The Mechanism of Micrografting With Salt-Tolerant Rootstock in Improving the Salt Tolerance of Scion in Vitis vinifera
Funding: This work was supported by Gansu Provincial Key Talent Project (2023RCXM23) and Central Government - Guided Local Scientific and Technological Development Funds (25ZYJA033).
ABSTRACT
Grapevine (Vitis vinifera L.) is highly sensitive to soil salinization, which severely restricts its cultivation in salt-affected areas. In this study, “Pinot Noir” (V. vinifera “Pinot Noir”) was micro-grafted onto the salt-tolerant rootstock “Kangzhen No. 3” to explore the mechanisms by which rootstock-mediated micrografting enhances scion salt tolerance. Grafted seedlings, un-grafted scions, and rootstocks were irrigated with 200 mmol/L NaCl for 6 days. Physiological assessments and transcriptomic analysis revealed that grafted plants exhibited significantly improved salt tolerance compared to ungrafted “Pinot Noir.” Differentially expressed genes were mainly enriched in plant hormone signal transduction, MAPK signaling, and phenylpropanoid biosynthesis pathways. Two key genes, VvFLS and VvGSTU14, were selected for functional validation. Overexpression in grapevine calli enhanced antioxidant capacity and significantly improved salt tolerance. These findings demonstrate that micrografting with a salt-tolerant rootstock can enhance scion performance under saline stress by modulating key signaling and metabolic pathways, providing a theoretical foundation for grapevine improvement and sustainable production on saline soils.
1 Introduction
Grapevine (Vitis vinifera L.) is a historically important fruit crop, playing a critical role in both agriculture and economic development (Zhou et al. 2022; Deng et al. 2021). However, grape cultivation is highly vulnerable to environmental stressors, particularly soil salinization, which has become a major challenge in grape production. Salt stress, a significant abiotic factor, severely limits agricultural productivity, affecting approximately 20% of irrigated lands globally (Zhao et al. 2021). This stress induces the production of reactive oxygen species (ROS), leading to cellular damage and physiological dysfunction, which ultimately compromise grape yield and quality (Meggio et al. 2014). Consequently, efficient removal of excess ROS, inhibition of lipid peroxidation, and maintenance of membrane integrity are critical for enhancing grapevine tolerance to salt stress (Fu et al. 2018). In response to salt-induced challenges, grapevines have developed a range of adaptive mechanisms, with antioxidant enzymes playing a central role in mitigating ROS. Key enzymes such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) help protect membrane integrity and sustain normal cellular function under stress (Cui et al. 2023).
Grafting is an effective approach for grapevines to mitigate salt stress (Shinde et al. 2019). The selection of appropriate salt-tolerant rootstocks for grafting can substantially improve the scion's resistance to stress (Gajjar et al. 2023). Micrografting, a more refined and efficient technique compared to traditional grafting, provides a better interface, simplified procedures, and higher success rates, while reducing resource consumption and experimental time (Wang 2020). Additionally, micrografting avoids pest and disease interference and is not constrained by seasonal or geographical limitations (Yang 2023). By enhancing the union between the scion and rootstock, micrografting optimizes water and nutrient transport, thereby improving grapevine survival under stress conditions. This technique offers an efficient and reliable platform for studying grapevine salt tolerance and holds significant promise for advancing research. Since the 1930s, research has identified varying levels of salt tolerance among grapevine rootstocks, leading to differential sensitivity to salinity (Gajjar et al. 2023). Studies under NaCl stress have shown that “Kangzhen No. 3,” “101–14,” and “Beta” exhibit relatively high salt tolerance, while “5BB,” “3309 M,” and “SO4” are more sensitive to salt stress (Chen et al. 2023). A comparative study indicated that the salt tolerance order among these rootstocks is as follows: “101–14” > “Kangzhen No. 3” > “Beta” (Chen 2022). For the present study, “Kangzhen No. 3” was selected primarily due to its availability in our experimental germplasm collection. Although “101–14” displays slightly superior salt tolerance, its introduction would require complex procedures and a lengthy preparation period, which could not be accommodated within the current research timeline. Thus, “Kangzhen No. 3” was chosen as the representative salt-tolerant rootstock for further investigation. Previous studies have demonstrated that under salt stress, “Kangzhen No. 3” significantly enhances the activities of antioxidant enzymes such as CAT, POD, and SOD, thereby scavenging ROS and exhibiting salt tolerance characteristics (Chen et al. 2023). In recent years, micrografting has gained considerable attention and shown promising applications in in vitro grapevine research, such as early virus detection and the evaluation of different scion-rootstock combinations on photosynthesis in micrografted grapevine seedlings (Guo 2020). This study aims to investigate the effects of micrografting with salt-tolerant rootstocks on grapevine salt tolerance, providing theoretical and practical insights for grapevine cultivation in saline-alkali soils. The combination of proper rootstock selection and micrografting offers an effective strategy for enhancing grapevine resilience to salt stress and provides a reliable technique for improving salt tolerance.
The mitogen-activated protein kinase (MAPK) signaling pathway is a key regulatory mechanism in plant responses to both biotic and abiotic stresses (Neill and Burnett 1999). By transmitting extracellular signals into the cell, this pathway regulates the expression of various genes, thereby triggering physiological changes such as cell proliferation, apoptosis, and antioxidant responses (Manna and Stocco 2011). Under salt stress, these responses help plants maintain homeostasis, enhancing their adaptability and survival. The plant hormone signal transduction pathway is closely intertwined with the MAPK signaling pathway, jointly modulating plant stress responses (Zhu 2021). Plant hormones such as abscisic acid (ABA) and auxin (IAA) interact with the MAPK pathway, coordinating the plants' response to salt stress and improving their physiological adaptability and stress resistance (Singh and Jwa 2013). Additionally, the phenylpropanoid biosynthesis pathway plays a critical role in plant stress responses, particularly in the synthesis of secondary metabolites such as flavonoids and lignin, which are essential for enhancing salt tolerance (Dong and Lin 2021). Phenylpropanoid metabolites not only strengthen cell walls but also support physiological processes and signal transduction, thereby promoting plant survival under salt stress (Cesarino et al. 2012).
This study aims to explore innovative strategies for improving salt tolerance in grapevines. Specifically, we will identify the genes and regulatory pathways involved in the micrografting of “Pinot Noir” and “Kangzhen No. 3” rootstocks to elucidate the potential mechanisms underlying enhanced salt tolerance. In addition, by examining the effects of VvFLS and VvGSTU14 gene overexpression on key physiological parameters, such as osmotic regulators and antioxidant enzyme activities, we aim to clarify their roles in boosting salt tolerance in grapevines. The ultimate goal of this study is to provide new theoretical insights and practical guidance for grapevine cultivation under salt stress.
2 Materials and Methods
2.1 Seedling Cultivation and Treatment
In this study, in vitro seedlings of Vitis vinifera L. “Pinot Noir” and the salt-tolerant rootstock “Kangzhen No. 3” were used as experimental materials, both of which were obtained from the Plant Tissue Culture Laboratory of the College of Life Science and Technology, Gansu Agricultural University.
For micrografting, healthy “Pinot Noir” seedlings cultured for 20–30 days were selected as scions, using single-bud stem segments from the middle to lower parts of the plant, with leaves removed but buds retained. Healthy “Kangzhen No. 3” seedlings cultured for 30–40 days were used as rootstocks, prepared as single-bud stem segments with both leaves and buds removed. Grafting was performed using the wedge method with a No. 23 surgical blade under aseptic conditions on a laminar flow workbench. The graft union was secured with aluminum foil strips (1.0 × 0.5 cm), and the grafted seedlings were cultured upright in GS medium (Feng 2012) (Figure 1). After 30 days of growth in the medium, the grafted seedlings, along with “Pinot Noir” and “Kangzhen No. 3” seedlings, were hardened and transplanted according to the protocol outlined by Dai Caihong (Dai 2014).
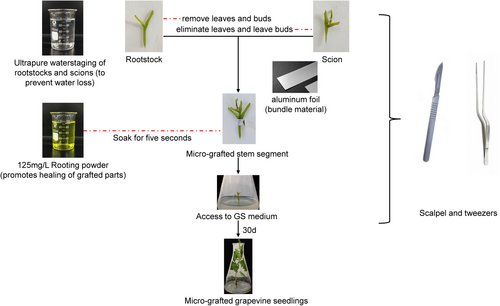
Prior to the onset of salt stress treatment, grafted plants, as well as transplanted seedlings of “Pinot Noir” and “Kangzhen No. 3,” were regularly irrigated with Hoagland nutrient solution for 30 days. Salt stress was applied by irrigating the treatment group with 200 mL of 200 mol·L−1 NaCl solution every 2 days. In the control group, the same volume of Hoagland solution was applied at identical intervals. Each treatment group consisted of four biological replicates. On Days 6 of salt treatment, mixed samples were collected from each group, including material from both Day 0 (pre-treatment) and Days 6 (post-treatment), for subsequent physiological, biochemical, and transcriptomic analyses.
2.2 Measurement of Antioxidant Enzymes, Superoxide Anion (O2•−), and Hydrogen Peroxide (H2O2) Contents
The activities of SOD, POD, CAT, and APX were determined following the methods described in Plant Physiology Experiments (Chen and Li 2016). The contents of O2•− and H2O2 were measured using commercial assay kits from Solarbio Technology Co. Ltd. and Kemin Biotech Co., respectively.
2.3 Determination of Osmotic Adjustment Substances in Leaves
The determination of proline (Pro), malondialdehyde (MDA), and relative electrical conductivity (REC) referred to the methods in Plant Physiology Experiments (Chen and Li 2016). The standard curve for proline is: C = 3.2811A + 0.0305, R2 = 0.9986.
The content of soluble sugar (SS) is detected using the kit from Suzhou Kemin Biotech Co.
2.4 Determination of Hormone Content
The determination of endogenous hormone content was conducted following the method of Yang et al. (2019), using the Waters Acquity Arc high-performance liquid chromatography (HPLC) system for analysis. The calibration of plant hormones is shown in Table 1.
| Plant hormone | Standard curve | R 2 |
|---|---|---|
| SA | Y = 7.9196X–28.018 | 0.9948 |
| IAA | Y = 17.857X–41.126 | 0.995 |
| ABA | Y = 98.601X–347.31 | 0.9941 |
2.5 Determination of Flavonoids and Lignin Content
The flavonoid and lignin content was measured using the Suzhou Kemin reagent kits (Kemin Biotech Co.).
2.6 Determination of Na+ and K+ Content
The determination of Na+ and K+ concentrations followed the method of Chen et al. (2019). First, the dried grape leaf samples were ground into powder. A quantity of 20 mg of the powder was placed into a test tube with 15 mL of deionized water, and the tube was sealed and boiled for 2 h. Then, an FP6410 flame photometer was used to measure the Na+ and K+ levels in the extract, and the Na+ and K+ content was subsequently calculated based on the dry weight of the tissue.
2.7 Transcriptome Analysis
After messenger RNA (mRNA) enrichment using mRNA Capture Beads, the mRNA was purified and then fragmented through high-temperature treatment. The fragmented mRNA was used as a template to synthesize the first strand of complementary DNA (cDNA) in a reverse transcription reaction. During the synthesis of the second strand of cDNA, the process also included end repair and the addition of an A-tail. Next, adapters were ligated, and target fragments were purified and selected using Hieff NGSDNA Selection Beads. The library was then amplified via polymerase chain reaction (PCR), and sequencing was performed using the Illumina NovaSeq X Plus platform. Quality control for the library construction was as follows: (1) Agarose gel electrophoresis was used to analyze the RNA integrity and check for DNA contamination; (2) Nanodrop spectrophotometer was used to assess RNA purity (OD260/280 and OD260/230 ratios); (3) The RNA integrity was precisely assessed using the Agilent 2100.
To ensure data quality, raw data was filtered before information analysis to minimize interference from invalid data. First, raw reads were quality-controlled using fastp to filter low-quality data and obtain clean reads. The specific filtering steps included: (1) Removal of reads containing adapters; (2) Removal of reads with more than 10% N; (3) Removal of reads consisting entirely of A bases; (4) Removal of low-quality reads (where more than 50% of bases have a quality value ≤ 20). Then, the paired-end sequences were aligned to the grape reference genome (Ensembl_release54) using HISAT 2 with default parameters. Gene expression levels were normalized by transcript abundance and measured in terms of transcripts per million (TPM). Differentially expressed genes (DEGs) were identified based on |log2FC| > 1 and false discovery rate (FDR) < 0.05. Gene functional annotation was based on the following databases: Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG).
2.8 Quantitative Real-Time PCR Analysis
To validate the RNA-Seq results, 11 DEGs were selected for quantitative real-time PCR (qRT-PCR) analysis to assess their consistency with transcriptomic data. These genes, which were significantly upregulated in HK, are associated with plant hormone signaling, MAPK signaling, and phenylpropanoid biosynthesis pathways. Total RNA was reverse-transcribed using the FastKing gDNA Dispelling RT SuperMix kit (TianGen Biotech Co. Ltd.) following the manufacturer's protocol. qRT-PCR was performed using Talent qPCR PreMix (SYBR Green; TianGen Biotech Co. Ltd.) on a LightCycler 96 Real-Time PCR System (Roche). Gene-specific primers and detailed procedures are provided in Data S1. Relative expression levels were calculated using the method (Livak and Schmittgen 2001), with VvGAPDH used as the reference gene. Each gene was analyzed with three technical replicates to ensure data reliability.
2.9 Functional Verification of Salt-Tolerance Genes
In this study, embryogenic calli of V. vinifera “Pinot Noir” were used as the starting material to generate VvFLS and VvGSTU14 overexpression lines via molecular cloning and Agrobacterium-mediated genetic transformation. The procedure was as follows: First, VvFLS and VvGSTU14 coding sequences were amplified from grape cDNA and ligated into the pCAMBIA1301 vector after gel purification. The recombinant plasmids were transformed into Escherichia coli DH5α, and positive clones were identified via colony PCR and verified by sequencing.
Subsequently, the confirmed plasmids were introduced into the Agrobacterium strain GV3101. Positive transformants were screened by PCR and cultured in 1/2 B5 liquid medium. Embryogenic grape calli were infected with the bacterial suspension and co-cultivated for 48 h. After removing the bacteria, the calli were transferred to B5 selection medium supplemented with hygromycin to obtain transgenic tissues.
Wild-type (WT), VvFLS-, and VvGSTU14-overexpressing calli were then cultured on B5 medium containing either 0 mM or 150 mM NaCl for salt stress treatment. After 15 days of culture, samples were collected for physiological and biochemical analyses. Primer sequences, PCR conditions, and media compositions are provided in Data S2.
2.10 Data Analysis
Data were collected from three biological replicates, each consisting of pooled samples from four individual plants. Technical triplicates were performed for all experiments. Data were processed using Excel 2024 and analyzed by one-way analysis of variance with Duncan's multiple range test (p < 0.05) in SPSS 26.0 to compare means among groups. GraphPad Prism 8.0 and TBtools 2.11 were used for data visualization, and figures were edited with Adobe Photoshop 2024, BioRender, and Adobe Illustrator 2024.
3 Results
3.1 Phenotypic Changes in Grafted Grapevine Seedlings, Scions, and Rootstocks Under Salt Stress
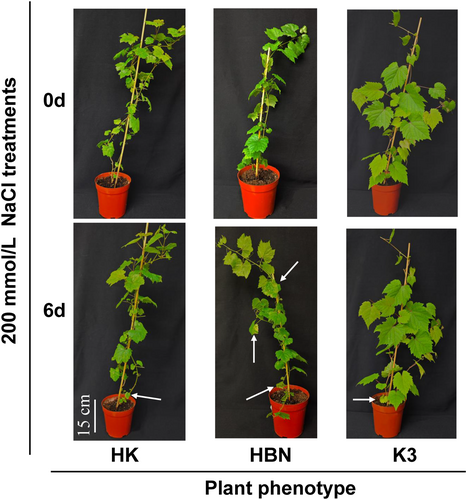
Compared with the control, “Pinot Noir” scion seedlings (HBN) exhibited more severe leaf chlorosis and defoliation after 6 days of treatment with 200 mmol/L NaCl. In contrast, the rootstock “Kangzhen No. 3” (K3) and the grafted seedlings (HK) showed only mild chlorosis on a few leaves (Figure 2). In total, 24 grapevine plants were subjected to salt stress, among which 16 plants (66.67%) displayed obvious salt-induced symptoms such as chlorosis, while the remaining eight plants showed relatively minor phenotypic changes. These data provide a clear overview of the phenotypic variation induced by salt stress and serve as a statistical basis for subsequent experimental analyses.
3.2 Pro, SS, MDA, and REC
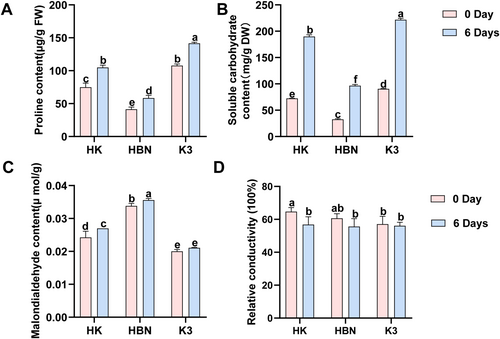
Under salt stress, the changes in the osmotic adjustment substances in the leaves of the micrografted grapes with “Kangzhen No. 3” as the rootstock are shown in Figure 3. Compared with HBN, the contents of Pro and SS in the leaves of the HK plants increased significantly, while the contents of MDA and REC decreased significantly under salt stress. Specifically, under salt stress, the Pro, SS, MDA, and REC values in HK increased significantly by 34.9% (p < 0.05), 96.3% (p < 0.05), 24% (p < 0.05), and 2.1% (p < 0.05), respectively.
3.3 Correlation Analysis of Physiological Indicators of “Pinot Noir,” Grafted Seedlings and “Kangzhen No. 3” Grape Seedlings Under Salt Stress
After 6 days of salt stress treatment, physiological analyses of the three grapevine materials, HBN, HK, and K3, revealed that grapevines employ multi-level, synergistic mechanisms to cope with salt stress (Figure 4). Pro and SS displayed a strong positive correlation (r = 0.85), suggesting their coordinated role in osmotic adjustment and water retention. Antioxidant enzymes showed notable co-regulation, with correlation coefficients ranging from 0.75 to 0.84, indicating their concerted action in scavenging ROS and alleviating oxidative damage.
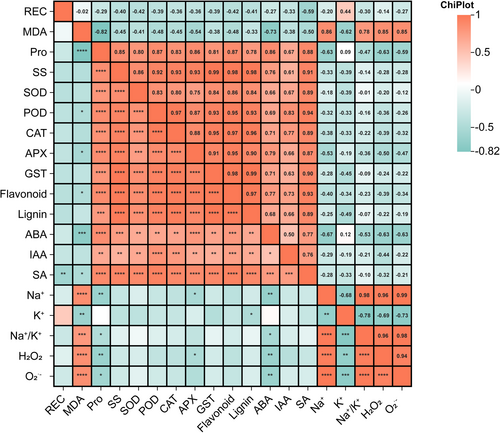
A particularly strong correlation was observed between SS and antioxidant enzyme activities (r = 0.92–0.99), implying that SS may serve as both an energy source and metabolic substrate for antioxidant enzyme synthesis. Furthermore, SS was highly correlated with flavonoid and lignin contents (r = 0.98–0.99), indicating its involvement in promoting secondary metabolite biosynthesis, which contributes to enhanced antioxidant capacity and cell wall reinforcement.
In addition, SA showed a significant positive correlation with flavonoid accumulation (r = 0.93), suggesting that SA may regulate signaling pathways to induce flavonoid biosynthesis, thereby enhancing stress defense mechanisms. Collectively, these findings highlight that grapevine salt tolerance is achieved through the integrated actions of osmotic regulation, antioxidant defense, secondary metabolism, and hormone-mediated signaling.
3.4 Transcriptome Analysis
To investigate the effects of salt-tolerant rootstock micrografting on gene expression in grapevine seedlings under salt stress, transcriptome sequencing was conducted using leaves from HK, HBN, and K3 plants subjected to 200 mmol L−1 NaCl for 6 days, with Hoagland nutrient solution-treated plants as controls. Each treatment included three biological replicates. Sequencing data for all samples are summarized in Table S3. The results indicated high sequencing quality, with Q30 values ranging from 93.53% to 95.35%, and clean read GC contents between 43.50% and 46.32%. Alignment to the grapevine reference genome showed high mapping rates, ranging from 88.16% to 95.27% (Table S3). The distribution of gene expression levels is illustrated in Figure 5A. Violin plots highlight expression density, with white dots representing median TPM values. The expression profiles exhibited a non-normal distribution but were highly consistent among biological replicates within each treatment group. These results confirm the reliability and quality of the transcriptome data for subsequent analyses.
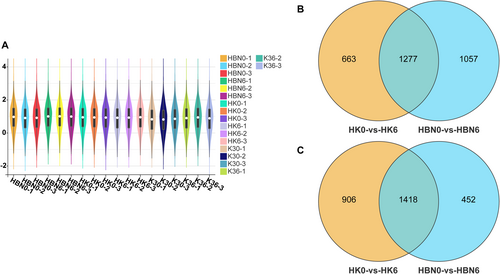
Using the thresholds of FDR < 0.05 and |log2FC| > 1, 4264 (1940 upregulated, 2324 downregulated) and 4204 (2334 upregulated, 1870 downregulated) differentially expressed genes (DEGs) were identified in the HK0 versus HK6 and HBN0 versus HBN6 comparisons, respectively. A Venn diagram revealed 2704 common DEGs between the two groups, with 1560 and 1500 unique DEGs in the HK0 versus HK6 and HBN0 versus HBN6 groups, respectively. These results suggest that salt-tolerant rootstock micrografting effectively induces gene expression in grapevine leaves under salt stress (Figure 5B,C and Table S4).
3.5 GO Annotation and KEGG Pathway Enrichment Analysis of DEGs
GO enrichment analysis of the 2704 common DEGs identified from the Venn diagram revealed significant enrichment across three major categories: biological process (BP), molecular function (MF), and cellular component (CC) (Figure 6A and Table S5). In the BP category, prominent enriched terms included phosphorylation (GO:0016310), hormone level regulation (GO:0010817), and phenylpropanoid biosynthesis (GO:0009699), all of which are closely associated with plant responses to salt stress. In the MF category, enriched terms such as oxidoreductase activity (GO:0016491), DNA-binding transcription factor activity (GO:0003700), and UDP-glycosyltransferase activity (GO:0008194) suggest roles in antioxidant defense and transcriptional regulation. For CC, the enriched genes were primarily associated with the plasma membrane (GO:0005886), cell wall (GO:0005618), and membrane-anchored components (GO:0046658), indicating their involvement in structural stability and stress signal perception. Collectively, these results suggest that the common DEGs are mainly involved in phosphorylation, hormone signaling, phenylpropanoid metabolism, and antioxidant defense, highlighting the multifaceted mechanisms through which salt-tolerant rootstock micrografting enhances salt tolerance in grapevine seedlings.
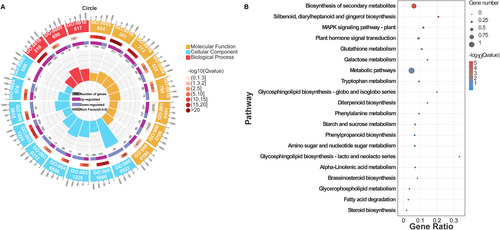
KEGG pathway enrichment analysis of the 2704 common DEGs revealed significant enrichment in the plant hormone signal transduction pathway (ko04075), the MAPK signaling pathway (ko04016), and the phenylpropanoid biosynthesis pathway (ko00940) (Figure 6B). These pathways play essential roles in plant adaptation to salt stress. In particular, the plant hormone signal transduction pathway enhances salt tolerance by regulating endogenous hormone levels, while the MAPK signaling pathway promotes stress adaptation through the activation of defense responses. Collectively, these findings provide important insights into the molecular mechanisms of salt stress adaptation in grapevine and identify potential targets for future functional studies.
3.6 Effects of Salt-Tolerant Rootstock Micrografting on the Antioxidant System Under Salt Stress
Salt-tolerant rootstock micrografting markedly enhanced the antioxidant system in grapevine under salt stress. A total of 24 DEGs associated with the MAPK signaling pathway were identified. Among them, the CAT1 gene (Vitvi00g04624), encoding CAT [EC:1.11.1.6], was upregulated in both HBN and HK. Similarly, RBOHE (Vitvi11g00037), encoding a respiratory burst oxidase, showed increased expression in HBN and HK, while RBOHB (Vitvi14g00183) was significantly upregulated across all three groups—HBN, HK, and K3. The GSTU14 gene (Vitvi07g00736), which encodes glutathione S-transferase [EC:2.5.1.18], also exhibited increased expression in HBN and HK, suggesting its involvement in ROS detoxification under salt stress. Additionally, in HK, several genes involved in antioxidant regulation, including SAG113 (Vitvi06g00533), PP2C51 (Vitvi16g01985), and CHIT1B (Vitvi03g00206), were significantly upregulated. These results indicate that micrografting enhances antioxidant defense by modulating the expression of key stress-responsive genes (Figure 7A).
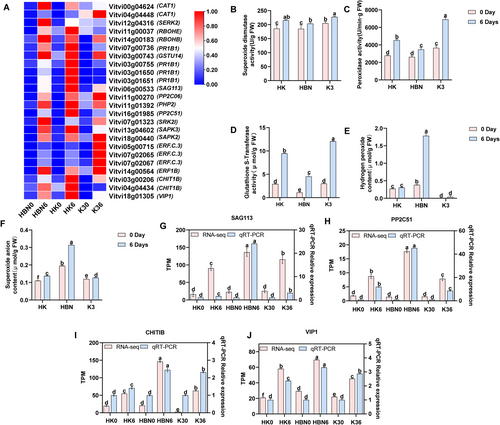
Physiological measurements confirmed increased activities of SOD, POD, and GST in HK, with levels exceeding those in HBN by 5% (p < 0.05), 30% (p < 0.05), and 109% (p < 0.05), respectively (Figure 7B–D). Concurrently, the accumulation of ROS, including H2O2 and O2·−, was markedly suppressed in HK, with reductions of 72% (p < 0.05) and 56% (p < 0.05), respectively (Figure 7E,F), indicating that micrografting effectively alleviates oxidative stress.
qRT-PCR analysis of SAG113, PP2C51, CHIT1B, and VIP1 genes showed expression patterns consistent with the RNA-Seq data, further validating the transcriptomic findings (Figure 7G–J). Overall, these results demonstrate that micrografting enhances salt tolerance in grapevine by modulating the MAPK signaling pathway and antioxidant gene expression, increasing antioxidant enzyme activity, and reducing ROS accumulation.
3.7 Effects on Plant Hormone Signal Transduction Under Salt Stress
Salt-tolerant rootstock micrografting markedly influenced the hormone signaling system of the grapevine under salt stress. A total of 21 DEGs associated with this pathway were identified (Figure 8A). Notably, the PR1B1 gene (Vitvi03g00743) was upregulated in HBN, HK, and K3, while its isoforms PR1B1 (Vitvi03g00755, Vitvi03g00650, and Vitvi03g00651) were exclusively upregulated in HK, suggesting a specific enhancement of SA-mediated defense signaling in grafted plants. Additionally, TGA9 (Vitvi06g01480), a transcription factor involved in SA signaling, was upregulated across all groups, and ABA-responsive gene GBF4 (Vitvi12g01667) showed elevated expression in HK and K3. The auxin-responsive GH3.1 gene (Vitvi07g01644) was upregulated in HBN and HK but downregulated in K3, indicating differential auxin signaling responses among treatments.
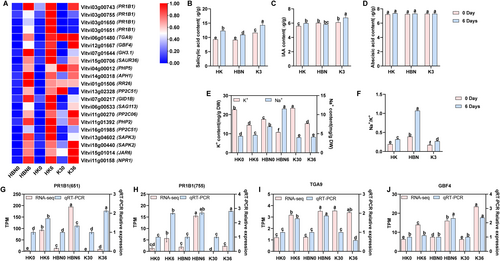
Hormone quantification further supported these findings: SA, IAA, and ABA levels were significantly higher in HK compared to HBN, with increases of 12% (p < 0.05), 3% (p < 0.05), and 3% (p < 0.05), respectively (Figure 8B–D). Meanwhile, HK exhibited improved ion homeostasis, as evidenced by a 59% (p < 0.05) reduction in Na+, a 25% (p < 0.05) increase in K+, and a 70% (p < 0.05) decrease in the Na+/K+ ratio (Figure 8E,F), which likely contributed to improved hormonal signaling and stress resilience.
qRT-PCR analysis of PR1B1 (Vitvi03g00651 and Vitvi03g00755), TGA9, and GBF4 showed expression patterns consistent with RNA-Seq data, confirming the reliability of the transcriptomic results (Figure 8G–J). Collectively, these findings demonstrate that micrografting enhances salt tolerance by modulating phytohormone signaling pathways, increasing hormone accumulation, and improving ion balance.
3.8 Regulation of the Phenylpropane Biosynthesis Pathway in Grapevine Under Salt Stress by Micrografting of Salt-Resistant Rootstocks
Salt-tolerant rootstock micrografting significantly influenced the phenylpropanoid biosynthesis pathway in grapevine under salt stress. A total of 12 DEGs involved in this pathway were identified (Figure 9A). The gene Vitvi06g00771, encoding POD [EC:1.11.1.7], was upregulated in both HK and K3. PER17 (Vitvi07g01502) was specifically upregulated in K3 but downregulated in HBN and HK, whereas PER12 (Vitvi18g01608) showed the opposite trend, being upregulated in HBN and HK but downregulated in K3. The PAL gene (Vitvi00g04609), which encodes phenylalanine ammonia-lyase [EC:4.3.1.24], was upregulated in K3 but downregulated in HBN and HK. Similarly, SHT (Vitvi11g00742), encoding shikimate O-hydroxycinnamoyltransferase [EC:2.3.1.133], was induced in K3 but suppressed in HBN and HK. COMT1 (Vitvi02g00263) and HOMT1 (Vitvi15g00874), both encoding caffeic acid 3-O-methyltransferase [EC:2.1.1.68], were upregulated in HBN and HK but downregulated in K3. OMT1 (Vitvi16g02061) was upregulated in all three materials. Notably, FLS (Vitvi18g02538), encoding flavonoid synthase [EC:1.14.20.6], was exclusively upregulated in HK. These results suggest that micrografting modulates the expression of key genes in the phenylpropanoid biosynthesis pathway, thereby enhancing antioxidant capacity and cell wall fortification to improve salt stress tolerance in grapevine (Figure 9A).
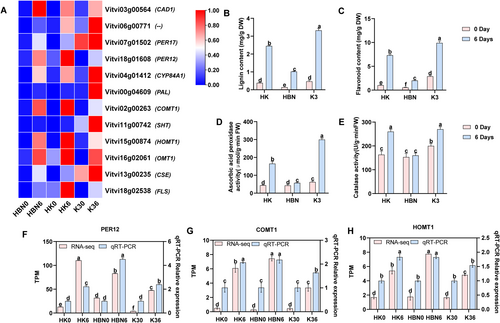
In this study, the key genes identified in the phenylpropanoid biosynthesis pathway were primarily associated with lignin synthesis. To further investigate how salt-tolerant rootstock micrografting modulates grapevine responses to salt stress, a series of validation experiments were performed. After 6 days of salt treatment, lignin content in the HK group increased by 140% compared to HBN (p < 0.05), contributing to enhanced cell wall stability and improved salt tolerance (Figure 9B). Flavonoid levels, representing a major class of antioxidant compounds, were also elevated by 72% (p < 0.05), supporting oxidative stress mitigation (Figure 9C). POD assays showed that CAT and APX activities in HK increased by 62.5% and 55%, respectively (p < 0.05), effectively reducing oxidative damage (Figure 9D,E). qRT-PCR validation of PER12, COMT1, and HOMT1 revealed expression patterns consistent with RNA-Seq data, confirming the reliability of transcriptomic analysis (Figure 9F–H). Collectively, these findings demonstrate that micrografting with a salt-tolerant rootstock enhances lignin biosynthesis, POD activity, and flavonoid accumulation, thereby improving salt stress resilience and survival in saline environments.
3.9 Expression of DGEs in Roots, Stems and Leaves of “Pinot Noir” Grafted Seedlings and “Kangzhen No. 3”
To analyze the molecular response mechanism of grapes under salt stress, this study verified the expression patterns of 6 key genes through qRT-PCR (Figure 10). Under salt stress, FLS (Vitvi18g0538) and GSTU14 (Vitvi07g00736) exhibited distinct expression enhancement across different materials. GSTU14 expression was markedly upregulated in K3 roots (3.18-fold compared to the control), with moderate expression observed in HBN roots, stems, and leaves (2.28-, 13.1-, and 0.72-fold, respectively). In HK, a strong induction was detected specifically in the leaves, with expression reaching 9.74-fold that of the control (Figure 10A). This gene responded to salt stress in multiple tissues, with particularly strong induction in HK leaves, suggesting a potential role in antioxidant defense.
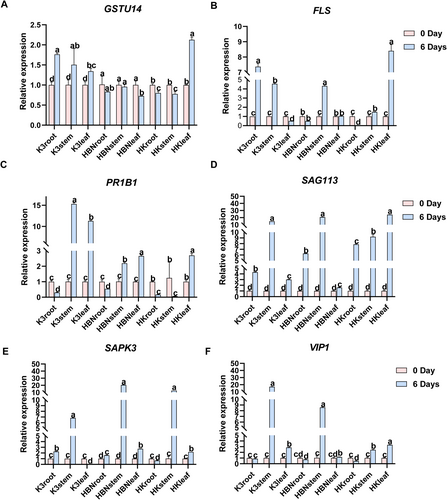
FLS expression was elevated in K3 roots and stems (6.36- and 3.5-fold, respectively), and also detected in HBN roots and stems (0.51- and 3.29-fold). In HK leaves, expression increased significantly, reaching 7.4-fold of the control, indicating a tissue-specific response to salt stress that may contribute to enhanced stress tolerance in HK (Figure 10B).
In addition, PR1B1, SAG113, SAPK3, and VIP1 showed salt stress-induced expression changes in various tissues of K3, HBN, and HK. However, these genes did not exhibit enhanced expression in HK leaves (Figure 10C–F).
Due to their distinct expression profiles—strongly induced in K3 roots and HK leaves but moderately expressed in HBN tissues—FLS and GSTU14 were selected for further functional characterization, as their patterns suggest enhanced scion gene activation triggered by micrografting with the salt-tolerant rootstock.
3.10 Functional Verification of the FLS and GSTU14 Gene
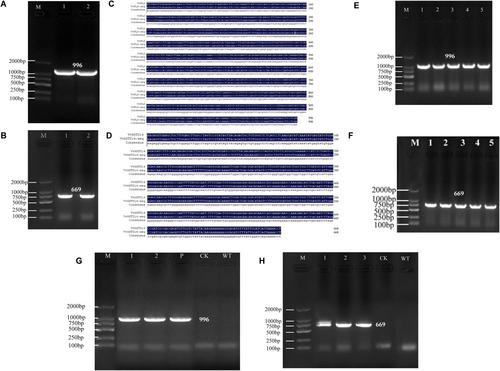
The open reading frame of the VvFLS gene is 996 bp in length, and the amplified CDS fragment was also 996 bp, consistent with the expected size observed in agarose gel electrophoresis (Figure 11A). Similarly, the ORF of VvGSTU14 is 669 bp, and the corresponding amplified CDS fragment is 669 bp. Agarose gel analysis showed a clear and distinct band at 669 bp, matching the expected size (Figure 11B). Sequence alignment using DNAMAN software revealed 100% similarity between the bacterial sequencing results and the CDS fragments of VvFLS and VvGSTU14, confirming the accuracy of amplification (Figure 11C,D). The correctly constructed VvFLS and VvGSTU14 expression vectors were subsequently introduced into Agrobacterium tumefaciens strain GV1301, and colony PCR confirmed successful transformation (Figure 11E,F). Transgenic grape calli carrying the VvFLS or VvGSTU14 gene were obtained through DNA extraction and PCR verification (Figure 11 G, H).
Salt stress tolerance of VvFLS-overexpressing grape calli was evaluated in this study. After 15 days of growth under normal conditions, no significant differences in growth status or morphology were observed between transgenic and WT calli (Figure 12A). However, following treatment with 150 mM NaCl for 15 days, VvFLS-overexpressing calli exhibited markedly better growth performance than WT (Figure 12B), indicating enhanced salt tolerance and improved adaptability under salt stress conditions. As shown in Figure 12C, the expression level of VvFLS in transgenic calli was significantly higher than in WT under normal conditions and increased even more prominently after salt stress treatment.
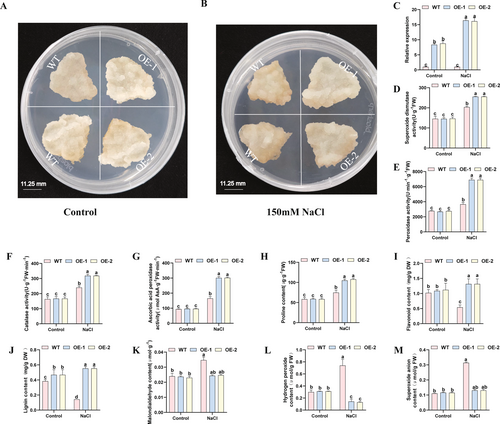
Under control conditions, there were no significant differences between WT and transgenic lines in antioxidant enzyme activities or in the levels of flavonoids, lignin, MDA, Pro, H2O2, and O2·− (Figure 12D–M). In contrast, under salt stress, transgenic lines exhibited significantly higher activities of antioxidant enzymes and increased Pro content compared to WT (Figure 12D–J), while the contents of MDA, H2O2, and O2·− were significantly lower (Figure 12K–M). Specifically, in the OE-1 line, SOD, POD, CAT, and APX activities, and the contents of Pro, flavonoids, and lignin were 1.25-, 1.89-, 1.33-, 1.82-, 1.4-, 1.42-, and 2.88-fold of those in WT, respectively. In the OE-2 line, the corresponding values were 1.25-, 1.88-, 1.32-, 1.83-, 1.43-, 1.42-, and 2.89-fold of WT. The MDA, H2O2, and O2·− levels in OE-1 were 0.70-, 0.19-, and 0.42-fold of WT, respectively, and in OE-2 were 0.71-, 0.17-, and 0.41-fold of WT.
The salt tolerance of VvGSTU14-overexpressing grape calli was assessed. After 15 days under normal conditions, no significant morphological or growth differences were observed between transgenic and WT calli (Figure 13A). However, upon exposure to 150 mM NaCl for 15 days, transgenic calli exhibited notably better growth performance than WT (Figure 13B), suggesting enhanced salt tolerance. As shown in Figure 13C, the expression level of VvGSTU14 was significantly higher in transgenic lines under normal conditions and further upregulated under salt stress.
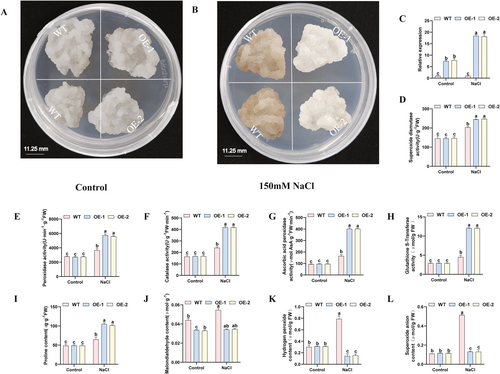
Under control conditions, no significant differences were observed between transgenic and WT calli in antioxidant enzyme activities or in the levels of MDA, Pro, H2O2, and O2·− (Figure 13D–L). Under salt stress, antioxidant enzyme activities and Pro content were significantly higher in transgenic lines (Figure 13D–I), while MDA, H2O2, and O2·− levels were significantly lower than in WT (Figure 13J–L). In the OE-1 line, SOD, POD, CAT, APX, and GST activities and Pro content were 1.2-, 1.57-, 1.74-, 2.42-, 2.66-, and 1.62-fold of WT, respectively. In the OE-2 line, these values were 1.21-, 1.53-, 1.74-, 2.43-, 2.66-, and 1.58-fold of WT. MDA, H2O2, and O2·− contents in OE-1 were reduced to 0.63-, 0.18-, and 0.25-fold of WT, and to 0.63-, 0.20-, and 0.25-fold in OE-2, respectively.
4 Discussion
4.1 Effects of Salt-Tolerant Rootstock Micrografting on Grape Antioxidant Systems
Salt stress typically inhibits grapevine growth and reduces biomass (Bistgani et al. 2019). Studies have shown that grafting with salt-tolerant rootstocks significantly alleviates growth inhibition caused by salt stress in crops like tomato, pepper, and watermelon, thereby enhancing salt tolerance (Wu et al. 2023). Our findings demonstrate that under 200 mmol·L−1 NaCl stress, antioxidant enzyme activities and the content of endogenous hormones and metabolites in grapevines were significantly reduced. In contrast, antioxidant enzyme activities were significantly elevated, and the content of endogenous hormones and metabolites was effectively modulated in grafted seedlings with salt-tolerant rootstocks. These changes alleviated ROS-induced damage to cell membranes, significantly enhancing salt tolerance. These results align with previous studies (López-Serrano et al. 2020; Zhen et al. 2010).
The MAPK signaling pathway comprises enzymes that are induced under various stress conditions and respond to stimuli (Gehart et al. 2010; Miransari et al. 2013). RNA-seq data from this study identified 22 DEGs enriched in the MAPK signaling pathway (ko04016) across grafted seedlings, scions, and rootstocks under salt stress. Notably, in HK, the gene CHIT1B, encoding basic endochitinase B, was significantly regulated (Figure 14A). Chitinase activity is closely associated with stress resistance in plants (Mészáros et al. 2014). For example, chitinase proteins were induced in Jatropha curcas callus under NaCl stress, and the ChI11 gene in tomato has been shown to play a critical role in salt stress response (Nuchadomrong and Pengthaisong 2012).
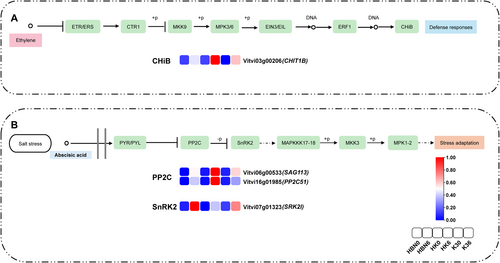
Importantly, CHIT1B is downstream of the ethylene (ETH) biosynthesis pathway, which is regulated by MAPK signaling (Li et al. 2018). During ETH biosynthesis, phosphorylation of MPK3 and MPK6 requires receptor-like kinases (RLKs) associated with salt tolerance, suggesting that the MAPK pathway may mediate ETH signaling activation under salt stress (Li et al. 2014).
Additionally, the ABA core signaling pathway involves PYR/PYL receptors, PP2C phosphatases, and SnRK2 kinases. This study observed that salt stress significantly upregulated SAG113 and PP2C51 expression in HK while downregulating the serine/threonine protein kinase gene SRK2I (Figure 14B). The upregulation of PP2C-encoding genes in response to salt stress enhances the regulation of antioxidant defense systems and related enzyme activities.
Under salt stress, plants typically generate excessive O2·− and H2O2, causing oxidative damage to cells (Fu and Yang 2023). However, salt-tolerant rootstock micrografting enhanced antioxidant enzyme activities, effectively reducing ROS production and accumulation, thereby alleviating oxidative damage. These findings underscore the effectiveness of salt-tolerant rootstock micrografting in mitigating salt stress in grapevines. This aligns with previous studies (Bai et al. 2019; Guo et al. 2023), further validating the reliability of our results.
Phenylpropanoid biosynthesis is a critical secondary metabolic pathway in plants, encompassing the general phenylpropanoid pathway and its specific branches that produce lignin, flavonoids, and phenolic compounds, which serve as resistance-related substances (Dong and Lin 2021). In this study, under salt stress, the expression levels of PER12, HOMT1, and COMT1 were significantly upregulated in the HK group, predominantly in the lignin biosynthesis pathway. This indicates that lignin accumulation is a key mechanism in the response of salt-tolerant rootstock micrografting to salt stress (Figure 15).
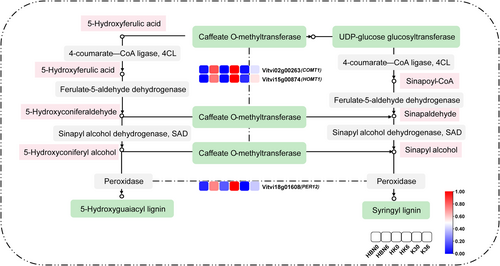
It is noteworthy that the phenylpropanoid biosynthesis pathway provides precursors for both lignin and flavonoid biosynthesis, indicating a strong synergistic relationship between the two in response to salt stress. Flavonoids contribute to maintaining intracellular homeostasis, which supports the expression of lignin biosynthesis-related genes and enzyme activities, ensuring normal lignin production (Shi et al. 2022). Meanwhile, lignin accumulates extensively in grapevine cell walls, enhancing their strength and stability, effectively reducing salt ion-induced cellular damage, and providing a stable environment for flavonoid functionality (Wang et al. 2024). Together, lignin and flavonoids form a coordinated defense system against salt stress in grapevine, deepening our understanding of the molecular mechanisms by which salt-tolerant rootstock micrografting enhances grapevine salt tolerance.
4.2 Effects of Salt-Tolerant Rootstock Micrografting on Plant Hormone Signal Transduction
Plant hormones play critical roles in regulating growth, development, and stress responses (Song et al. 2022; Koramutla et al. 2022). In this study, salt stress significantly altered the balance of endogenous hormones, with ABA, SA, and IAA levels markedly increasing, consistent with previous findings (Yang 2022; Sun 2023).
ABA, a key hormone in stress responses, plays a central regulatory role under salt stress conditions (Golldack et al. 2014). ABA accumulation is closely associated with intracellular ion homeostasis, particularly the dynamic balance of Na+ and K+. Salt stress causes a massive influx of Na+ into cells, severely disrupting physiological and biochemical processes (Zhang 2020). Through a complex signaling network, ABA actively regulates ion transporters on the plasma membrane, such as promoting Na+ extrusion or sequestration into vacuoles, thereby reducing cytosolic Na+ levels and mitigating its toxicity (Janicka-Russak and Kłobus 2007).
Transcription factors play pivotal roles in stress responses, with the ABF family emerging as critical regulators in the ABA signaling pathway (Wei et al. 2017). Studies have shown that ABF4 significantly activates downstream ABA-responsive genes, thereby enhancing plant tolerance to salt, osmotic, and drought stresses (Yoshida et al. 2014, 2015). In this study, salt stress markedly upregulated the expression of the GBF4 transcription factor in HK, which further promoted ABA biosynthesis (Figure 16A). This upregulation synergistically activated ABA-responsive genes through the PYR/PYL receptors and PP2C phosphatases, thereby enhancing grapevine tolerance to salt stress. SA, a phenolic hormone, also plays vital roles in physiological and biochemical processes (Shan et al. 2024; Hashempour et al. 2014). Studies have shown that SA enhances plant cell resistance to salt stress and contributes to improved stress tolerance by regulating various physiological processes, such as cellular homeostasis and metabolic adjustments under adverse conditions (Zhou et al. 2023; Mimouni et al. 2016; Gupta and Seth 2021; Gao et al. 2023). SA, a phenolic hormone, also plays vital roles in physiological and biochemical processes (Shan et al. 2024; Hashempour et al. 2014) (Figure 16B).

4.3 Research on VvFLS and VvGSTU14 Gene Enhancing the Tolerance of Grapes to Salt Stress
FLS is a key rate-limiting enzyme in the flavonoid biosynthesis pathway and plays a crucial role in regulating tissue colouration and flavonoid accumulation in plants (Yang 2015). Flavonoids are known to enhance plant tolerance to both biotic and abiotic stresses (Li 2011). Under salt stress, grapevine accumulates ROS, which causes lipid peroxidation and membrane damage, typically reflected by elevated MDA levels (Shi et al. 2011).
In this study, VvFLS-overexpressing grape calli exhibited enhanced salt tolerance compared to WT calli, as evidenced by lower levels of MDA, H2O2, and O2·−, along with higher activities of key antioxidant enzymes (SOD, POD, CAT, APX, and GST). These findings suggest that VvFLS overexpression mitigates oxidative membrane damage and reinforces the antioxidative defense system under salt stress, consistent with previous reports (Cai 2022; Li 2011; Qi 2016). Additionally, flavonoids are known to scavenge ROS when enzymatic antioxidant defenses are insufficient (Zhu et al. 2017). Our results showed that transgenic calli accumulated significantly more flavonoids and lignin under salt stress, which likely contributed to improved ROS detoxification and stress resilience, in agreement with earlier findings (Wei 2023). Taken together, VvFLS overexpression enhances salt tolerance through both enzymatic and non-enzymatic antioxidant pathways, providing theoretical insights into the molecular basis of grapevine salt stress adaptation.
Similarly, VvGSTU14-overexpressing calli showed reduced MDA levels and elevated Pro content under salt stress, indicating alleviated membrane lipid peroxidation and improved osmotic adjustment. These results align with reports on GpGSTU17, MsGSTU8, and AtGSTU19, which enhance salt tolerance through modulation of osmolyte accumulation (Gu 2023; Zhang 2017; Xu et al. 2016). The elevated activities of SOD, POD, CAT, and APX in VvGSTU14-overexpressing lines further suggest that VvGSTU14 contributes to ROS detoxification by enhancing enzymatic antioxidant capacity. Notably, SOD and POD may primarily scavenge superoxide radicals, while CAT and APX target H2O2, a pattern similar to that observed in the ThGSTZ1 gene from Tamarix hispida (Gao et al. 2016). Moreover, GST activity was significantly higher in transgenic lines than in WT, consistent with the roles of ThGSTZ1, GsGSTU13, and AtGSTU19 in boosting GST activity under salt stress in other species (Gao et al. 2016; Jia et al. 2016). Collectively, these findings highlight VvGSTU14 as a positive regulator of salt stress tolerance via enhancement of both antioxidant enzyme activities and osmotic adjustment capacity.
4.4 Application Prospects and Limitations of This Study in Grape Cultivation
The functional characterization of VvFLS and VvGSTU14 provides valuable insights into the molecular mechanisms underlying salt tolerance in grapevine and offers promising genetic resources for the development of stress-resilient cultivars. These genes could be harnessed through transgenic or gene-editing approaches to enhance antioxidant capacity and osmotic regulation in commercial grape varieties grown in salinized areas. However, current findings are based on callus-level studies, and further validation in whole plants under field-like conditions is essential to assess agronomic performance, stability, and potential trade-offs. Additionally, regulatory and public acceptance issues surrounding genetically modified grapevines may pose limitations to large-scale application.
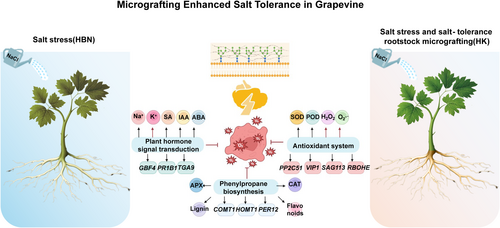
5 Conclusion
Salt stress significantly affects the growth and morphology of grapevines. However, micrografting with salt-tolerant rootstocks can effectively alleviate these negative impacts and significantly improve the salt tolerance of grapevines (Figure 17). At the physiological level, micrografting with salt-tolerant rootstocks significantly increases the activities of antioxidant enzymes in grapevines, optimizes the homeostasis of Na+ and K+ ions, and increases the contents of substances such as ABA, SA, IAA, lignin, and flavonoids. At the transcriptomic level, it regulates the expression of key genes in pathways such as “Plant Hormone Signal Transduction (ko04075),” “MAPK Signaling Pathway (ko04016),” and “Phenylpropanoid Biosynthesis (ko00940).” These mechanisms enhance the adaptability and tolerance of grapevines to salt stress by scavenging ROS and reducing membrane damage. In addition, overexpression of the VvFLS and VvGSTU14 genes can significantly enhance the salt tolerance of grape callus tissues, providing new gene resources and theoretical support for the cultivation of salt-tolerant grape varieties.
Author Contributions
Designed the experiments, analyzed the data, and wrote the manuscript: Ying Lai and Sheng Li. Performed the experiments: Ying Lai, Haokai Yan, Ping Sun, Guiping Chen, Zhilong Li, Jingrong Zhang, Jianping Wang, and Lei Ma. Provided experimental equipment and sites: Shaoying Ma. Revised the manuscript: Sheng Li, Guojie Nai, and Jinyu Bao. All authors read and approved the manuscript.
Acknowledgements
This work was supported by the Project funded by the Central Government - Guided Local Scientific and Technological Development Funds (25ZYJA033) and Gansu Provincial Key Talent Project (2023RCXM23).
Open Research
Data Availability Statement
To support the results, the RNA-seq raw data set has been deposited in the NCBI database (https://www.ncbi.nlm.nih.gov/bioproject/, released on 2025-07-07), with an accession of PRJNA1284448.




