Differences Between High- and Low-Motility Buffalo Sperm Identified by Comparative Proteomics
Contents
This study was undertaken to investigate differences in protein expression between high- and low-motility sperm of swamp buffalo. The research used two-dimensional gel electrophoresis (2DE) coupled to matrix-assisted laser desorption/ionization time-of-flight tandem mass spectrometry (MALDI-TOF/TOF-MS) to analyse the different proteins. The results showed 18 different expression protein spots between high- and low-motility buffalo sperm; eight of these proteins were up-regulated in low-motility sperm, five were down-regulated, one deleted and four proteins specifically expressed. Finally, four proteins were successfully identified by MS as belonging to three unique proteins; they are outer dense fibre of sperm tails protein 2 (ODF2), ATP synthase subunit alpha (ATP5A1) and succinyl-CoA synthetase subunit beta (SUCLG2). In summary, these results help to develop an understanding of the molecular mechanisms associated with low-motility sperm and provide clues for finding molecular markers associated with sperm motility.
Introduction
Sperm cells have various special properties. They are produced in the testes through a differentiation process called spermatogenesis, which involves marked genetic, functional, cellular and chromatin changes, and is responsible for delivering the paternal genome to the oocyte (Mezquita 1985; Poccia 1986). Fertilization of eggs by spermatozoa sets the stage for mammalian development, and viable sperm are a prerequisite for successful fertilization and embryo development. Motility is a key element of good quality sperm cells, as they need to pass through the female reproductive tract to the site of fertilization (Govindaraju et al. 2012). Despite its importance, we still do not have a complete understanding of the molecular mechanisms controlling sperm motility. There are some proteins associated with sperm motility as reported in literature. Ren et al. (2001) have reported that the sperm cation channel protein (CatSper) plays an important role in sperm motility and that low expression of CatSper in mouse sperm results in slow and less directed movements. The sperm from male mice lacking transaldolase (TAL) do not have forward motility and are sterile (Perl et al. 2006). JAM-A is a member of the family of the junctional adhesion molecules (JAM) membrane proteins and is essential for normal motility after the final maturation of sperm in the oviduct. Disruption of JAM-A in mice exhibits a significant reduction in percentages of motile sperm and progressive and hyperactivated motility, whilst there is no functional impairment with JAM-B deletion (Sakaguchi et al. 2006; Shao et al. 2008). Outer dense fibre protein 2 (ODF2) is a component of the outer dense fibres (ODFs) that are located in the flagellum of spermatozoa, and ODF forms a unique sperm tail structure and is involved in motility functions (Tarnasky et al. 2010). Furthermore, the expression level of the tyrosine phosphatase non-receptor type 14 (PTPN 14) protein was significantly lower in both mRNA and protein levels from moderate-motile human sperm; it was predicted that PTPN 14 was likely a novel sperm-motility biomarker (Chao et al. 2011). In a recent issue, De Canio et al. (2014) suggested that several different proteins between bovine X- and Y-chromosome-bearing sperm cells were essential for sperm-motility regulation, in particular testis-specific glyceraldehyde-3-phosphate dehydrogenase, as well as calmodulin.
To overcome the static analysis of the genome, proteomics, by focusing on differential protein expression, could help to discover the novel biomarkers about buffalo sperm motility (Soggiu et al. 2013). Recently, more studies identified the proteins related to various sperm functions using proteomic approaches (Martínez-Heredia et al. 2006; de Mateo et al. 2007; Liao et al. 2009), further studies focused on the candidate proteins correlated with sperm motility (Erikson et al. 2007; D'Amours et al. 2010; Novak et al. 2010; Siva et al. 2010). By allowing the simultaneous detection of thousands of proteins at the same time, proteomic technologies constitute efficient tools to help reveal molecular regulatory mechanisms, and sperm motility is not an exception (Amaral et al. 2014). In this study, we applied the 2DE and MALDI-TOF/TOF-MS strategies to identify proteins, which are differentially expressed in high- and low-motility buffalo sperm separated by Percoll; then, we used Western blotting to confirm the down-regulation of ODF2 and ATP5A1 from the previous approaches. In addition, we showed that ODF2 and ATP5A1 maybe the novel sperm-motility biomarkers.
Material and Methods
Sperm Separation
The semen samples were random collected from normal fertile swamp buffalo from the Livestock Breeds Improvement Station in Nanning (Guangxi, China) and frozen in 0.2 ml straws. All animals ranged between 3 and 10 years of age, and the number of male buffalo enrolled in this study was 6. High- and low-motility buffalo sperm were separated by Percoll gradient centrifugation: 100% Percoll solution was diluted to 90% and 45% with Dulbecco's phosphate-buffered saline (D-PBS). A density gradient of Percoll was prepared in an Eppendorf tube (0.4 ml of the 90% fraction under 0.4 ml of the 45% fraction). Spermatozoa were thawed at 38°C for 1 min and layered on top of the Percoll gradient. Samples were then centrifuged at 2500 × g for 30 min at 4°C. Sperm were collected from the two layers, washed separately in PBS three times, and their motility measured by computer-assisted sperm analysis (CASA).
Protein Extraction
0.4 ml frozen semen was centrifuged at 3000 × g for 10 min at 4°C and discarded the supernatants. The pellets were resuspended in 1 ml of PBS and centrifuged at 3000 × g for 10 min at 4°C. Supernatants were discarded, and the pellets solubilized in lysis buffer consisting of 7 m urea, 2 m thiourea, 4% CHAPS and 1% DTT. After an extraction period of 1 h on ice, the homogenates were centrifuged at 12000 × g for 10 min at 4°C and the supernatants collected. Subsequently, 300 μl from the resulting mixture was processed using 900 μl cold acetone at −20°C for 2 h or overnight, and the resulting suspensions were then centrifuged at 12000 × g for 30 min at 4°C. After centrifugation, the supernatants were discarded and the pellets were washed twice in 400 μl cold acetone. The precipitated proteins were dissolved in lysis buffer after drying at room temperature for 10 min., and the protein concentrations of all samples were measured using the Bio-Rad Bradford protein assay with bovine serum albumin (Sigma, St. Louis, MO, USA) as a standard.
Two-Dimensional Electrophoresis
The protein samples were diluted in rehydration buffer containing 8 m urea, 2% CHAPS, 0.5% IPG buffer (pH 3–10) and 20 mm DTT. First-dimensional electrophoresis was performed on an Ettan IPGphor II isoelectric focusing apparatus (GE healthcare, Germany), using 24 cm IPG strips with a pH range of 3–10 and 350 μg proteins were loaded for each IPG strip. Isoelectric focusing (IEF) was performed according to the following steps: 30 V for 6 h, 60 V for 6 h, 200 V for 1 h, 500 V for 1 h, 1000 V for 1 h and 8000 V for 10 h to reach a total of 40 000 Vh. After IEF electrophoresis, the strips were equilibrated in equilibration buffer (6 m urea, 87% glycerol, 2% SDS and 1.5 m Tris-HCl, pH 8.8) containing 1% DTT for 15 min. This was followed by a further equilibration step in the same buffer without DTT but with 4% iodoacetamide (IAA) for 15 min. Second-dimensional electrophoresis was carried out in 1 mm thick 12.5% polyacrylamide gels (PAAG) using an Ettan DALTsix electrophoresis unit (GE healthcare) according to the procedures in the operating manual, except that the electrophoresis conditions were modified to 19°C, 15 mA/gel for 15 min and then 30 mA/gel until the end of the electrophoresis. The gels were stained using silver staining (Yan et al. 2000).
Image Analysis
All images were acquired using a UMAX Image Scanner (GE healthcare) in transparency mode at 600 dpi. Gel images were analysed by 2-D ImageMaster 2.0 software (GE healthcare, Germany). Spot detection, background subtraction, normalization and spot matching were performed using the automated tools of the software. And all spots were manually reviewed and validated to ensure proper detection and matching.
In-Gel Digestion
Differentially expressed protein spots were manually excised. Gel pieces were washed with 400 μl of Milli-Q water twice to remove excess acid solution and destained in 50 μl of a solution containing 30 mm potassium ferricyanide (K3Fe(CN)6) and 100 mm sodium thiosulfate (Na2S2O3) for 2 min. All destained spots were dehydrated with 120 μl acetonitrile (ACN), then dried in a speed vacuum pump (Eppendorf, 5301, Germany), and incubated overnight at 37°C with trypsin digestion solution (10 ng/μl). After incubation, solutions were collected from each tubes, and peptides were extracted twice with 50% ACN and 50% Milli-Q water with 0.1% trifluoroacetic acid (TFA) added. All of the extracted solutions were mixed and dried in a speed vacuum, then dissolved in 1 μl of Milli-Q water with 5% TFA. The resuspended solutions were spoted onto a 384-cell MALDI-TOF target covering 0.5 μl of α-cyano-4-hydroxy-cinnamic acid (CHCA) (10 mg/mL; Sigma-Aldrich, USA) matrix for MS analysis.
MALDI-TOF-MS Analysis
The MALDI-TOF-mass spectrometer 4800 system (ABSciex, USA) was operated to acquire MS spectrum range from 800 to 4000 in reflector mode in each spot scan, the 10 most intense precursor ions were selected for tandem mass spectrometry to obtain the spectra resulting from collision-induced dissociation (CID) in the presence of air. Each spectrum represents the sum of 1500 laser pulses from randomly chosen spots per sample position. Raw data were analysed using the computer software provided by the manufacturers and are reported as monoisotopic mass. Database searches were performed against the NCBInr database using gps software (ABSciex, Canada). Search parameters were typically set to one missed cleavage site for tryptic peptides allowed, the modifications accepted were carbamidomethylation with IAA of cysteines and possible artefactual oxidation of methionines, the mass tolerance was 0.3 Da for both precursor and fragment ions. Protein hits were validated if the protein scores were above the Mascot software (ABSciex, Canada) default significance threshold (p < 0.05).
Western Blot Analysis
Approximately 60 μg protein samples per lane resolved by mini SDS-PAGE were electrophoretically transferred to Immobilon-P PVDF membrane in a Milliblot-Graphite Electro-blotter System (Millipore, Billerica, MA, USA) for 1 h at 20 mA. Membranes were taken out and incubated for 30 min at room temperature in TBS-T-blocking solution containing 5% blocking reagent and washed twice in 0.1% TBS-Tween solution for 5 min. They were then incubated overnight at 4°C with primary antibodies against rabbit IgG (diluted 1 : 2000), and the blots were visualized using the ECL system (Amersham) as described by the manufacturers.
Statistical Analysis
Statistical analysis was carried out using spss software (SPSS Statistics 18.0, Armonk, NY, USA). T-test was used to compare the sperm-motility data. One-way anova was used to evaluate the results from 2DE gels. Each experiment was repeated at least three times, and differences were considered significant with p < 0.05.
Results
Motility of Buffalo Sperm Separated by Percoll
Upstream and downstream sperm were obtained from the 90% and 45% Percoll gradient solution, and 10 glass slides were made, respectively. Their motility was measured by CASA by observing 4–5 visual fields in each of the slides. The results showed that the motility rate of upstream and downstream sperm was (12.25 ± 2.76)% and (42.67 ± 3.56)%, there were extremely significant differences between upstream sperm (low motility) group and downstream sperm (high motility) group (p < 0.01). As shown in Table 1.
| Group | Motility rate (%) |
|---|---|
| Upstream sperm | 12.25 ± 2.76a |
| Downstream sperm | 42.67 ± 3.56b |
- Different letters (a and b) differ extremely significantly (p < 0.01). The upstream sperm and downstream sperm were separated by the 90% and 45% Percoll gradient solution. The motility rate of upstream sperm was (12.25 ± 2.76)%, the motility rate of downstream sperm was (42.67 ± 3.56)%.
Comparative Analysis of 2D Protein Maps of High- and Low-Motility Buffalo Sperm
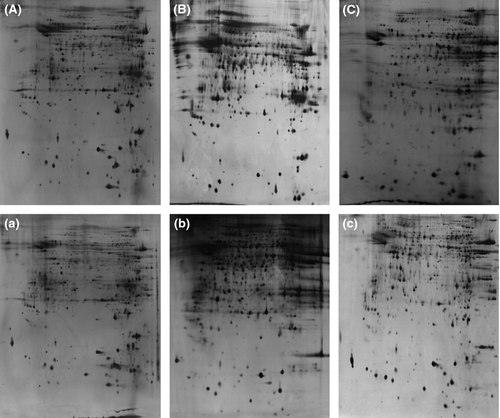
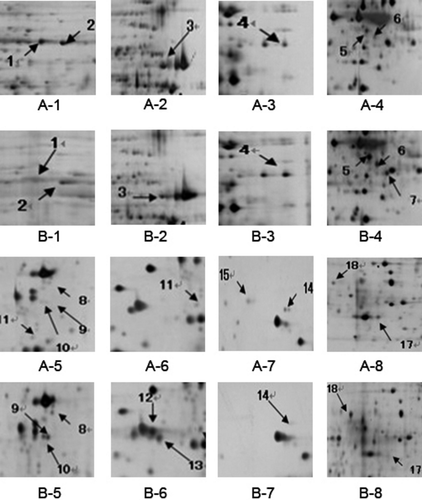
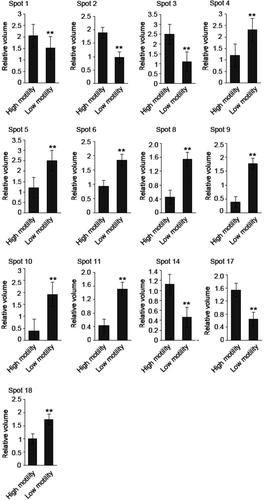
2D protein maps of high- and low-motility sperm from six normal swamp buffalo were revealed by high-resolution 2D electrophoresis after silver staining. There are six 2D protein maps in total from high- and low-motility sperm group with two biological replicates and three technical replicates. All the 2D protein maps showed in Fig. 1. Total protein spots from six maps were matched and detected automatically using 2-D ImageMaster 2.0 software, and the quantitative data were normalized according to all of the matched spots. After the comparative analysis, 18 different protein spots were selected. Compared with the high-motility buffalo sperm 2DE map, eight proteins (No. 4, 5, 6, 8, 9, 10, 11, 18) were up-regulated in low-motility buffalo sperm, five proteins (No. 1, 2, 3, 14, 17) were down-regulated, one protein (No. 15) was deleted and four new proteins (No. 7, 12, 13, 16) were specifically expressed in low-motility buffalo sperm (Figs 2, 3 and 4).

Mass Spectrometry
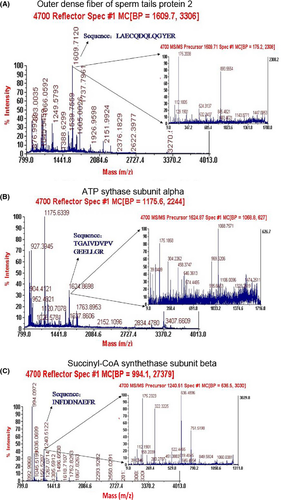
Eighteen differentially expressed protein spots were excised from 2D gels for in-gel digestion and subsequently identified by mass spectrometry. Four spots (1, 2, 3, 4) were successfully identified as belonging to three unique proteins, which may be related to sperm motility; they are outer dense fibre of sperm tails protein 2, ATP synthase subunit alpha and succinyl-CoA synthetase subunit beta (SUCLG2). A detailed list of the three proteins identified in this study is provided in Table S1. The outer dense fibre of sperm tails protein 2 and ATP synthase subunit alpha were down-regulated in the sperm with low motility, whereas SUCLG2 was up-regulated (Table 2). And their corresponding mass spectrum diagram as shown in Fig. 5.
| Spot | Protein definition | Accession number | Isoelectric point (pI) | Molecular weight (Mw) | Regulation |
|---|---|---|---|---|---|
| 1, 2 | Cenexin (Outer dense fibre of sperm tails protein 2) | gi|2996006 | 6.23 | 70660.2 | Down |
| 3 | ATP synthase subunit alpha, mitochondrial | gi|27807237 | 9.21 | 59682.7 | Down |
| 4 | Succinyl-CoA synthetase subunit beta | Q3MHX5 | 7.51 | 46661.7 | Up |
- Compared with the high-motility buffalo sperm, ‘Up’ means that spot expressed higher in low-motility buffalo sperm; ‘Down’ means that spot expressed lower in low-motility buffalo sperm.
Western Blotting
Western blotting analysis was used to confirm the results acquired from the 2DE study. We selected the outer dense fibre of sperm tails protein 2 (ODF2) and ATP synthase subunit alpha (ATP5A1) to perform Western blots, and the results showed that ODF2 and ATP5A1 proteins expression level was lower in the low-motility sperm than in the high-motility sperm (Fig. 6). Thus, the Western blots results were consistent with the results from mass spectrometry.
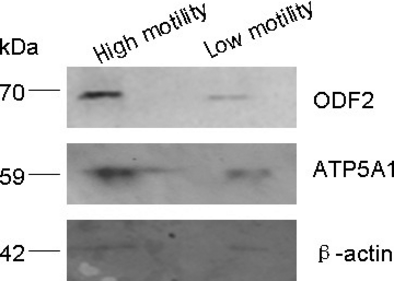
Discussion
Spermatozoa contain three main movement forms: straight-line motion, circles and swing. The higher the proportion of straight-line motion, the higher the sperm motility. The level of sperm motility directly affects the fertilization rate, so sperm with the higher motility will have better chances of penetrating the zona pellucida and hence have higher fertilization success rate. On the contrary, low-sperm motility can lead to asthenospermia and result in low fertility or even infertility. Consequently, the study of sperm motility is beneficial to the diagnosis and treatment of some male reproduction diseases such as low fertility and infertility. The present research has used 2DE coupled to MALDI-TOF/TOF-MS to analyse proteins that are differentially expressed between high- and low-motility sperm of swamp buffalo and has successfully identified four protein spots as belonging to three unique proteins; they are outer dense fibre of sperm tails protein 2 (ODF2), ATP synthase subunit alpha (ATP5A1) and SUCLG2. ODF2 and ATP5A1 were down-regulated in the sperm with low motility, whereas SUCLG2 was up-regulated.
ODF2, also known as Cenexin, was first discovered in 1995 (Lange and Gull 1995); it is especially expressed in testis and is described as a molecular marker of functional maturation of centrioles. The duplication and structural maturation of centrioles are intimately connected with spermatogenesis, and involve mitosis, meiosis and metamorphosis. Thus, Cenexin, as a centriole protein, may play an important role in spermatogenesis and the formation of normal sperm structure. Maturation of the centrosome in gametes may participate in the formation of sperm flagella (Krioutchkova and Onishchenko 1998), and the general description of the function of Cenexin in Uniprot KB data includes the following: outer dense fibres of sperm tails protein are a main component of sperm tail outer dense fibres (ODFs), and ODFs seem to be filamentous structures at the outside of axoneme in the principal and midpiece piece of mammalian sperm tail. Moreover, ODFs can contribute to maintain the passive elastic structures and elastic recoil of sperm tail then may have a modulation effect on sperm motility. It was recently demonstrated that inhibition of tyrosine phosphorylation of ODF2 adversely impacts sperm motility and ODF2 protein has also been implicated in sperm motility (Mariappa et al. 2010; Tarnasky et al. 2010). Our experimental results indicate that ODF2 was down-regulated in the low-motility buffalo sperm, so the protein is associated with sperm motility. It could affect the normal structure of sperm, especially the flagella and tail, which lead to functional changes in sperm motility, etc.
ATP synthase includes three main types: P, V and F. The F type is a key enzyme in the synthesis of ATP and consists of two structural domains in Escherichia coli: F1, which contains the extramembrane catalytic core, and Fo, which contains the membrane proton channel. These are linked by a central and peripheral stalks. F1 is composed of five subunits, such as alpha and beta, whilst Fo consists of three main subunits, a, b and c (McCarty et al. 1988; Boekema and Böttcher 1992). ATP synthase is widely found in mitochondrial inner membrane, chloroplast thylakoid membrane and the plasma membrane of heterotrophic bacteria and photosynthetic bacteria, which produce ATP mainly by photophosphorylation or oxidative phosphorylation to provide energy for metabolic activity (Walker et al. 1982). The α-subunit of ATP synthase from mitochondria is a key component of the outer membrane of the enzyme. It produces ATP from ADP in the presence of a proton gradient, which is generated across the membrane by electron transport complexes of the respiratory chain (Walker et al. 1989). In the 1940s to 1950s, scientists observed that ATP concentrations in mitochondria or chloroplasts increased dramatically during cellular respiration or photosynthesis, which indicated that the concentrations of ATP must correlate with biological activity (Boyer 1998). In the present trial, the concentration of the ATP synthase subunit alpha in low-motility buffalo sperm was lower than in the high-motility buffalo sperm, thus suggesting that high-motility sperm use more energy, that is the increased quantity of the ATP synthase subunit alpha expressed in the high-motility sperm provides more energy for sperm movement, and the enzyme may represent a new molecular marker of evaluation of sperm motility.
The biochemical function of succinyl-CoA synthetase is to catalysis the aerobic metabolism reaction of succinyl-CoA and GDP or ADP to generate succinate, GTP or ATP, and CoA. Succinyl-CoA synthetase has two main subunits: α and β, which are encoded by sucD and sucC, respectively. The alpha subunits tend to be conservative in the system of biological evolution, and the homology is >88% in a wide range of species including pigeons, swine and rat. In contrast, the beta subunits have experienced major changes during evolution and determined the nucleotide specificity (Johnson et al. 1998). Succinyl-CoA synthetase has been linked with some diseases; for example, disruption in the genetic code in beta subunits caused encephalomyopathy and mitochondrial DNA depletion syndrome (Elpeleg et al. 2005). In addition, two-dimensional electrophoresis technology showed that the quantity of succinyl-CoA synthetase expressed in heat-shocked Myxococcus xanthus was higher than in the control group (Otani et al. 2001). The current work has found that SUCLG2 was up-regulated in sperm with low motility, but there is still no evidence of a direct relationship between the enzyme and the sperm motility. Therefore, the enzyme may affect sperm motility through metabolic processes.
Conclusion
Eighteen different expression protein spots were found between high- and low-motility buffalo sperm, and four were successfully identified by MS as belonging to three unique proteins. They are ODF2, ATP5A1 and SUCLG2, which are related to energy metabolism. These results help to develop an understanding of the molecular mechanisms involved in sperm with low motility and provide clues for finding molecular markers associated with sperm motility. In addition, we showed that ODF2 and ATP5A1 maybe the novel sperm-motility biomarkers.
Acknowledgements
We acknowledge Dr. Bernard A Goodman for providing language help and writing assistance. This study was jointly supported by the National Natural Science Foundation of China (30860192) and the Natural Science Foundation of Guangxi (0991047).
Conflict of interest
None of the authors have any conflict of interest to declare.
Author contributions
Ming Zhang coordinated the project and supervised the manuscript. Yu-lin Huang was responsible for Western blotting analysis and data collection and drafted the manuscript. Qiang Fu and Li Yang were responsible for protein extraction and MALDI-TOF/TOF identification and analysis. Jun-liang Guan, Hong Pan and Fu-mei Chen were responsible for sperm separation and 2DE analysis. Ke-huan Lu supervised the project and overworked the manuscript.




