sperm penetration and embryo development
To evaluate fertilization parameters, a randomly selected set of the presumptive zygotes were fixed and stained as described above. The oocytes were considered penetrated when they contained one or more swollen sperm heads and/or male pronuclei with their corresponding sperm tails and two polar bodies. The fertilization parameters evaluated were the post-fertilization maturation rate (matured and/or penetrated oocytes/total inseminated), the penetration rate (oocytes penetrated/total matured), monospermy (oocytes containing only one male pronucleus/total penetrated) and the efficiency of fertilization (monospermic oocytes/total inseminated).
At 2 and 7 days post-insemination, the cleavage rate (oocytes cleaved to two to four cells/total cultivated) and blastocyst formation (blastocysts/total cultured) were evaluated under a stereomicroscope. The total cell number, as an indicator of embryo quality, was evaluated by mounting each blastocyst on a slide in 4 μl of Vectashield (Vector Laboratories, Burlingame, CA, USA) containing 10 μg/ml Hoechst-33342 under a coverslip. Samples were analysed by fluorescence microscopy.
2.7 Experimental design
2.7.1 Experiment 1. Interactive effects of MINs and Gns on nuclear maturation of oocytes
This experiment was designed to evaluate the effects of the three MINs and their interaction with Gns on meiotic maturation. A total of 2,058 oocytes were matured for 22 hr in the absence (control) or presence of dbcAMP (1 mM), cilostamide (20 μM) (Enzo Life Sciences, Madrid, Spain) or cycloheximide (7 μM) with or without Gns, and for another 22 hr in the absence of MINs and Gns. Nuclear progression was assessed at 22 and 44 hr of maturation.
2.7.2 Experiment 2. Interactive effects of MINs and Gns on developmental competence of oocytes
In this experiment, 2,515 oocytes matured as in experiment 1 were inseminated and cultured to evaluate their developmental competency. In each replicate, a random subset of presumed zygotes was fixed and stained at 18 hr post-insemination (n = 1,155) to assess fertilization parameters. The remaining presumed zygotes (n = 1,360) were cultured to assess the in vitro embryo development at 2 and 7 days of culture.
2.8 Statistical analysis
The data were analysed using IBM SPSS 19 Statistics (SPSS, Chicago, IL, USA). The mean ± standard deviation (SD) of the binary variables (maturation, penetration and monospermic rates, efficiency data, cleavage rates and blastocyst formation) was determined after calculating the proportion in each group and in each replicate. The Kolmogorov–Smirnov test was used to assess the assumption of normality, and the groups were compared using a mixed-model ANOVA. The ANOVA model included the fixed effects of MINs, Gns supplementation, their interaction and the random effect of the replicate in each experiment. When the ANOVA revealed a significant effect, the values were compared using a Bonferroni test. The threshold for significance was set at p < .05. The results are expressed as the mean ± SD.
3 RESULTS
3.1 Interactive effects of MINs and Gns on nuclear maturation of porcine oocytes
The presence of MINs affected (p < .005) the proportion of oocytes in the GV and intermediate stages at 22 hr of maturation. In contrast, the supplementation of Gns and the MIN-Gn interaction had no significant effect on these parameters. In the absence of MINs, the Gns produced a decrease (p < .02) in the proportion of oocytes remaining at the GV stage. Yet, most oocytes cultured in the presence of MINs were arrested at the GV stage (range: 93.1 ± 5.7%–99.4 ± 1.2%) regardless of the presence of Gns in the medium (Figure 1a).
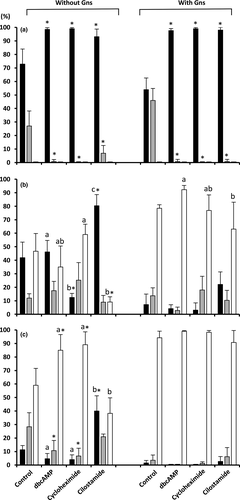
The maturation rates of oocytes cultured in the absence or presence of MINs with or without Gns at 44 hr are shown in Figure 1b. There was a significant effect of MINs, Gns and the interaction between MINs and Gns on the proportion of oocytes at the MII and GV stages. At 44 hr, the rates of oocytes reaching MII in the groups cultured with Gns were higher (p < .03) compared with those without Gns regardless of the presence or absence of MINs during culture. Only 46.6 ± 13.2% of the oocytes cultured in the absence of Gns and MINs achieved the MII stage by the end of culture, and the addition of MINs to the maturation medium was insufficient to increase that percentage. In the absence of Gns, the cultures supplemented with cilostamide showed a larger number of oocytes at the GV stage (80.3 ± 8.3%) and consequently a lower number of oocytes at the MII stage (9.1 ± 3.8%) than the controls (p < .05).
In the presence of Gns, 78.5 ± 2.6% of the oocytes cultured without MINs were at MII by the end of culturing. The supplementation of the maturation medium containing MINs failed to increase the rates of nuclear maturation (range: 63.1 ± 19.9%–92.3 ± 3.1%), although the dbcAMP inhibitor increased (p < .05) the proportion of MII oocytes compared with the cilostamide-treated group (Figure 1b).
3.2 Interactive effects of MINs and Gns on developmental competence of oocytes
After IVF (Figure 1c), the oocyte maturation rates were affected by MINs, Gns and the interaction between both factors (p < .001). Notably, following fertilization, the oocytes cultured without Gns and in the presence of dbcAMP and cycloheximide increased (p < .05) the maturation rate compared with the control oocytes without Gns by 1.5- to 2.5-fold. In contrast, this rate remained very low (p < .02) in oocytes cultured in the presence of cilostamide and without Gns (38.2 ± 11.6%).
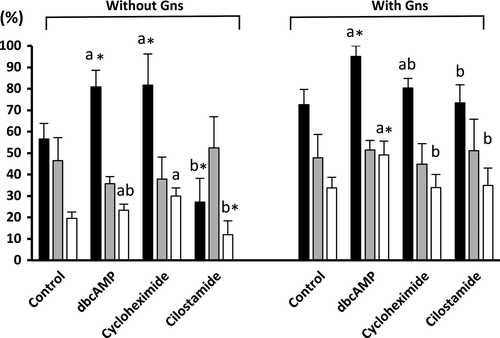
The presence of MINs, Gns and the interaction between both factors (p < .01) affected the penetration rate and the efficiency of fertilization of the inseminated oocytes. As shown in Figure 2, in the presence of Gns, only oocytes cultured in the presence of dbcAMP resulted in a higher (p < .05) penetration rate (95.5 ± 6.5%) and efficiency of fertilization (49.4 ± 6.4%) compared with those cultured with Gns in the absence of MINs (72.6 ± 7.1% and 33.7 ± 5.0%, respectively). In the absence of Gns, although oocytes cultured with dbcAMP and cycloheximide showed higher penetration rates (p < .05) than oocytes cultured without MINs, no difference was observed in the efficiency of fertilization (23.4 ± 2.8%, 30.0 ± 3.7% and 19.6 ± 2.9%, respectively). Again, decreased penetration rates and efficiency of fertilization (p < .05) were observed in the group of oocytes cultured in the presence of cilostamide without Gns (27.2 ± 11.1% and 12.0 ± 6.4%, respectively) compared with oocytes cultured in absence of MINs and Gns (56.5 ± 7.3% and 19.6 ± 2.9%, respectively).
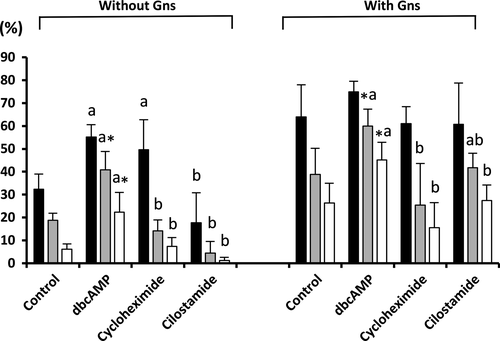
The presence of MINs, Gns and the interaction between both factors affected (p < .05) all of the embryo development parameters evaluated. As shown in Figure 3, the presence of dbcAMP during maturation increased (p < .05) blastocyst formation rates and the efficiency of blastocyst formation in both the presence and absence of Gns compared with the controls. Moreover, the oocytes cultured with dbcAMP and Gns had a higher (p < .001) efficiency of blastocyst formation (45.1 ± 7.7%) compared with the other treatment groups (range: 1.2 ± 1.4%–27.4 ± 6.8%). No differences were observed in the total number of blastocyst cells among the groups (range: 31.7 ± 2.8–46.3 ± 5.8).
4 DISCUSSION
Maintaining oocytes at the germinal vesicle stage could improve the synchrony of nuclear and cytoplasmic maturation and therefore the developmental competence of the oocytes. In the present study, we tested three different MINs: dbcAMP, cycloheximide and cilostamide. Although the effects of these MINs on in vitro maturation have already been studied in porcine oocytes, we were particularly interested in evaluating both the intrinsic potential of these MINs, which can only be evaluated in the absence of Gns, and the interaction between these MINs and Gns on the maturation and developmental competence of the oocytes, which has not been fully investigated. The MIN concentrations used for the experiments were chosen based on those used in previous studies with porcine oocytes (Funahashi et al., 1997; Laforest et al., 2005; Le Beux et al., 2003). We used a chemically defined medium for the IVM of oocytes for a precise analysis of the physical actions of the inhibitors and hormones on oocyte maturation.
As expected, the three MINs tested were able to block the spontaneous resumption of meiosis regardless of the presence/absence of Gns. At 22 hr of maturation, the groups treated with MINs comprised a significantly more homogeneous population of oocytes than the untreated groups because a higher proportion of oocytes (>93%) were arrested at the GV stage compared with the oocytes cultured in the absence of MINs. Meiotic resumption is controlled by phosphatases that lead to the activation of M-phase promoting factor (MPF) and is associated with a decreased intracellular concentration of cAMP and subsequent inactivation of protein kinase A (Kim & Menino, 1995). Consequently, the addition of cAMP in the maturation medium inhibits meiosis resumption. Cilostamide also indirectly increases cAMP. It is a specific phosphodiesterase inhibitor, and it is well documented that phosphodiesterases influence oocyte maturation by decreasing cAMP levels (Sasseville et al., 2006). Finally, the mechanism of action of cycloheximide is to inhibit protein synthesis, which is necessary to activate MPF (Motlik & Kubelka, 1990). Therefore, although different mechanisms of action are involved, all the MINs used in this study prevented meiotic resumption.
In contrast to the MIN groups, the groups cultured in the absence of MINs contained a heterogeneous population of oocytes. That heterogeneity was higher in the presence than in the absence of Gns (more than 45% and 27%, respectively, had progressed to an intermediate stage at 22 hr of culture). These results confirm the asynchronous meiotic progression repeatedly observed during the IVM of porcine oocytes.
Previous studies have shown the reversibility of these MINs in the presence of Gns. However, in the present study, we have demonstrated that in the absence of Gns, the inhibitory effect of dbcAMP and cycloheximide is also fully reversible because the GV-stage oocytes were capable of the same final maturation rate as non-arrested oocytes and oocytes cultured without Gns. This reversibility was not observed in the cilostamide group. At 44 hr of maturation, few of the oocytes cultured with cilostamide but without Gns had reached the MII stage, and almost all oocytes were still arrested at the GV stage (80.3%). The reason for this cilostamide-maintained GV stage is presently unclear, and interestingly, it was not observed in the presence of Gns, suggesting that Gns are needed for the reversion of the cilostamide-induced blocking of meiosis.
The present study revealed that the proportion of oocytes that reached MII at 44 hr of maturation in the groups cultured with Gns was significantly higher compared with the groups without Gns, regardless of the presence or absence of MINs during culture. Two factors should be taken into account. First, our results support the hypothesis that in swine, spontaneous resumption oocyte meiosis or GVBD is substantially slower than in any other mammal (Santiquet, Robert, & Richard, 2013), and the greatest maturation occurs in the presence of Gns. Second, our results suggest that the presence of MINs themselves in the maturation medium does not improve the rate of nuclear maturation. Also, the presence of Gns during the first half of culture is essential to maintain the inter- and intracellular processes required to support nuclear maturation on time when meiosis is blocked by the MINs. As mentioned above, most MIN studies add Gns to the maturation medium. Only Akaki, Yoshioka, Noguchi, Hoshi, and Funahashi (2009) reported that in the absence of Gns, the addition of epidermal growth factor (EGF) to the maturation medium worked with dbcAMP to improve the nuclear maturation of porcine oocytes, resulting in levels of maturation similar to oocytes cultured in a standard IVM system with Gns and dbcAMP. It has been demonstrated that EGF functionally mimics the action of FSH, and its exogenous supplementation into an IVM medium can replace FSH for the purpose of nuclear maturation, having a synergistic effect with FSH on cytoplasmic maturation of porcine oocytes (Uhm et al., 2010). Our basic IVM medium was also supplemented with the same concentration of EGF (10 ng/ml); however, it did not stimulate meiotic resumption and progression to MII with or without MINs to the same degree as Gns. Differences in the chemically defined IVM medium and the supplements used could at least partially explain the differences found between these studies.
Interestingly, after 18 hr of IVF, the maturation rates of oocytes incubated with dbcAMP and cycloheximide in the absence of Gns were higher than those achieved before IVF and similar to those obtained in the presence of Gns at 44 hr of maturation. These results suggest that in addition to inducing meiotic resumption, Gns interacts with some MINs to accelerate meiotic progression. Only 22 hr was needed for oocytes cultured with MINs and Gns to mature from GV to MII, whereas oocytes incubated with MINs but without Gns required more time. It should also be noted that in the absence of Gns, the rate of later maturation (after 18 hr of IVF) was approximately 1.5 times higher in the presence of dbcAMP and cycloheximide than in the absence of these two MINs. The outcome of the retarded meiotic progression produced by these MINs in the absence of Gns is questionable. According to Son, Lee, and Lim (2005), the delay of meiotic progression results in a negative outcome, and embryos derived from late-maturing oocytes are of lower quality compared with oocytes that mature on time.
If, as mentioned above, these oocytes cultured in the absence of MINs contained a heterogeneous population of oocytes, this asynchrony may have resulted in the susceptibility of some of these oocytes to the effects of ageing. It is well documented that the ageing of oocytes results in some cytoskeletal anomalies (Somfai et al., 2011) and decreases MPF/histone H1 kinase activity (Kikuchi et al., 2000), which probably leads to polyspermic fertilization and defects in early development. Presumably, the in vitro synchronization of nuclear maturation should prevent ageing and it should also allow more time for some of the oocytes to undergo structural changes, to accumulate more molecules essential for early development and therefore potentially improve the efficacy of in vitro embryo production (Dieleman et al., 2002). Nevertheless, in our experiment, meiotic inhibition by cycloheximide and cilostamide did not improve the efficiency of fertilization as expected; only dbcAMP + Gns was found to improve the efficiency of fertilization and the development of oocytes to the blastocyst stage.
Previous studies have demonstrated that oocytes pre-treated with cycloheximide and dbcAMP can be fertilized successfully and develop to the blastocyst stage. Our results are consistent with studies reporting an improvement in the fertilization parameters using dbcAMP (Kim et al., 2008; Somfai et al., 2003) and with those that showed no effect with cycloheximide (Ye et al., 2005). To the best of our knowledge, this is the first study to report the fertilization parameters of porcine oocytes cultured in the presence of cilostamide. We did not find any beneficial effect on the efficiency of fertilization when meiosis was synchronized with this MIN culture in the presence of Gns. Moreover, in the absence of Gns, cilostamide produced a negative effect on the fertilization parameters, probably due to the deficient maturation rate observed. Regarding embryo development to the blastocyst stage, our results are also consistent with studies that showed no significant effect after the parthenogenetic activation of oocytes treated with cilostamide (Dieci et al., 2013). However, in contrast to Ye et al. (2005), who observed a higher proportion of oocytes developing to the blastocyst stage following cycloheximide pre-treatment, we did not find any beneficial effect from the cycloheximide culture. Differences in the experimental conditions could explain the improvement in oocyte developmental competence found by those authors. In the study of Ye et al. (2005), the oocytes were treated with 5 μg/ml of cycloheximide for 12 hr and then matured on a medium supplemented with FSH for 36 hr. In our experiment, we cultured the oocytes for 22 hr with 2 μg/ml of cycloheximide in the presence or absence of Gns and for 22 hr in the absence of Gns.
Interestingly, the efficiency of blastocyst formation in the oocytes treated with dbcAMP and Gns was almost twice (45.1%) that of oocytes cultured only with Gns (26.3%). These results are consistent with previous studies that demonstrated that the temporary arrest of meiosis by dbcAMP increases the incidence of porcine embryos that develop to the blastocyst stage (Kim et al., 2008; Somfai et al., 2003). Moreover, in our study, although cycloheximide and cilostamide inhibitors in combination with Gns did not affect oocyte development to the blastocyst stage, those MINs in the absence of Gns produced low blastocyst outcomes (1.2% and 7.4%, respectively), which might be due to an inadequate cytoplasmic ability to support embryo development. We also noted that in the absence of Gns, only the culture with dbcAMP was able to improve the efficiency of blastocyst formation at rates similar to the group of oocytes matured in a standard IVM system with Gns. This evidence confirms the importance of dbcAMP as a key molecule controlling meiotic resumption and the developmental capacity of porcine oocytes.
In summary, in the present study, we demonstrate that Gns interaction with the three MINs tested accelerated meiotic progression to the MII stage and that oocytes cultured with cycloheximide and cilostamide required Gns for adequate fertilization and subsequent embryonic development. In addition, our results confirm the intrinsic role of dbcAMP as a regulator of the porcine oocyte meiotic cell cycle. Regardless of the presence/absence of Gns, the presence of dbcAMP during the first period of maturation may increase or even double the capacity for oocyte development to the blastocyst stage.
ACKNOWLEDGEMENTS
The Fundación Séneca (19892/GERM/15), Murcia, Spain, supported this study. MINECO (Madrid, Spain) is acknowledged for grant-based support to C.A. Martinez and A. Nohalez (BES-2013-064087 and BES-2013-064069, respectively). CONACYT is acknowledged for financial assistance granted to J.R. Ake-Villanueva (ID# 730037).
DECLARATION OF INTERESTS
None of the authors have any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
All authors contributed equally.




