Characterization of mesenchymal stem cells in bovine endometrium during follicular phase of oestrous cycle
Contents
Stem cells have been postulated as responsible for cell regeneration in highly and continuously regenerative tissues such as the endometrium. Few studies in cattle have identified and specified the presence of stem cells in the endometrium during the oestrous cycle. The aim of this study was to investigate the presence of mesenchymal stem cells (MSCs) in the bovine endometrium during the follicular phase (FP) of the oestrous cycle. Uterine tissue was collected in the time-frame comprising day 18 of the cycle and ovulation (day 0). We isolated, cultured and expanded four primary cell lines from endometrium and identified byRT-qPCR the expression of OCT4, SOX2 but not NANOG (undifferentiated/embryonic markers), CD44 (MSCs marker) and c-KIT (stem cell marker) genes; and the encoded Oct4, Sox2 and Cd44 proteins by Western blot or immunostaining of paraffin-embedded tissue in endometrium. We demonstrated that cells isolated from bovine endometrium displayed essentially the same gene expression pattern; however, at the protein level, Oct4 and Cd44 were not detected. Besides, they showed typical functional characteristics of MSCs such as fibroblast-like morphology, plastic adherence, high proliferative capacity, clone formation in vitro and the ability to differentiate into chondrogenic, osteogenic and adipogenic lineages. We obtained for the first time an extensive characterization of undifferentiated cells populations contained in the bovine endometrium during the FP of the oestrous cycle.
1 INTRODUCTION
The endometrium is a highly regenerative tissue which undergoes a large number of growth cycles, differentiation and shedding during women's reproductive life (Gargett, 2007). Meanwhile during the bovine oestrous cycle, the endometrium goes through remodelling implying morphological and functional changes with cyclic patterns of cell proliferation and apoptosis (Arai, Yoshioka, Tasaki, & Okuda, 2013). This has been observed in distinct dynamic expression profiles and numerous differentially expressed genes in the bovine endometrium during the oestrous cycle related to extracellular matrix remodelling transport and cell growth among others (Mitko et al., 2008). The cyclic endometrial modifications are globally controlled by variations in oestradiol and progesterone concentrations (Henriet, Chevronnay, & Marbaix, 2012). From the follicular phase (FP) to the early luteal stage, the percentage of positive proliferation marker cells is higher in all types of endometrial cells that may result from oestradiol-17B-(E2) stimulated growth factors (Arai et al., 2013). Likewise, an increase in follicular size in each follicular wave, meaning a maximum size of preovulatory follicle, has been associated with an E2 plasmatic concentration peak (Chasombat, Nagai, Parnpai, & Vongpralub, 2014).
The wide cell renewal ability present in endometrial tissue is similar to the cellular turnover in bone marrow hematopoietic tissue, epidermis and intestinal epithelium which are known for being highly and continuously regenerative tissues and where adult stem cells are responsible for cellular production conforming a small population of stromal and endometrial cells with similar properties and phenotypes to bone marrow or adipose tissue mesenchymal stem cells (MSCs) (Gargett & Masuda, 2010). Stem cells are rare undifferentiated cells present in many adult tissues and organs within cell niches and their behaviour is regulated by the coordination of intrinsic and extrinsic cellular mechanisms which regulate the balance of self-renewal and differentiation in them (Li & Xie, 2005). Most adult stem cells lack distinctive morphological characteristics which makes hard to locate them in tissues, and these are defined by their functional properties rather than their marker expression patterns (Gargett, 2007). The functional properties of MSCs are plastic adherence ability, colony formation and multipotency by differentiating in vitro into mesodermal-derived lineages such as adipogenic, chondrogenic and osteogenic with a characteristic surface phenotype (Dominici et al., 2006).
Stem cells have been identified in the endometrium of humans, mice, monkeys (Cervelló, Martinez-Conejero, Horcajadas, Pellicer, & Simon, 2007; Chan & Gargett, 2006; Padykula, 1991) and farm animals, such as pigs, sheep and cows (Bodek, Bukowska, Wisniewska, & Ziecik, 2015; Donofrio, Franceschi, Capocefalo, Cavirani, & Sheldon, 2008; Letouzey et al., 2015; Mehrabani et al., 2015; Miernik & Karasinski, 2012; Nogueira de Moraes et al., 2016). There are few studies in cattle that have identified putative mesenchymal progenitor stem cells in the endometrium, and we were the first to report the presence of these cells in the early and late luteal phase of the oestrous cycle (Cabezas et al., 2014). The objective of this work was to investigate the presence of MSCs in the bovine endometrium during the FP, through culture isolation, characterization and determination of the molecular properties from uteri of slaughtered cycling cows. These findings might contribute to better understanding of their presence in the endometrium throughout the oestrous cycle, and the biological properties and therapeutic potential of bovine MSCs.
2 MATERIALS AND METHODS
All the experimental procedures involving animal handling and/or sampling were approved by the Bioethics Committee of the Universidad de Concepción, Chile under the approval number CE-6-2010 and were conducted according to the normative for Animal Well-being of the Faculty of Veterinary Sciences of same university. All unspecified reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.1 Uterine sampling
Genital tracts were collected from healthy non-pregnant Angus cows (n = 4) slaughtered in a local animal abattoir. The cycling status was determined by evaluation of both ovaries following the criteria of Ireland, Murphee, and Coulson (1980) and confirmed by RIA quantification of serum progesterone and E2. It is considered for the FP those surrounding the day 18 to the ovulation or day 0 of the oestrous cycle. The uterine sampling was obtained from horn ipsilateral to the corpus luteum containing caruncular regions and intercaruncular and washed twice with phosphate-buffered saline (PBS) supplemented with 1% antibiotic antimicotic (AAM) solution (10 IU/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B.
2.2 Cell isolation
Tissue samples were digested with sterile 1 mg/ml collagenase type I, in PBS for 2 hr at a temperature of 37°C, centrifuged at 200 g for 10 min. Cells were plated at a density of 0.5 × 105 cells/ml in DMEM-F12 with 10% foetal calf serum (FCS) supplemented with 1% AAM solution, 1 mM of pyruvate and 2 mM of l-glutamine, and cells were cultured in a chamber with 5% CO2, 39°C and 100% humidity. To separate the epithelial cells from stromal cells, the supernatant after first seeding was removed approximately 18 hr after and re-seeded in the same way, later then medium was changed every 2–3 days until cells reached confluence.
2.3 Colony formation
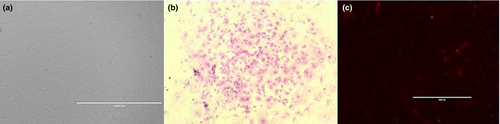
The cells were seeded in 60 mm plates at density of 30 cells/cm2 in same environmental and medium conditions that in primary cells culture, changing medium every 15 days. The experiments were performed in triplicate for all the cell lines derived. The plates were microscopically examined to make sure that each colony was originated from a single cell. After 1 month were fixed with 2% paraformaldehyde (PFA) and stained with Giemsa to visualize clones. Colonies containing more than 20 cells were counted. To evaluate the ability of cells to form colonies or clonal efficiency (CE), the following formula was used: (number of clonal cells/number of inoculated cells) × 100% (Chan, Schwab, & Gargett, 2004). Colonies were stained with alkaline phosphatase (AP) according to protocol (Vector® Red substrate; Vector Laboratories, Burlingame, CA, USA) with a positive AP staining which was characterized by a red coloration and observed with digital inverted microscope fluorescence EVOS FL (Life Technologies, Carlsbad, CA, USA).
2.4 Cell proliferation
Cells were seeded in triplicate in 60 mm plates at density of 2,000 cells/cm2 and cultured at same environmental and medium conditions that in primary cells culture. Cell-doubling time (CDT) was calculated using the following formula: CD = ln (Nf/Ni)/ln 2, with Nf being the final and Ni the initial number of cells and DT = CT/CD, where DT is the CDT and CD is cell-doubling number, being CT is cell culture time (Vidal et al., 2006).
2.5 In vitro differentiation into mesodermal derivatives
Cells were cultured in passage 3 at 4 × 104 cells/per well in six-well dishes in triplicates, standard conditions until they reached 90% confluence and then changed to differentiation media (DM). Differentiation media were based on DMEM low glucose with 10% FCS and supplemented to induce chondrogenic lineage (CL), with 100 nM dexamethasone (Dex), 35 μg/ml vitamin C, 1× insulin selenium transferrin (ITS), osteogenic linage (OL) with 50.9 μM Dex, 10 mM β-glycerophosphate, 0.1 mM vitamin C, and adipogenic linage (AL) with 1 μM Dex, 22 μg/ml 1× ITS, 0.25 mM 3-isobutyl-1methylxanthine (3-IBM), 100 mM indometacin. Control time-mated cells were cultured in DMEM low glucose + 10% FCS, without inducers or other supplements for exactly the same time periods as experimental groups. In all cases, media were changed every 3 days. At days 0, 7 and 14, cells were fixed with 2% PFA and stained with Alcian blue for CL, alizarin red for OL and Oil Red for AL to detect the expression of glycosaminoglycan, calcium deposition or lipid vacuoles, respectively.
2.6 RT-PCR
The total RNA was extracted with tri-reagent extraction according to manufacturer's instructions. The DNA was transcribed from 200 μg of total RNA with M-MLV reverse transcriptase. Gene expression analysis was performed by real-time PCR using the standard curve method. In all qPCRs, beta-actin (ACTB) was used as an internal control. Only those PCR experiments with an efficiency within the range of 90–110% with a correlation coefficient of 0.97 were used for gene expression analysis. Also, all samples within the quantification range of the standard curve were considered for the analysis. Samples were run in triplicates using SYBR Green on MX3000P real-time PCR device (Agilent, Santa Clara, CA, USA). PCR was performed with the primer sequences as shown in Table 2.
2.7 Immunohistochemistry
Fresh tissue samples were sectioned at 4 μm and mounted on poly-L-lysine-covered slides. Antibodies against the same proteins as above were used for in situ detection in paraffin-embedded fixed samples, following routine procedures published (Gómez-Villamandos et al., 1998). In addition, vimentin and cytokeratin were used as markers of correct immunoreactivity of the uterine biopsies and marked stromal and epithelial cells, respectively. The following primary antibodies and dilutions were used: Oct-4, mouse monoclonal (Santa Cruz Biotechnologies, Dallas, TX, USA; sc-9081, clon H-134), dilution 1:200; Sox-2, mouse monoclonal (Santa Cruz Biotechnologies; sc-20088), dilution 1:200; Cd44, goat polyclonal (Santa Cruz Biotechnologies; sc-31043), dilution 1:100; cytokeratin, mouse monoclonal (Dako, Santiago de Chile, Chile; M3515 clone AE1/AE3), dilution 1:300; and vimentin, mouse monoclonal (Dako; M0725, clone V6), dilution 1:300. Final steps included enzymatic incubation using I mm PRESS Universal Antibody (anti-mouse Ig/anti-rabbit Ig, peroxidase).
2.8 Western blot (WB)
The extraction of protein samples was performed by RIPA buffer (25 mM Tris–HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxy cholate, 0.1% SDS) and separated on SDS–PAGE gel (10% acrylamide), transferred onto nitrocellulose membranes and subjected to WB analysis with the same primary antibodies used in immunohistochemistry. Secondary anti-species antibodies conjugated to horseradish peroxidase were from Santa Cruz Biotechnologies, donkey anti-goat IgG-HRP: sc-2033 and donkey anti-mouse IgG-HRP: sc-2318. All WBs were normalized with ACTB.
2.9 Data analysis
Statistical analysis of quantitative real-time PCRs, clonogenic and proliferation assays was conducted using a nonparametric test (Kruskal–Wallis), and the data were expressed as mean ± error. All statistical analyses were tested for α = 0.05.
3 RESULTS
3.1 Ability to form colonies and proliferate
Cells showed a fibroblast-like morphology, adherence to plastic and when seeded at low confluence proliferated and produced colonies (Figure 1). The accounted colonies yielded 4.25 ± 0.49 clones per dish and CE = 0.51 ± 0.06%; meanwhile, the average DT was of 33.2 ± 0.86 hr, showing no differences between cell lines (Table 1).
| Cell line | Numbers of colonies | CE (%) | CDT (hours) |
|---|---|---|---|
| FP1 | 5.3 | 0.63 | 31.2 |
| FP2 | 3 | 0.36 | 35.2 |
| FP3 | 4.7 | 0.56 | 32.5 |
| FP4 | 4 | 0.48 | 33.9 |
3.2 Multilineage in vitro differentiation
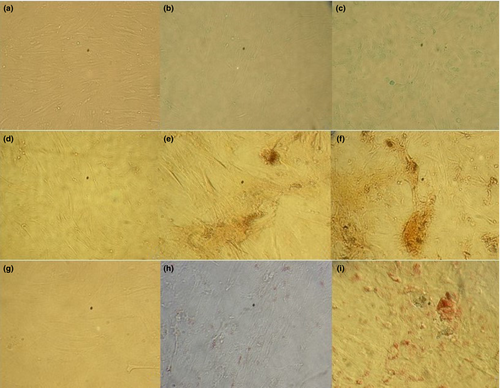
Cells were differentiated in vitro into chondrogenic, osteogenic and adipogenic lineages being observed a higher intensity of cell staining at day 14 than at day 7. No staining was observed in non-induced stained controls (Figure 2). Additionally, through reverse transcriptase–reverse transcription quantitative PCR (RT-qPCR) and agarose gel electrophoresis, the presence of AGGRECAN (chondroitin sulphate proteoglycan 1) and SOX9 (sex determining region Y-box 9) for chondrogenic, SPARC (secreted protein acidic and cysteine rich) and RUNX2 (runt-related transcription factor 2) for osteogenic and PAPF1 (preadipocyte factor 1) for adipogenic differentiation (data not shown, primer sequences in Table 2) was evaluated.
| Gene name | Primer sequences | Annealing T (°C) | GenBank accession no. |
|---|---|---|---|
| OCT4 | F:5′-GGAGAGCATGTTCCTGCAGTGC 3′ | 58 | NM_174580 |
| R:5′-ACACTCGGACCACGTCCTTCTC 3′ | |||
| NANOG | F:5′-TTCCCTCCTCCATGGATCTG 3′ | 58 | NM_001025344 |
| R:5′-ATTTGCTGGAGACTGAGGTA 3′ | |||
| SOX2 | F:5′-CGAGTGGAAACTTTTGTCCG 3′ | 55 | NM_001105463 |
| R:5′-GGTATTTATAATCCGGGTGTT 3′ | |||
| CD44 | F:5′-GACTGTACATCGGTCACGGACC 3′ | 58 | S63418.1 |
| R:5′-GGTATAACGGGTGCCATCACGG 3′ | |||
| c-KIT | F:5′-TCCAAAACTCATCTGTCTCACC 3′ | 58 | AF263827.1 |
| R:5′-CCCACATCGTTATAAGCCCTG 3′ | |||
| ACTB | F:5′-GGCCAACCGTGAGAAGATGACC 3′ | 58 | BT030480.1 |
| R:5′-GAGGCATACAGGGACAGCACAG 3′ | |||
| AGGRECAN | F:5′-CGCATTTCCAAGGAGAAGGAG 3′ | 62 | XM_018251445 |
| R:5′-GAGCGCATGTTCTGGATTTC 3′ | |||
| SOX9 | F:5′-AAGTTCCCCGTGTGCATC 3′ | 63 | AF278703.1 |
| R:5′-TGCGGCTTGTTCTTGCTC 3′ | |||
| SPARC | F:5′-ATCCACGAGAATGAGAAGCG 3′ | 62 | NM_174464 |
| R:5′-GCACAGGGAAGATGTACATGT 3′ | |||
| RUNX2 | F:5′-TGTGGTTACTGTCATGGCG 3′ | 62 | XM_010799141 |
| R:5′-TCGTTGAACCTGGCTACTTG 3′ | |||
| PAPF1 | F:5′-ACCAGTGCGTGACCTTTC 3′ | 62 | AB009278.1 |
| R:5′-AGCCGTCCTTGCAGATG 3′ |
3.3 Expression of gene and protein markers
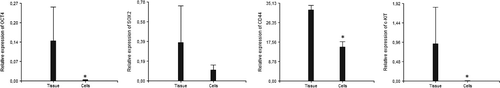
Tissue biopsies and cells isolated from the bovine endometrium and cultured in vitro expressed OCT4, SOX2, CD44 and c-KIT but not NANOG. OCT4 and c-KIT showed significant differences between tissues and cells with p < 0.05 being higher in endometrial tissue (Figure 3). In negative controls, there was not amplification of any of the tested genes.
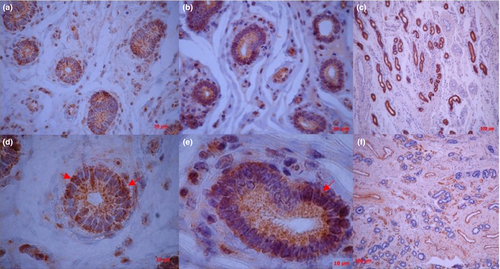
In tissue, the Oct4 and Sox2 proteins were detected in WB and immunohistochemically, being located in the nuclear and perinuclear areas, also spreading into the glandular lumen transmembrane area of glandular as well as in some stromal cells; meanwhile, Cd44 was detected only by WB. In cells, only Sox2 was found through WB (Figures 4 and 5).

4 DISCUSSION
It has been proposed that resident stem cell populations in the uterus are responsible of cyclic endometrial regeneration (Chan et al., 2004). Upon in vitro culture, all primary cell cultures derived here displayed a fibroblast-like morphology with plastic adherence, high proliferative capacity, clone formation and the ability to differentiate into chondrogenic, osteogenic and adipogenic lineages in vitro. These are indicators of functional stromal-derived MSCs (Gargett, 2007). We did not attempt to dissect out the two main components of the functionalis, that is the caruncular and intercaruncular subpopulations present on it; therefore, must likely we dealt with a mixed primary culture of whole endometrial tissue of cows in the FP of the oestrous cycle. Although our standardized laboratory cell culture procedures dramatically reduce the number of epithelial cells, due to their delayed ability to bind to plastic, we cannot rule out their presence in the cultures, and thus, these two cell populations might have responded differentially to oestrogens (Gargett, Schwab, Zillwood, Nguyen, & Wu, 2009). Besides humans, only in mice, researchers had detected putative stem cell populations in the epithelial and stromal tissue of the endometrium (Gargett, 2007). Interestingly, these cells expressed the oestrogen receptor-1 (ERα) differentially, (only in the stromal cells). Putative epithelial stem cells did not, in spite of being located in close neighbourhood to normal luminal epithelial cells that expressed the ERα gene. After 17-β oestradiol administration to ovariectomized mice, the entire putative epithelial stem population underwent proliferation as well as many stromal cells. This is indicative of recruitment of stem cells from niches under the effect of oestrogen, which act as stem/progenitor cells and proliferate during physiological endometrial growth. Same phenomenon had not been described for cattle. The present study demonstrated that in bovine endometrium, estrogens and probably their metabolites did not impair multipotent capacity of endometrial cells, which must likely would be of stromal origin.
Adult stem cells have properties such as self-renewal, high proliferation and plasticity which need to be examined in vitro (Eckfeldt, Mendenhall, & Verfaillie, 2005). The presence of clonogenic cells in tissues has been shown to support the existence of a MSC niche in the endometrium (Chan et al., 2004). We report in this study that approximately 0.51% of cells isolated from the bovine endometrium in FP formed clones. Similar clonogenic efficiency has been found in early and late luteal phase of bovine oestrous cycle (Cabezas et al., 2014). Studies in porcine uterus and human endometrium have shown that the clonogenic activity of cells does not vary between phases of the cycle, suggesting that these cells are ovarian steroid-independent hormones (Miernik & Karasinski, 2012; Schwab, Chan, & Gargett, 2005).
When the cells were seeded at a density of 2,000 cells/cm2, these proliferated rapidly with a CDT ranging 31.2–35.2 hr. In heifers, Mehrabani et al., 2015 described that endometrial MSCs doubled in a short time (37 hr). In their experiment, the initial seeding was more than 10 times higher than the one in our study. MSC derived from human decidua basalis were classified as highly proliferative cells and showed a DT of 2.21 ± 0.21 days (Lu et al., 2011). Mesenchymal stem cells have considerable plasticity but the mesodermal in vitro differentiation potential into adipocytes, chondrocytes and osteoblasts is generally used as criterion and is easily achieved (Lindner, Kramer, Rohwedel, & Schlenke, 2010). In this study, we demonstrated for the first time that bovine endometrial cells were efficiently induced to differentiate into three lineages: chondrogenic, osteogenic and adipogenic.
There are no defined gene markers for endometrial MSC in cattle or other species other than humans. Here, we detected the expression of two surface markers of MSC, CD44 and the proto-oncogene c-KIT. While the former might be present in contaminating mature fibroblast populations (Halfon, Abramov, Grinblat, & Ginis, 2010), the latter has not been reported in such cells. Besides, functional assays, such as colony-forming capacity and differentiation potential, are important specific properties that allow discrimination between MSCs and fibroblasts (Alt et al., 2011). The presence of expressed c-KIT as ligand of a stem cell factor might be indicative of the endometrial origin of the MSCs described here. In bovines, c-KIT is expressed in both myometrial and endometrial epithelial and stromal cells cultures, in which authors define as a marker of multipotent cells, suggesting that stem cells from bone marrow migrate to the uterus throughout the animal's life and is independent of its age and ovarian hormonal status (Łupicka, Bodek, Shpigel, Elnekave, & Korzekwa, 2015). Meanwhile, CD44 has been expressed in endometrial MSCs of some farm animal species such as porcine, ovine and bovine (Cabezas et al., 2014; Letouzey et al., 2015; Miernik & Karasinski, 2012; Nogueira de Moraes et al., 2016). mRNAs for CD44, C-KIT and OCT4 were detected both in tissue and cells, but their expression was lower in the cells. Proteins encoded by these mRNAs were found only in the analyzed tissues. It is suggested that adhesion to plastic and in vitro culture generates a loss of characteristics and cell markers, or well that other cells are selectively excluded during culturing on plastic plates such as epithelial cells and/or vascular endothelial cells as has been observed in adipose-derived adherent stromal cells (Yoshimura et al., 2006).
Embryonic stem cell gene markers such as OCT4 and SOX2 were expressed in endometrial tissue and cells during the FP of bovine oestrous cycle, while NANOG was not. The expression of Oct4, Sox2 and Nanog is specific to pluripotent cells and its regulation is key for the self-renewal ability of embryonic stem cells in an undifferentiated state (Young, 2011). However, while pluripotent stem cell markers have been found in several adult tissues, they are not fully pluripotent from a functional stand point, but instead are kept quiescent by an as yet not defined physiological mechanisms aimed probably to avoid the risk of teratoma formation (Ratajczak, Liu, Ratajczak, Kucia, & Shin, 2011). Several studies have reported the expression of OCT4 mRNA in uterus of healthy and diseased women (Matthai et al., 2006; Ono et al., 2010; Pacchiarotti, Caserta, Sbracia, & Moscarini, 2011), and endometrial OCT4 is not involved in or modulated by hormone-induced cyclical changes in the endometrium (Bentz et al., 2010). There are reports of the joint expression of OCT4 with SOX2 and NANOG in human endometrium, myometrium and endometriotic tissues (Götte et al., 2011), and in cultured stromal cells of porcine endometrium (Bodek et al., 2015). In bovine, only one study has shown Nanog expression associated with Oct4 and Sox2, in epithelial, stromal and myometrial cells between days 8 and 10 of the oestrous cycle, being highest mRNAs in the ipsilateral horn, suggesting the influence of ovarian hormones, and also revealing a decrease in pluripotent transcription factor expression in older cows (Łupicka et al., 2015).
The presence and expression of markers in the endometrium during the FP were similar to our previous findings in putative mesenchymal progenitor cells during the early and late luteal phase of the oestrous cycle (Cabezas et al., 2014). In this study, Oct4 and Sox2 were detected in the nuclear, perinuclear and transmembrane area of glandular as well as in some stromal cells. It has been reported in deer antlerogenic MSCs expression of Oct4, not only in the nucleus, but also in perinuclear areas and within far-ranging intercellular connections, indicating a possible mechanism of expansion in a resident stem cell niche by induction of pluripotency in surrounding non-niche cells via transfer of transcription factors through intercellular connections (Rolf et al., 2012).
There seems to be a resemblance over the wide range of markers expressed in endometrium and bone marrow, thereby suggesting their relevance in tissue repair and regeneration (Indumathi, Harikrishnan, Rajkumar, Sudarsanam, & Dhanasekaran, 2013). The presence of undifferentiated markers could indicate that endometrial stem cells might come from residual foetal stem cells bonded in various organs (Ratajczak et al., 2011) or alternative from the oviduct where we have detected Oct4 as well (E. Lara, manuscript in preparation). The functional characteristics of the endometrial cells are similar but not identical throughout each stage of the cycle, suggesting that transit cells under certain circumstances could behave like actual stem cells while they undergo maturation in specific conditions, this implies differences in the plasticity of stem cells (Potten & Loeffler, 1990). It seems that E2 during FP stimulates the growth of endometrial cells through the Wnt/β-catenin pathway, which in the case of porcine endometrium governs the cyclical cell regeneration process (Bukowska, Ziecik, Laguna, Gawronska-Kozak, & Bodek, 2015).
From the above discussed, it seems that bovine endometrium contains undifferentiated cell populations with properties similar to those of MSCs. We confirmed the presence of stem cells in bovine endometrium and obtained for the first time an extensive characterization of these cells alongside the FP of the oestrous cycle, which would be of great interest for the understanding of stem cell biology in farm animal species and for regenerative therapy in uterine or pelvic organ diseases.
ACKNOWLEDGEMENT
This work was funded by research grant FONDECYT REGULAR 1110642 from the Government of Chile.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
E.L., conceived the idea and performed experimental design, sampling of animals, RT-qPCR, cell and protein work alongside data analysis, manuscript writing and critical reading; N.R., involved in assistantship during most of experiments, proofreading and redaction of manuscript; D.R., involved in immunostainings; L.R.-A., involved in critical reading, statistical analysis of PCR; F.O.C, contributed to critical reading and supervised Ph.D students and manuscript writing. All authors approved the final article.




