Aducanumab for the treatment of Alzheimer's disease: a systematic review
Abstract
Aducanumab is a novel disease-modifying anti-amyloid-beta (Aβ) human monoclonal antibody specifically targeted to the pathophysiology of Alzheimer's disease (AD). It was granted for treating AD in June 2021 by the United States Food and Drug Administration. We systematically analyzed available trials to evaluate the efficacy and safety of aducanumab treating AD. We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines. We conducted an extensive literature search using the electronic databases MEDLINE through PubMed, EMBASE, Cochrane, Web of Science, and Scopus for suitable studies on aducanumab. We considered human clinical trials of aducanumab, assessing its efficacy and adverse effects in treating AD, excluding any experimental animal studies. We included three randomised controlled trials. Studies reported that aducanumab reduced brain amyloid-beta plaques in a time- and dose-dependent manner (dose–response, P < 0.05) and a slowed decline in cognition (22% reduction) in the high-dose treated group, difference of −0.39 versus placebo in Clinical Dementia Rating Scale Sum Boxes (95% CI, −0.69 to −0.09; P = 0.012) along with a reduced amyloid positron emission tomography standard uptake value ratio score (P < 0.001) and plasma p181-tau (phosphorylated tau) level. Amyloid-related imaging abnormality was reported as a serious adverse event and was profound in high-dose treated group (425/1029 in 10 mg/kg). Aducanumab has been reported to affect two main pathophysiologic hallmarks (Aβ and tau) of AD. We suggest future studies addressing aducanumab's efficacy and safety to confirm that the benefit of this drug outweighs the risk.
INTRODUCTION
In the current world, one of the main reasons older adults become disabled and dependent is dementia, which is the seventh greatest cause of death among all diseases.1 Alzheimer's disease (AD) is the most common cause of dementia, the global frequency of which ranges 60–70%.1 It is a fatal, progressively degenerative, and irreversible brain disorder affecting almost 6 million people in the United States and claimed more than a hundred thousand lives in 2017.2, 3 Each year, about 500 000 Americans become diagnosed with AD, and the prevalence keeps rising with age.3 The worldwide prevalence rate of AD is approximately 36 million and is exponentially rising.8 Apart from its effects on health, AD has been shown to have negative social, economic and psychological effects on patients and their caregivers. Despite the tremendous increase in AD prevalence and severity, only a few licensed medications are available to treat dementia symptoms of the disease and provide only symptomatic relief without providing a long-term, disease-modifying benefit.4
A few psychiatrists first recognised the brain deposition of aberrant protein, which characterises AD development, about a hundred years ago.5 But the exact pathophysiology behind this disease remains unclear to date. Over the last two decades, it has been evident that the amyloid-beta (Aβ) and tau proteins creating deposits in the brain are crucial to AD pathogenesis. Aβ and tau, typically soluble proteins, combine into amyloid-like filaments to form the plaques and tangles that are key pathological characteristics of AD.2, 5 Therefore, many therapeutic development initiatives for AD have placed a significant emphasis on addressing the amyloid cascade.2
Aducanumab is a disease-modifying human monoclonal antibody (mab) approved by the United States Food and Drug Administration (US FDA), which is the first AD medication to be licensed since 2003 and the first drug that is specifically directed to the pathophysiology of the disease.6 It explicitly targets Aβ aggregates such as insoluble fibrils and soluble oligomers.2, 7 In a double-blinded, placebo-controlled phase 1b study (PRIME), monthly infusion of aducanumab for 1 year reduced brain Aβ plaques in a time- and dose-dependent manner.7 This result was supported by two more randomised phase 3 studies (ENGAGE and EMERGE) of aducanumab.2 These studies also reported delayed clinical deterioration in the aducanumab-treated group.2, 7
However, the safety concern of aducanumab and some reported adverse events have created some controversies since its approval.4 The main adverse event reported in clinical trials of aducanumab was ARIA (amyloid-related imaging abnormality). This brain imaging abnormality exhibited two types of findings on MRI (magnetic resonance imaging), that is, ARIA-E (brain oedema or sulcal effusion) and ARIA-H (microhaemorrhage or hemosiderin deposition into brain parenchyma).2 Seizures, among other severe symptoms, happened in the context of ARIA-E, and they rarely necessitated hospitalisation.2 Other less severe symptoms reported in the studies were headache, dizziness, confusion, and nausea,2 but no fatal events were reported in any of the studies.2 Due to the controversies and criticisms from many clinicians, this novel drug was disapproved until 2021, when it was approved again by the US FDA, requiring a post-approval clinical trial to confirm that aducanumab delivers the anticipated therapeutic benefit.6
The development of the first disease-modifying drug, aducanumab, was a revolution in treating AD, given the enormous need for a therapeutic option for this disease. Therefore, many experimental studies, a few randomised controlled trials, and some systematic reviews have been conducted to discover the mechanisms, efficacy, and safety of mab for treating AD.2, 4, 7, 8 Among all the anti-Aβ mabs (aducanumab, solanezumab, bapineuzumab, gantenerumab, and lecanemab) developed and undertaken in clinical trials so far, aducanumab was found to be the most effective until recently when lecanemab showed some promising result on a phase 3 clinical trial in treating AD.9, 16 Based on the results from the clinical trial, the US FDA granted accelerated approval of lecanemab for treating AD in January 2023.17 However, the study reports that lecanemab was found to be effective in reducing amyloid markers and delaying cognitive decline only in early AD.16 The researchers reported no efficacy and safety data for later-stage AD. Moreover, the phase 3 study of lecanemab has some limitations like limited study population, shorter duration, obstacles due to the Covid-19 pandemic (missed doses, delayed assessments, and intercurrent illnesses), high dropout rate, and an extension study is still ongoing.16 Because of this, this systematic review aims to explore available clinical trials on the effectiveness and safety of aducanumab for the treatment of AD and provide a concise message, which might shed light on future advancement of AD treatment and help in future research on it.
METHOD
We have conducted this systematic review following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines.13 The protocol is registered in PROSPERO (CRD42022358265).
Data sources
A detailed search strategy was built using the keywords ‘Aged’, ‘Elder’, ‘Old’, ‘Debilitated’, ‘Humanized Monoclonal Antibody’, ‘Aducanumab’, ‘Efficacy’, ‘Drug safety profile’, ‘Alzheimer disease’, ‘Temporoparietal atrophy’, ‘Alzheimer's dementia’, ‘Alzheimer syndrome’ to search separate electronic databases which includes MEDLINE through PubMed, the Cochrane Library (Cochrane Central Register of Controlled Trials-CENTRAL), EMBASE, Web of Science, and Scopus. We searched the databases on 7 September 2022. The comprehensive search strategy for all the databases has been provided in Appendix S1.
Study selection
We looked for studies assessing the benefit and adverse effects of aducanumab for the treatment of AD. Randomised controlled trials (RCTs), quasi-experimental studies, non-randomised trials, controlled before-after studies, analytic studies (cohort study, case–control study), and comparative cross-sectional studies were considered. We included the articles published in the English language only.
We excluded the studies not considering aducanumab, not focusing on AD, animal studies, laboratory experiments, studies not conducted on humans, ongoing/ incomplete studies, case reports, case series, modelling studies, review articles, systematic reviews, editorials, comments, letters, book chapters, conference abstract, and opinions.
Two reviewers screened the title and abstract of the redeemed articles separately, and a third reviewer solved any dispute. We used the online software ‘Rayyan’ to screen the articles.14 Then two independent teams went through the full-text articles, and the lead reviewer solved any disagreement. We excluded the articles following the ‘prioritisation and sequential exclusion’ technique15 Reasons for exclusion were reported.
Data extraction
We extracted data based on the study population, time of intervention, the dosage of intervention, positive and negative outcomes, the severity of negative outcomes, and so forth. Two teams extracted data independently, and the lead reviewer cross-checked to resolve any dispute.
Data analysis
We have performed a narrative synthesis here. A meta-analysis could not be conducted due to heterogeneity of outcome.
Risk of bias assessment
The Cochrane Risk of Bias (ROB) assessment tool was used to assess the ROB. Two teams of review authors assessed the ROB separately, and any dispute was solved by discussion. The domains considered in ROB were random sequence generation, blinding of the participants and personnel, allocation concealment, blinding of outcome assessors, incomplete outcome data, selective reporting, and other biases.
RESULTS
Search results
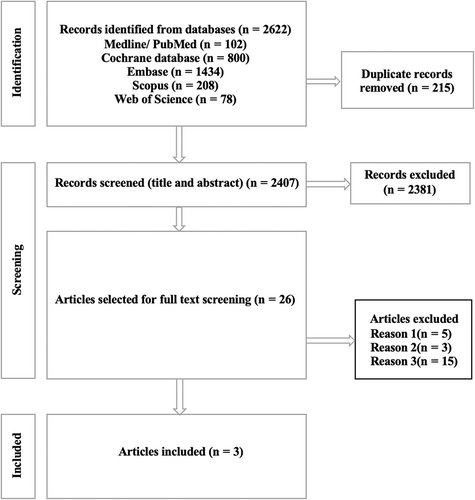
The comprehensive search from five databases retrieved 2622 articles. After removing 215 duplicates, we listed a total of 2407 articles for screening the title and abstract. At this stage, 2381 articles were excluded due to not meeting inclusion criteria as mentioned in the methods section. Among the remaining articles, 23 were excluded in the phase of full-text screening. These articles were excluded due to irrelevance to the study intervention. In the end, we included three RCTs for final analysis. Fig. 1 shows the PRISMA flow diagram of the detailed selection process of included articles.
ROB assessment of clinical trials
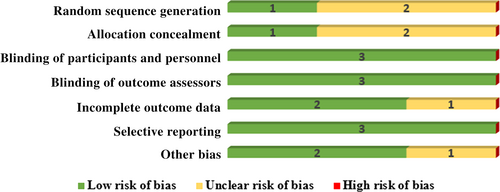
We assessed the risk of bias in included articles using the Cochrane ROB tool. Two out of three trials did not give adequate information on random sequence generation. The same scenario is observed in allocation concealment; two were marked as unclear, whereas only one study was marked as low ROB. All the studies mentioned the blinding of participants and personnel, so we ranked them as having a low ROB. Three trials maintained the blinding of outcome assessment which categorised them as having a low ROB. The majority of the studies (two out of three) were rated as having a low ROB for attrition bias, whereas only one study was tagged as having an unclear ROB due to a lack of information about attrition. There was no selective reporting in any trials, so we marked them as low ROB. We recognised two studies with a low ROB in the domain of other biases. One study did not give enough information in this domain, marking an unclear ROB. Assessment of ROB is shown in Fig. 2 in the graphical evaluation.
Characteristics of included studies
All the included studies were published between 2016 and 2022. Two studies were conducted globally, whereas one was in the United States. All of them were RCTs. All the trials included both males and females. Participants' ages in the included trials ranged from 50 to 90 years. Intervention in three studies compared the effects of doses of aducanumab with a placebo. The duration of the intervention in two studies was 76 weeks, and 52 weeks in one study. Two studies focused on both aspects of positive and negative outcomes, but one only described the intervention's adverse effects. A summary of characteristics of included publications, along with summarised outcomes, are described in Table 1.
| Author | Year of publication | Sample size | Study design | Country | Age in years | Gender | Intervention | Duration for intervention | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Salloway et al.10 | 2021 | Control = 1087 Intervention = 2198 |
RCT, Phase 3 | Global | 50–85 | Male and female | Low dose (3, 6 mg/kg) aducanumab vs. high dose (10 mg/kg) aducanumab vs. placebo | 76 weeks | 30%, 16.2%, 25.9% more ARIA in 10, 6, 3 mg/kg aducanumab-treated group respectively than placebo group |
| Haeberlein et al.2 | 2022 | EMERGE Control = 548 Intervention = 1090 ENGAGE Control =545 Intervention = 1102 |
RCT, Phase 3 | Global | 50–85 | Male and female | Low dose (3 or 6 mg/kg) aducanumab vs. high dose (10 mg/kg) aducanumab vs. placebo | 76 weeks | Primary end point: in EMERGE, 22% decline in high dose (−0.69 to −0.09), 15% decline in low dose. In ENGAGE, 2% increase in high dose (−0.26 to 0.33), 12% decrease in low dose. Dose-dependent reduction in Alzheimer's disease markers |
| Sevigny et al.7 | 2016 | Control = 40 Intervention = 125 |
RCT, Phase 1b | United States | 50–90 | Male and female | Low dose aducanumab vs. high-dose aducanumab vs. placebo | 52 weeks | 10 mg/kg aducanumab declined CDR-SB at 1 year (P = 0.07 vs. placebo). 3 mg and 10 mg/kg aducanumab slowed clinical progression significantly in MMSE. 10 mg/kg aducanumab showed greatest affect in MMRM (P < 0.05 vs. placebo) |
- Abbreviations: ARIA, amyloid-related imaging abnormalities; CDR-SB, Clinical Dementia Rating Scale Sum Boxes; MMSE, Mini-Mental State Examination; MMRM, mixed model repeated measures.
Description of the intervention
Salloway et al.10 explored the three different doses of aducanumab, including 3, 6, and 10 mg/kg. Participants completed 20 doses of low or high aducanumab or placebo intravenously (IV) monthly over 76 weeks. Budd Haeberlein2 and his team followed the same intervention: 3 or 6 mg as a low dose and 10 mg/kg as a high dose of aducanumab and placebo. Participants got IV infusion diluted by saline 4 weekly over 76 weeks, a total of 20 doses. Sevigny et al.7 measured outcome of four different doses (1, 3, 6, 10 mg/kg) of aducanumab. Patients received IV infusions of placebo or aducanumab monthly for 52 weeks.
Description of outcomes
Table 2 demonstrates the in-detail outcome measurements of the intervention with dose and summary.
| Authors | Intervention | Effectiveness measurement | Adverse effects | ||
|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||
| Salloway et al.10 | Placebo vs. low aducanumab vs. high aducanumab | - | - | Any ARIA: 274/756 in 3 mg/kg 104/392 in 6 mg/kg 425/1029 in 10 mg/kg ARIA-E: 223/756 (29.5) in 3 mg/kg 83/392 (21.2) in 6 mg/kg 362/1029 in 10-mg/kg Recurrent ARIA-E: 10.6% in 10 mg/kg ARIA-H: Microhaemorrhage: 141/756 in 3 mg/kg 50/392 in 6 mg/kg 197//1029 in 10 mg/kg Superficial siderosis: 91/756 in 3 mg/kg 23/392 in 6 mg/kg 151/1029 in 10 mg/kg |
Any ARIA: 111 (10.3)/1076 ARIA-E: 29/1076 Recurrent ARIA-E: 0 ARIA-H: Microhaemorrhage: 71/1076 Superficial siderosis: 24/1076 |
| Haeberlein et al.2 | Placebo vs. low aducanumab vs. high aducanumab | EMERGE: Low dose (3 mg/kg or 6 mg/kg) vs. high dose (10 mg/kg) vs. placebo (%) in Primary endpoint CDR-SB: −0.26 (−15%) in low −0.39 (−22%) in high Secondary endpoint MMSE: −0.1 (3%) in low dose, 0.6 (−18%) in high dose ADAS-Cog 13: −0.70 (−14%) in low dose, −1.40 (−27%) in high dose. ADCS-ADL-MCI: 0.7 (−16%) in low dose, 1.7 (−40%) in high dose. ENGAGE: low dose (3 mg/kg or 6 mg/kg) vs. high dose (10 mg/kg) vs. placebo (%) Primary endpoint CDR-SB: −0.18 (−12%) in low dose 0.03 (2%) in high dose Secondary endpoint MMSE: 0.2 (−6%) in low dose −0.1 (3%) in high dose ADAS-Cog 13: −0.58 (−11%) in low dose −0.59 (−11%) in high dose ADCS-ADL-MCI: 0.7 (−18%) in low dose 0.7 (−18%) in high dose |
EMERGE: (Placebo decline ± SE) Primary endpoint CDR-SB: 1.74 ± 0.11 Secondary endpoint MMSE: −3.3 ± 0.2 ADAS-Cog 13: 5.16 ± 0.40 ADCS-ADL-MCI: −4.3 ± 0.4 ENGAGE: (Placebo decline ± SE) Primary endpoint CDR-SB: 1.56 ± 0.11 Secondary endpoint MMSE: −3.5 ± 0.2 ADAS-Cog 13: 5.14 ± 0.38 ADCS-ADL-MCI: −3.8 ± 0.3 |
Safety MRI population: EMERGE: ARIA- E: 140 (26%) in low dose 188 (35%) in high dose Brain microhaemorrhage: 87 (16%) in low dose 108 (20%) in high dose Localised superficial siderosis: 52 (10%) in low dose 73 (13%) in high dose Safety population: Headache: 110 (20%) in low dose 107 (20%) in high dose Falls: 80 (15%) in low dose 86 (15%) in high dose Nasopharyngitis: 71 (13%) in low dose 89 (16%) in high dose Dizziness: 42 (8%) in low dose 55 (10%) in high dose Serious adverse events: In both doses, 13% Safety MRI population: ENGAGE: ARIA- E: 141 (26%) in low dose 199 (36%) in high dose Brain microhaemorrhage: 89 (16%) in low dose 104 (19%) in high dose Localised superficial siderosis: 51 (9%) in low dose 89 (16%) in high dose Safety population: Headache: 99 (18%) in low dose 115 (21%) in high dose Falls: 80 (15%) in low dose 86 (15%) in high dose Nasopharyngitis: 65 (12%) in low dose 68 (12%) in high dose Dizziness: 49 (9%) in low dose 54 (10%) in high dose Serious adverse events in both doses: 14%. |
Safety MRI population: EMERGE: ARIA-E: 13 (2%) Brain microhaemorrhage: 37 (7%) Localised superficial siderosis: 14 (3%) Safety population: Headache: 84 (15%) Falls: 71 (13%) Nasopharyngitis: 91 (17%) Dizziness: 44 (8%) Serious adverse event: 15% Safety MRI population: ENGAGE: ARIA-E: 13 (2%) Brain microhaemorrhage: 37 (7%) Localised superficial siderosis: 14 (3%) Safety population: Headache: 84 (15%) Falls: 71 (13%) Nasopharyngitis: 91 (17%) Dizziness: 44(8%) Serious adverse events: 13% |
| Sevigny et al.7 | Placebo vs. low aducanumab vs. high aducanumab | CDR-SB: Dose-dependent slowing of clinical progression at 1 year (dose–response, P < 0.05), with the greatest slowing for 10 mg/kg (P < 0.05 vs. placebo) MMSE: dose-dependent slowing of clinical progression at 1 year (dose–response, P < 0.05), with the greatest effects at 3 and 10 mg/kg (P < 0.05 vs. placebo), results were same irrespective of ApoE4 carrier status. |
CDR-SB: Dose–response P > 0.05 versus aducanumab MMSE: Dose–response P > 0.05 versus aducanumab |
ARIA: 32 Headache: 25 Urinary tract infection: 9 Upper respiratory tract infection: 12 Treatment discontinued due to adverse effects: 183 of 118 evaluable patients (3%) in the combined aducanumab groups developed treatment-emergent anti-aducanumab antibodies within the first year of treatment, with no apparent effect. ARIA-E which is dose dependent 0, 3%, 6%, 37%. |
Any adverse effect: 39/40 ARIA-E: 41% Serious events: 15/40 Discontinuing treatment due to an adverse effect: 4/40. |
- Abbreviations: ARIA, Amyloid-Related Imaging Abnormalities; ARIA-E, ARIA-oedema; ARIA-H, ARIA-haemorrhage; CDR-SB, Clinical Dementia Rating Scale Sum Boxes; MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; ADAS-Cog 13, Alzheimer Disease Assessment Scale Cognitive 13 Item Scale; ADCS-ADL-MCI, Alzheimer's disease co-operative study activities of daily living scale for mild cognitive impairment.
Effectiveness
Budd Haeberlein2 and his team evaluated the positive outcomes of low and high-dose aducanumab in a 76-week trial. In EMERGE, for the primary endpoint, the clinical decline was more observed in CDR-SB (Clinical Dementia Rating Scale Sum Boxes) in high-dose aducanumab (difference of −0.39 vs. placebo (95% CI, −0.69 to −0.09; P = 0.012; 22% decrease)) but not in ENGAGE (difference of 0.03, (95% CI, −0.26 to 0.33; P = 0.833; 2% increase)). For secondary endpoint (EMERGE) MMSE (Mini-Mental State Examination), ADAS-Cog 13 (Alzheimer Disease Assessment Scale Cognitive 13 Item Scale) showed the most (27%), ADCS-ADL-MCI (Alzheimer's disease co-operative study activities of daily living scale for mild cognitive impairment) showed 18%, 27%, 40% clinical decline of Alzheimer's disease respectively. But the latter two scales’ marks were noy different in both doses in ENGAGE. Sevigny et al.7 measured outcome of 1, 3, 6, 10 mg/kg of aducanumab versus placebo. It showed dose-dependent slowing of clinical progression of AD at 1 year (dose–response, P < 0.05), with the highest deceleration for 10 mg/kg (P < 0.05 vs. placebo) in both CDR-SB and MMSE scale. But on the composite NTB (neuropsychological test battery) or the FCSRT (Free and Cued Selective Reminding Test) free recall, there were no changes from baseline after 1 year of trial. Still, skewed non-normal (floor) effects at baseline were observed.
Safety
Salloway et al.10 investigated different doses of aducanumab to detect the adverse effects. The mean age was 70.4 years. It showed that after completing 20 doses, ARIA are prominent in 41.3% of 10 mg/kg group patients. ARIA-E was more observed than ARIA-H at 35.2% in 10mg/kg, which is greater than all. Recurrence of ARIA-E was also only seen in 10 mg/kg patients.
Microhaemorrhage (50/392) and superficial siderosis (23/392) were the least in patients who had taken 6 mg/kg aducanumab. Older participants had an increased hazard of ARIA-H (hazard ratio (HR) 1.06, 95% CI 1.02–1.09 per additional year). ApoE ε4 carriers had more risk than noncarriers (HR 2.5, 95% CI, 1.90–3.20). Budd Haeberlein2 and his team found negative impacts of low and high-dose aducanumab in their trial. ARIA-E was 9% and 10% higher in the high-dose groups compared with low-dose groups in EMERGE and ENGAGE, respectively. Adverse events with an incidence >8% in any dose group were brain microhaemorrhages, localised superficial siderosis (ARIA-H superficial siderosis), headache, falls, nasopharyngitis, and dizziness. Sevigny et al. observed detrimental effects of low and high doses of aducanumab versus placebo. Adverse effects were common: ARIA, headache, urinary tract infections, and upper respiratory tract infections. ARIA-E abnormalities were found in one (3%), two (6%), 11 (37%), and 13 (41%) patients receiving 1, 3, 6 and 10 mg/kg aducanumab respectively compared with 0% in placebo receivers. ARIA-E was generally observed early in the course of the treatment.
DISCUSSION
Summary of findings
Despite the rapidly rising worldwide prevalence of AD, only a few symptomatic medications were available to treat the disease.4 But over the past two decades, new hope for treating AD emerged with the development of the first disease-modifying mab aducanumab. Since its discovery, several experimental studies and clinical trials have been conducted to analyze the effectiveness and safety of this drug. Besides aducanumab, some other mab drugs have been developed and undertaken in clinical trials, but aducanumab was reported to be the most effective among them all.9 In a double-blinded, placebo-controlled phase 1b study (PRIME) involving 165 patients with mild or prodromal AD, aducanumab was infused monthly in doses of 1, 3, 6 and 10 mg/kg. After 54 weeks of treatment, the outcome revealed reduced brain Aβ plaques in a time- and dose-dependent manner.7 This result was supported by two more randomised phase 3 studies (ENGAGE and EMERGE) involving patients with early AD.2 This outcome was measured by PET (positron emission tomography) SUVR (standard uptake value ratio) score in both studies.2, 7 These studies also reported a dose-dependent slowing of cognitive decline and delayed clinical progression in the aducanumab-treated group.2, 7 The greatest slowing of cognitive decline was observed in the 10 mg/kg dose (P < 0.05 vs. placebo), and the greatest effect for delaying clinical progression was achieved with the 3 and 10 mg/kg doses.7 The EMERGE and ENGAGE studies also reported decreased plasma p-tau181 (phosphorylated tau) levels over time.2 The primary and secondary endpoints were not achieved in ENGAGE, and only the high-dose aducanumab-treated group reached all the endpoints in EMERGE.2 Therefore, futility analysis resulted in an early cessation of the study.2 Despite this, these studies demonstrated a positive correlation between plasma p-tau181 level reduction by aducanumab and delay in the clinical decline of AD.2 However, all three RCTs of aducanumab were funded by a pharmaceutical company (Biogen), so there is a potential concern for exaggerating the positive treatment outcomes.
In December 2021, Salloway et al.10 reported the combined safety outcomes from the ENGAGE and EMERGE trials. The most common adverse event encountered during these studies was ARIA-E; other adverse events were ARIA-H, headache, nasopharyngitis, falls, and dizziness.2, 7 Compared to low-dose groups, the number of reported ARIA-E was greater in the high-dose groups and ApoE ε4 carrier groups than in noncarriers.2 However, no fatal events were reported due to ARIA, and serious adverse events were also minimal.2, 7
Agreement and disagreement with contemporary research
Aducanumab is still under trial as a new drug, with limited data supporting its effectiveness and some questionable adverse events such as amyloid-related imaging abnormalities (ARIA-E and ARIA-H).7, 11 While the US FDA granted accelerated approval in June 2021, requiring a post-approval clinical trial to confirm that aducanumab delivers the anticipated therapeutic benefit, the EMA (European Medical Agency) revoked the commercial approval for aducanumab in April 2022.6, 12 Therefore, some review studies and meta-analyses have been conducted to evaluate the effectiveness of aducanumab and other available mabs for treating AD while analyzing the adverse outcomes of individual drugs.8, 9, 11 Mo et al.8 concluded in their systematic review and meta-analysis of anti-Aβ immunotherapy for AD that aducanumab is the most effective among all the mabs, with improved cognition while having ARIA as an adverse event. This outcome was supported by another systematic review and meta-analysis of phase 3 RCTs by Avgerinos et al.9 analyzing the efficacy of anti-Aβ mabs in AD. However, in their review paper, Vaz et al.11 raised questions about the US FDA's approval of aducanumab and supported the EMA's decision to disapprove. The EMA's committee considered that a decrease in brain Aβ aggregates is insufficient as a biomarker to anticipate clinical benefit.11 The reviewers concluded the disparate outcomes between ENGAGE and EMERGE, as well as its post hoc analysis, did not provide enough support for a therapeutic advantage. Therefore, they suggested future studies on combination therapy that targets both the upstream and downstream pathologies of AD.11
Research implications
Although aducanumab significantly reduced brain Aβ plaques and plasma tau levels in different clinical trials, there is not enough clinical evidence linking the decrease in Aβ plaques to clinical effectiveness.11 The pathophysiology of AD is also significantly influenced by another harmful protein, tau. Although the ENGAGE and EMERGE studies reported a decrease in plasma tau levels in the aducanumab-treated group, overall, this effect was not reported with great significance.2 Also, the available RCTs were conducted only in patients with a mild or early phase of AD.2, 7 Therefore, in patients with advanced AD, aducanumab may not have a significant effect, nor may it reverse cognitive decline and brain damage.
Hence, future studies should be designed targeting the global population with an extended period of trials to evaluate the efficacy of aducanumab in different pathophysiologic parameters of AD. Also, as this drug targets two main pathologic hallmarks of AD, future research needs to discover if it can be used to prevent or modify the pathophysiologic pathway of AD.
Strengths and limitations
We conducted this systematic review by strictly following the PRISMA guidelines. Only RCTs were included in this study. The included articles were critically assessed for risk of bias using the Cochrane ROB assessment tool.
This study also has some limitations. We considered articles written only in the English language. So, there is a chance that potential studies published in any other language will be missed. Second, we had a very limited number of included RCTs with small sample sizes. So, the results might be exaggerated in some cases.
CONCLUSION
Aducanumab, the first disease-modifying mab, has been the most enticing medication for treating AD in the last decade. While it gave new hope for the advancement of AD treatment, some controversies were also raised with its approval. Notably, the contradictory results of two identically designed studies (ENGAGE and EMERGE) and the lack of evidence that brain Aβ reduction correlates with clinical improvement made the EMA disapprove this drug.11 Additionally, the huge cost burden and reported adverse events of aducanumab raised questions about its safety and cost-effectivity.11 Despite the controversies, the US FDA approved this novel drug in June 2021, requiring a post-approval clinical trial.6 Aducanumab has been reported to have a significant effect on reducing brain Aβ content along with plasma tau levels and a slower deterioration of cognitive function in different clinical trials.2, 7 However, there is not enough scientifically evidenced correlation between reducing brain Aβ plaque and clinical improvement of AD.11 Moreover, the adverse events (ARIA) and high-cost burden is a questionable drawback for its global acceptance. Therefore, in this systematic review, we tried to assemble available data on aducanumab's efficacy and safety concerns for treating AD to analyze if the benefit outweighs the detriment.
We conclude that beyond the controversies, aducanumab has been the first drug targeting the AD pathologic hallmark and has effectively reduced the clinical progression of the disease.2, 7 However, it was only effective in the mild or early stages of AD.2, 7 Therefore, future research should be implemented targeting the global population with a large sample size and an extended period to evaluate aducanumab's efficacy and safety balance for the treatment of AD.
AUTHOR CONTRIBUTIONS
Study conceptualisation: the conceptualisation was a collaborative effort among the authors. First draft of the protocol: Afroza R. Protocol review and finalisation: KM Saif-Ur-Rahman. Literature search and screening: Afroza R., Md Anwar, Mirza F., Sadia B., Nuzhat T., Syeda S., Sharar N. Data extraction: Afroza R., Mirza F., Sadia B., Nuzhat T., Syeda S., Sharar N. Quality assessment: Afroza R., Mirza F., Sadia B., Nuzhat T., Syeda S., Sharar N., KM Saif-Ur-Rahman. Data synthesis and analysis: Afroza R., Md Anwar, Mirza F., Sadia B. First draft of the article: Afroza R., Md Anwar, Mirza F., Sadia B. Review and editing: KM Saif-Ur-Rahman, Abdullah A. Finalisation: KM Saif-Ur-Rahman. Final draft of the article: the final script was reviewed and approved by all the authors. The review's guarantee is provided by its corresponding author.
ACKNOWLEDGMENTS
We are grateful to the authors of the primary studies. Open access funding provided by IReL.
FUNDING INFORMATION
The authors have not disclosed any specific funding for this research from any governmental, private, or non-profit funding organisation.
DISCLOSURE
There is no conflict of interest in the submission of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




