CNGC2 Negatively Regulates Stomatal Closure and Is Not Required for flg22- and H2O2-Induced Guard Cell [Ca2+]cyt Elevation in Arabidopsis thaliana
Funding: This work was supported by Japan Society for the Promotion of Science, JSPS KAKENHI (grant numbers 23K23569 and 25H00921 to Y.M. and grant numbers 22K05560 and 25K01980 to S.M.).
ABSTRACT
In guard cells, cytosolic Ca2+ acts as a second messenger that mediates abscisic acid (ABA)- and pathogen-associated molecular pattern (PAMP)-induced stomatal closure. It was reported that Arabidopsis cyclic nucleotide-gated ion channel 2 (CNGC2) functions as hydrogen peroxide (H2O2)- and PAMP-activated Ca2+-permeable channels at the plasma membrane of mesophyll cells and mediates Ca2+-dependent PAMP-triggered immunity. In this study, we examined the role of CNGC2 in the regulation of stomatal movement because CNGC2 is also expressed in guard cells. We found that stomata of the CNGC2 disruption mutant cngc2-3 are constitutively closed even in the absence of ABA or the flagellar-derived PAMP, flg22. Consistently, leaf temperatures of the cngc2-3 mutant were higher than those of wild-type (WT) plants. The stomatal phenotype of the cngc2-3 mutant was restored by complementation with wild-type CNGC2 under the control of the guard cell preferential promoter, pGC1. Elevation of cytosolic free Ca2+ concentration in guard cells induced by flg22 and H2O2 remained intact in the cngc2-3 mutant. The introduction of the ost1-3 mutation into the cngc2-3 background did not alter the stomatal phenotype. However, the stomatal phenotype of the cngc2-3 mutant was successfully rescued in the double disruption mutant cngc2-3aba2-2. Taken together, these results suggest that CNGC2 negatively regulates stomatal closure response and does not function as flg22– and H2O2-activated Ca2+ channels in guard cells. Though CNGC2 is responsive for H2O2- and flg22-induced [Ca2+]cyt elevation in mesophyll cells, the involvement of CNGC2 in the response to H2O2 and flg22 in guard cells is questionable.
1 Introduction
Guard cells can sense various physiological stimuli, including light, drought, CO2, and phytohormones such as abscisic acid (ABA) and regulate stomatal apertures, which allow plants to optimize CO2 uptake for photosynthesis and transpirational water loss, and adapt to the surrounding environment (Schroeder et al. 2001; Assmann and Jegla 2016). In addition, guard cells can recognize pathogen infection via the pathogen-associated molecular patterns (PAMPs) and close stomatal pores to prevent pathogen entry into leaves, a process called stomatal immunity (Melotto et al. 2006).
Cytosolic calcium ion (Ca2+) in guard cells functions as a second messenger that controls stomatal movement. A rapid increase in cytosolic free Ca2+ concentration ([Ca2+]cyt) in guard cells occurs in response to ABA (McAinsh et al. 1990; Allen et al. 1999), reactive oxygen species (ROS) (Pei et al. 2000), and PAMPs (Thor and Peiter 2014). The guard cell [Ca2+]cyt increase activates the plasma membrane anion channel, slow-anion channel associated 1 (SLAC1) (Mori et al. 2006; Vahisalu et al. 2008; Brandt et al. 2015) and inactivates the plasma membrane (PM) H+-ATPase (Kinoshita et al. 1995), leading to plasma membrane depolarization and thereby stomatal closure. In addition, some other studies have reported the possible involvement of cytosolic Ca2+ as a positive regulator for stomatal opening. Blue light triggers guard cell [Ca2+]cyt increase via hyperpolarization of guard cell plasma membrane (Harada and Shimazaki 2009). Auxin-induced stomatal opening is suppressed by the Ca2+ chelator, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) in Commelina (Cousson and Vavasseur 1998). Arabidopsis calcineurin B-like (CBL)-interacting protein kinase 23 (CIPK23) and CBL complex functions as a [Ca2+]cyt sensor that is activated by increased Ca2+ concentration (Maierhofer et al. 2014) and positively regulates blue light-dependent stomatal opening (Inoue et al. 2020). Low CO2-induced stomatal opening is partially mediated by another type of [Ca2+]cyt sensor kinases, calcium-dependent protein kinases (CPKs) (Schulze et al. 2021).
It has been suggested that the major routes for the Ca2+ entry in guard cells are plasma membrane Ca2+-permeable ion channels (Hedrich 2012; Jezek and Blatt 2017). Patch-clamp analyses using Arabidopsis guard cell protoplasts revealed that hyperpolarization-activated Ca2+-permeable non-selective cation channels are activated by ABA via NAD(P)H oxidase-dependent ROS production (Pei et al. 2000; Murata et al. 2001; Kwak et al. 2003). Recently, Tan et al. (2023) reported that the four cyclic nucleotide-gated channels (CNGCs), CNGC5, 6, 9, and 12, function as ABA-activated plasma membrane Ca2+ channels that are required for ABA-induced stomatal closure in Arabidopsis. The ABA-activated protein kinase open stomata 1 (OST1) phosphorylates and activates the CNGCs (Yang et al. 2024), but the CNGCs are not the targets of ROS (Tan et al. 2023), implying that in addition to the CNGCs, molecularly unidentified ROS-activated Ca2+ channels are also involved in Ca2+-dependent ABA signaling. In addition to ABA, PAMPs, including the bacterial flagellar peptide (flg22), bacterial elongation factor (EF-tu) peptide (elf26), and lipopolysaccharide (LPS) induce stomatal closure via guard cell [Ca2+]cyt increase (Arnaud and Hwang 2015; Kim and Liang 2019). It was reported that Arabidopsis reduced hyperosmolality-induced Ca2+ increase 1.3 and 1.6 (OSCA1.3 and 1.6) function as guard cell plasma membrane Ca2+ channels that are required for flg22-induced stomatal closure (Thor et al. 2020). In the double gene disruption mutant osca1.3osca1.6, flg22-induced stomatal closure is impaired, but ABA-induced stomatal closure remains intact (Thor et al. 2020).
Arabidopsis CNGC2/DND1 was identified from a null mutant ‘defense, no death’ (dnd1), which exhibits reduced hypersensitive response (HR) cell death in effector triggered immunity (ETI) (Clough et al. 2000). The CNGC2 together with CNGC4 form a functional Ca2+ channel when co-expressed in Xenopus oocytes (Tian et al. 2019). The Arabidopsis CNGC2-defective mutant cngc2 shows reduction of flg22- and hydrogen peroxide (H2O2)-triggered calcium channel activation in mesophyll cells (Tian et al. 2019). In addition, the cngc2 mutant shows reduced flg22- and H2O2-induced [Ca2+]cyt increase, resulting in compromised resistance against pathogen attack. These results suggest that CNGC2 functions as flg22- and ROS-activated Ca2+ channels in mesophyll cells and mediates pathogen resistance. CNGC2 is also highly expressed in guard cells (Wang et al. 2013). A recent study shows that CNGC2 is involved in [Ca2+]cyt increase at a whole-plant level and regulation of stomatal opening induced by high humidity treatment (Hussain et al. 2024). However, the role of CNGC2 in stomatal response to other stimuli and its function in guard cells have not been well established. In this study, we evaluated the role of CNGC2 in the regulation of stomatal movement in response to several stimuli, including flg22 and H2O2. Our data suggest that CNGC2-mediated [Ca2+]cyt increase functions as a negative regulator of stomatal closure.
2 Materials and Methods
2.1 Plant Materials and Growth Conditions
Arabidopsis thaliana ecotypes Colombia-0 (Col-0) and Wassilewskija (Ws) were used as wild-type (WT) plants. In addition, T-DNA insertion mutants cngc2-3 (Col-0 background), cngc2-2 (Ws background) (Chan et al. 2003), cngc2-3ost1-3 (Col-0 background), cngc2-3aba2-2 (Col-0 background), and CNGC2 complementation line (cngc2-3pGC1::CNGC2) were used for this study. The CNGC2 and OST1 loss-of-function mutant cngc2-3ost1-3 was generated by crossing T-DNA insertion mutants cngc2-3 (SALK_066908) (Chin et al. 2013) and ost1-3 (SALK_008068) (Yoshida et al. 2002). Similarly, the double disruption mutant cngc2-3aba2-2 was also generated by crossing between the T-DNA insertion mutant cngc2-3 and fast neutron-generated mutant aba2-2 (Nambara et al. 1998). For the generation of the complemented line in the background of cngc2-3, the guard cell preferential promoter pGC1 (Yang et al. 2008), wild-type CNGC2 coding sequence, and Venus were cloned into the pHygII-UT vector (Waadt et al. 2008; Kunz et al. 2014). Agrobacterium tumefaciens GV3101 carrying the construct was used to transform the cngc2-3 mutant by the floral dip method (Clough and Bent 1998). All these Arabidopsis seeds were sown on a soil mixture of 1:1 soil: vermiculite (v/v) and grown in growth chambers with controlled conditions (16/8 h light/dark condition at 22°C temperature, 80 μmol m−2 s−1 light intensity, and 70% relative humidity) after being stratified at 4°C for 3 days. Plants were watered twice a week with 0.1% (v/v) Hyponex. For all experiments, rosette leaves from 3- to 5-week-old plants were used.
2.2 Measurement of Stomatal Aperture
Stomatal apertures were measured as described previously (Munemasa et al. 2019) with a few modifications. Fully expanded rosette leaves were detached and floated on stomatal bioassay buffer containing 5 mM KCl, 50 μM CaCl2, and 10 mM MES/Tris (pH 5.6). The leaves were incubated for 2 h in light to induce stomatal opening and then treated with 10 μM ABA for 2 h or 2 μM flg22 for 1 h in the stomatal assay buffer. To determine the stomatal opening by fusicoccin (FC), leaves were incubated in stomatal opening buffer for 2 h in the dark. Then 10 μM FC was added and incubated in the dark for 2 h. After incubation, the treated leaves were shredded in a blender, and the epidermal peels were collected with a nylon mesh. The peels were placed on a glass slide and photographed under an optical microscope (IX71, Olympus). At least 20 stomatal apertures of a leaf of one plant were measured per independent experiment using WinRoof3.0 software (Mitani Corporation) to calculate an average. The experiments were performed with at least three independent replications using different plants to obtain the average (n = 3, 60 stomata).
2.3 Determination of Leaf Temperature
For leaf temperature measurement, 3- to 4-week-old plants grown in the soil were considered. Leaf temperature was measured using an infrared thermography instrument (InfReC R550/R450; Nippon Avionics Co. Ltd.) and analyzed images using the InfReC Analyzer NS9500 standard software.
2.4 Cloning and cRNA Synthesis for Two-Electrode Voltage-Clamp Analysis
CNGC2 (AT5G15410), CNGC4 (AT5G54250), and AtCaM4 (AT1G66410) were cloned into the pNB1u oocyte expression vector by the USER cloning strategy (Nour-Eldin et al. 2006). The capped RNA (cRNA) was synthesized from 1 μg of linearized plasmid DNA template using the mMESSAGE mMACHINE T7 Transcription kit (Thermo Fisher Scientific), according to the manufacturer's instructions. The quality of synthesized cRNA was checked by agarose gel electrophoresis.
2.5 Two-Electrode Voltage-Clamp Analysis
Each Xenopus laevis oocyte was injected with 50 mL of water or cRNA (each 10 ng) and incubated in ND96 buffer (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM HEPES/NaOH, pH 7.5) at 18°C for a few days before electrophysiological recordings previously described by Tian et al. (2019). The glass pipettes were filled with 3 M KCl. The bath solution contained 5 mM CaCl2, 2 mM KCl, 1 mM MgCl2, and 10 mM MES/Tris to adjust the pH to 5.6. The osmolality was adjusted to 220 mM using D-mannitol. The oocytes were continuously perfused with the bath solution during the experiment. Currents were recorded with a holding potential of 0 mV and ranging from +40 to −180 mV in 20-mV decrements. Voltage-clamp recordings were performed using an Axoclamp 900A amplifier (Molecular Devices); data were recorded using a Digidata 1440A system (Molecular Devices) and analyzed using PCLAMP 10.7 software (Molecular Devices).
2.6 Guard Cell [Ca2+]cyt Imaging
Transgenic Arabidopsis plants expressing Nuclear Export Signal (NES)-fused Yellow Cameleon3.6 (NES-YC3.6) under the control of the guard cell preferential promoter pGC1 were used in this study (Yang et al. 2008; Krebs et al. 2012). The rosette leaves of 3- to 4-week-old seedlings were detached, and the abaxial side of the leaf was gently adhered to a cover glass with an adhesive. Then, abaxial epidermal strips of leaves were peeled off, and mesophyll tissues were removed with a sharp razor blade. The epidermal strips were immersed in stomatal opening buffer and incubated under light for at least 2 h at 22°C. Turgid guard cells were selected and used for [Ca2+]cyt imaging. 5 min after starting the experiment, guard cells were treated with 500 nM flg22, 500 μM H2O2, 50 μM ABA, or 10 mM CaCl2, and the response was monitored for 30 min. Fluorescence intensities (F535 and F480) from the cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) in guard cells were measured under a fluorescence microscope (IX73, Olympus) equipped with a dual-emission imaging system (W-View Gemini system; 440AF21 excitation filter, 445DRLP dichroic mirror and 2 emission filters, 480DF30 for CFP and 535DF25 for YFP, Hamamatsu Photonics) and a CMOS camera (Hamamatsu ORCA-Flash4.0 digital camera, Hamamatsu Photonics). The same exposure time was used for both CFP and YFP and for the analysis. [Ca2+]cyt elevation was counted when changes in fluorescence ratios (F535/F480) were ≥ 0.1 unit from the baseline.
2.7 ABA Content Measurement
Approximately 0.1 g (fresh weight) of rosettes were frozen in liquid nitrogen, ground, and extracted with 4 mL of extraction solvent (80% acetonitrile, 19% water, 1% acetic acid) containing isotope-labelled internal standards (d6-ABA; OlChemim s.r.o.). After incubation for 1 h at 4°C and centrifugation, the supernatant was combined with a rinse of the pellet and purified by sequential solid-phase extraction using Oasis HLB, MCX, and WAX cartridges (Waters Corporation), as described in Gupta et al. (2017). The final ABA fraction, eluted with extraction solvent from the WAX cartridge, was dried and reconstituted in 100 μL of 1% aqueous acetic acid. ABA levels were quantified using an Agilent 1260–6410 Triple Quadrupole LC–MS system equipped with a ZORBAX Eclipse XDB-C18 column. Chromatographic conditions followed Tsukahara et al. (2015), using a solvent gradient (3%–50% acetonitrile with acetic acid) over 20 min at a flow rate of 4 mL min−1. MS/MS transitions were m/z 263/153 for ABA and m/z 269/159 for d6-ABA, in negative ion mode, with a retention time of approximately 12.6 min.
2.8 Statistical Analysis
For data analysis, the chi-squared (χ2) test and one-way ANOVA with Tukey's test were used to assess significant differences between datasets. For significant differences, p values < 0.05 were considered.
3 Results
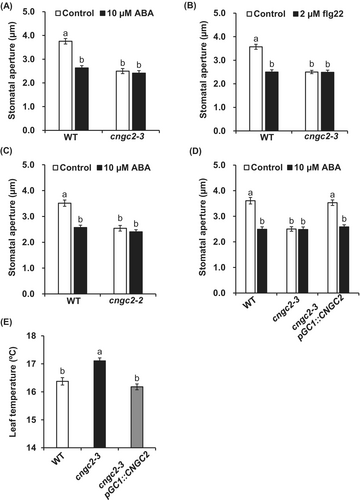
3.1 Disruption of CNGC2 Confers Constitutive Stomatal Closing Phenotype in Arabidopsis
To evaluate functions of the Arabidopsis plasma membrane Ca2+-permeable channel CNGC2 in stomatal movement, first, we examined the stomatal response of the cngc2-3 mutant (Col-0 background) and cngc2-2 mutant (Ws background). The experiment was carried out with detached leaves. Application of ABA at 10 μM or flg22 at 2 μM induced stomatal closure in wild-type plants, but the stomatal pores of the cngc2-3 mutant were closed even without ABA or flg22 treatment (Figure 1A,B). A similar phenotype was also observed in the cngc2-2 mutant (Figure 1C). Complementation of the cngc2-3 mutation with the wild-type CNGC2 fused with Venus under control of guard cell preferential promoter pGC1 (Yang et al. 2008) restored the stomatal response (Figure 1D), indicating that disruption of CNGC2 expressed in guard cells is responsible for the cngc2-3 phenotype. Consistent with the result of stomatal aperture measurement, the leaf temperature of the cngc2-3 mutant was higher than that of wild-type plants, and there was no significant difference in leaf temperature between wild-type plants and the guard cell CNGC2 complementation line (cngc2-3pGC1::CNGC2) (Figure 1E). To check if CNGC2 disruption causes a complete loss of stomatal opening ability, we performed stomatal aperture measurement with a fungal phytotoxin fusicoccin (FC) that causes stomatal opening through activation of the plasma membrane H+-ATPase in guard cells (Kinoshita and Shimazaki 2001). Similar to wild-type, the cngc2-3 mutant opened stomata in response to FC treatment (Figure S1), indicating that the cngc2-3 mutant is defective in guard cell signaling that regulates stomatal movement, but not in the development of functional stomata.
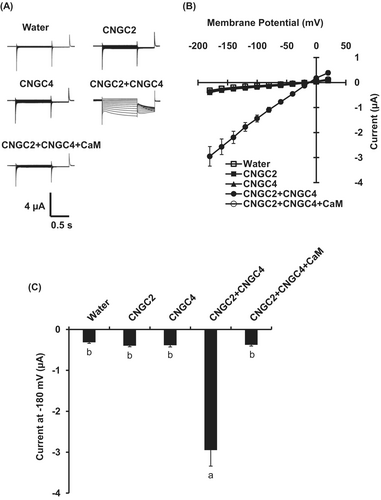
3.2 CNGC2 Is Not Involved in flg22- and H2O2-Induced [Ca2+]cyt Elevation in Guard Cells
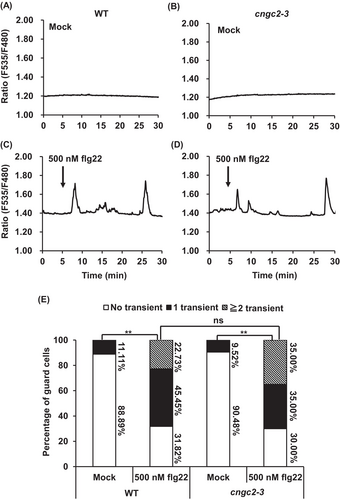
In vitro two-electrode voltage clamp analysis revealed that CNGC2, together with CNGC4, forms a functional Ca2+ channel in Xenopus oocytes (Figure 2; Tian et al. 2019). In mesophyll cells, CNGC2 is required for H2O2- and PAMP-induced [Ca2+]cyt elevation (Tian et al. 2019). Next, we tested whether CNGC2 is also involved in PAMP- and H2O2-induced [Ca2+]cyt elevation in guard cells using the genetically encoded [Ca2+]cyt indicator, NES-YC3.6. We monitored [Ca2+]cyt elevation in guard cells using isolated epidermal strips.
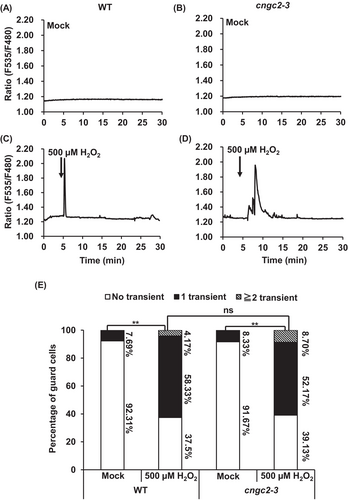
The [Ca2+]cyt spikes (transient [Ca2+]cyt elevations) were observed in 11.11% of wild-type guard cells treated without flg22 (n = 18) (Figure 3A,E) and 68.18% of guard cells treated with 500 nM flg22 (n = 22) (Figure 3C,E). The frequency of [Ca2+]cyt spikes in wild-type guard cells treated with 500 nM flg22 was significantly larger than that in the wild-type guard cells treated without flg22 (p < 0.01) (Figure 3E), as previously reported (Thor and Peiter 2014). In the cngc2-3 mutant, Ca2+ peaks were found in 9.52% of guard cells treated with no flg22 (n = 21) (Figure 3B,E) and 70.00% of guard cells treated with 500 nM flg22 (n = 20) (Figure 3D,E). The number of [Ca2+]cyt spikes in the guard cells of cngc2-3 treated with 500 nM flg22 was significantly higher than that in the cngc2-3 guard cells treated without flg22 (p < 0.01) (Figure 3E). The percentage of guard cells that showed [Ca2+]cyt spikes in the cngc2-3 guard cells treated with 500 nM flg22 was not significantly different from those in wild-type guard cells treated with 500 nM flg22 (Figure 3E). Next, we evaluated the H2O2-induced [Ca2+]cyt elevation in guard cells. Similar to flg22, 500 μM H2O2 treatment increased percentages of guard cells showing [Ca2+]cyt spikes in both wild-type and cngc2-3 guard cells, and there was no significant difference between wild-type and cngc2-3 guard cells showing the H2O2-induced [Ca2+]cyt spikes (Figure 4E). We also tested whether CNGC2 is involved in guard cells [Ca2+]cyt increases induced by other stimuli, including ABA and high extracellular Ca2+. We found that treatment with 50 μM ABA or 10 mM CaCl2 induced [Ca2+]cyt elevations in both wild-type and cngc2-3 guard cells (Figures S2 and S3) and no significant difference was observed in ABA- and high extracellular Ca2+-triggered guard cells [Ca2+]cyt elevation between wild-type and cngc2-3 mutant (Figures S2 and S3). A well-known plasma membrane channel blocker, lanthanum chloride (LaCl3) completely inhibited the flg22-, H2O2-, ABA-, and high extracellular Ca2+-induced [Ca2+]cyt spikes (Data not shown), indicating that all the stimuli trigger [Ca2+]cyt elevation through the activation of the plasma membrane Ca2+ channels, as previously reported (Pei et al. 2000; Murata et al. 2001; Thor et al. 2020). Taken together, these results suggest that CNGC2 is not required in flg22-, H2O2-, ABA-, and high extracellular Ca2+-induced [Ca2+]cyt increase in guard cells.
3.3 The cngc2-3 Phenotype Is Dependent on Endogenous ABA but Not OST1 Kinase
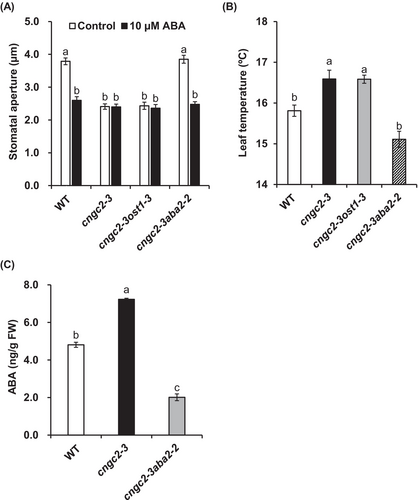
To analyze the mechanism that causes the cngc2-3 stomatal phenotype, we performed stomatal aperture measurements using the double disruption mutants cngc2-3ost1-3 and cngc2-3aba2-2. The Ca2+-independent protein kinase OST1 is one of the major components for Ca2+-independent ABA signaling in guard cells (Mustilli et al. 2002) and phosphorylates the downstream targets, including SLAC1 (Geiger et al. 2009; Lee et al. 2009) and CNGC5, 6, 9, and 12 (Yang et al. 2024). The short-chain alcohol dehydrogenase ABA2 converts xanthoxin to abscisic aldehyde, and ABA2 disruption causes a severe ABA-deficient phenotype (Cheng et al. 2002; González-Guzmán et al. 2002). We found that, similar to the cngc2-3 mutant, the cngc2-3ost1-3 mutant showed a constitutive closed stomatal phenotype (Figure 5A). However, unlike the cngc2-3 mutant and cngc2-3ost1-3 double mutant, the cngc2-3aba2-2 double mutant showed a wild-type-like stomatal phenotype (Figure 5A), indicating that the introduction of the aba2-2 mutation into the cngc2-3 background restored the stomatal phenotype. Consistently, the cngc2-3ost1-3 double mutant showed higher leaf temperature compared to the wild-type plant, but no significant difference was observed in leaf temperature between the wild-type and cngc2-3aba2-2 double mutant (Figure 5B). Next, to check whether the constitutive closed stomatal phenotype of the cngc2-3 mutant is due to ABA over-accumulation, we measured the ABA content in rosette leaves. We found that the ABA content in cngc2-3 rosette leaves was significantly, but only 1.5 times higher than that in wild-type rosette leaves (Figure 5C).
4 Discussion
Cytosolic Ca2+ plays a key role in regulating guard cell ion transport and stomatal movements (McAinsh et al. 1990; Mori et al. 2006; Siegel et al. 2009; Chen et al. 2010; Wang et al. 2013). The role of Ca2+ as a positive regulator in stomatal closure has been well established (De Silva et al. 1985; Pei et al. 2000; Hossain et al. 2011; Murata et al. 2015; Agurla et al. 2018; Tian et al. 2019). The involvement of Ca2+ as a positive regulator in stomatal opening has also been suggested in some studies (Shimazaki et al. 1992; Cousson and Vavasseur 1998; Harada and Shimazaki 2009; Inoue et al. 2020; Schulze et al. 2021). In guard cells, the plasma membrane Ca2+ channels act as the core players that trigger the stimuli-induced [Ca2+]cyt elevation. The Arabidopsis plasma membrane Ca2+ channel CNGC2 is involved in various physiological processes including pathogen resistance (Yu et al. 1998; Clough et al. 2000; Tian et al. 2019), calcium sensitivity (Chan et al. 2003; Wang et al. 2017), flower development (Chaiwongsar et al. 2009; Chin et al. 2013), heat tolerance (Finka et al. 2012; Lu et al. 2022), and auxin homeostasis (Chakraborty et al. 2021). Recently, Tian et al. (2019) reported that in mesophyll cells, CNGC2 functions as a plasma membrane Ca2+-permeable channel that mediates H2O2 and flg22 responses. CNGC2 is also expressed in guard cells (Wang et al. 2013) and guard cells close stomata in response to H2O2 (Pei et al. 2000) and flg22 (Melotto et al. 2006). Therefore, here we analyzed the role of CNGC2 in the regulation of guard cell signaling and stomatal movement.
In this study, we found that the cngc2 mutants showed constantly closed stomata (Figure 1A–C), as recently reported (Hussain et al. 2024). In addition, the complementation analysis revealed that disruption of guard cell-expressed CNGC2 is responsible for the cngc2 stomatal phenotype (Figure 1D). Consistently, the cngc2-3 mutant showed higher leaf temperature than that of wild-type and CNGC2 complementation line under control growth conditions (Figure 1E). These results suggest that guard cell CNGC2 is a negative regulator of stomatal closure response.
Although CNGC2 forms a functional Ca2+-permeable cation channel (Figure 2) and is responsive for H2O2- and flg22-induced [Ca2+]cyt elevation and immune responses in mesophyll cells (Tian et al. 2019), flg22- and H2O2-induced [Ca2+]cyt elevation remained intact in the cngc2-3 guard cells (Figures 3 and 4). Therefore, the function of CNGC2 as a flg22- and H2O2-activated Ca2+ permeable cation channel might be cell-type specific. In addition to flg22 and H2O2, ABA and high extracellular Ca2+, stimuli that induce stomatal closure, were able to induce guard cell [Ca2+]cyt elevation in the cngc2-3 mutant as in wild-type plants (Figures S2 and S3). This result suggests that CNGC2 does not function as Ca2+ permeable channels responsible for stomatal closure induced by ABA and high extracellular Ca2+.
The introduction of the aba2-2 mutation into the cngc2-3 background restored the stomatal phenotype of the cngc2-3 mutant (Figure 5A). On the other hand, the double disruption mutant cngc2-3ost1-3 showed the constitutively closed stomatal phenotype as the cngc2-3 mutant did (Figure 5A). These results suggest that the stomatal phenotype of the cngc2-3 mutant is dependent on endogenous ABA, but not the OST1 kinase. Overaccumulation of ABA may be the possible cause of the constitutive closed stomatal phenotype of the cngc2-3 mutant (Figure 5C). However, since it has been reported that ABA contents in Arabidopsis normally increase 10- to 100-fold in response to drought stress and other conditions (Kale et al. 2019; Sato et al. 2018; Gupta et al. 2017), solid conclusions cannot be drawn from the slight increase (1.5-fold) in ABA content observed in the cngc2-3 mutant.
A recent study reported that CNGC2 mediates high humidity-induced stomatal opening (Hussain et al. 2024). CYP707A3, encoding ABA 8′-hydroxylase, is responsible for the inactivation of the major ABA pool under high humidity conditions (Okamoto et al. 2009). High humidity triggers CNGC2-dependent [Ca2+]cyt increase at the outermost regions of leaves, which leads to the activation of calmodulin (CaM)-binding transcriptional activators (CAMTAs), resulting in the induction of CYP707A3 expression. The CNGC2/CAMTA-dependent transcriptional activation of CYP707A3 causes ABA catabolism and subsequent stomatal opening (Hussain et al. 2024). This is consistent with our data showing a slight increase in ABA contents in the cngc2-3 mutant grown under the condition where the relative humidity is 70% (Figure 5C). We found that the disruption of the guard cell core ABA signaling component OST1 kinase did not restore the stomatal phenotype of the cngc2-3 mutant (Figure 5A), suggesting that, unlike low humidity-induced stomatal closure (Merilo et al. 2013; Merilo et al. 2018), high humidity-induced stomatal opening is less dependent on the OST1 kinase. This could be explained by high humidity-activated ABA-dependent but OST1-independent signaling, but the details need to be studied in the future.
In conclusion, our findings suggest that, unlike mesophyll cells, guard cells do not utilize CNGC2 as a Ca2+-permeable channel that is required for flg22- and H2O2-induced [Ca2+]cyt increase. CNGC2 functions as a negative regulator of stomatal closure through ABA-dependent, but OST1-independent pathways.
Author Contributions
Rojina Akter: conceptualization, investigation, writing – original draft, writing – review and editing. Yasuhiro Inoue: methodology. Saori Masumoto: methodology. Yoshiharu Mimata: methodology. Takakazu Matsuura: investigation. Izumi C. Mori: investigation, writing – original draft. Toshiyuki Nakamura: supervision. Yoshimasa Nakamura: supervision. Yoshiyuki Murata: supervision, funding acquisition, writing – review and editing. Shintaro Munemasa: conceptualization, investigation, supervision, funding acquisition, writing – original draft, writing – review and editing.
Acknowledgements
This work was supported by the Joint Usage/Research Center, Institute of Plant Science and Resources, Okayama University. We are grateful to Rainer Waadt and Jörg Kudla (University of Münster) for kindly providing the pHygII-UT construct. We also thank Catherine W.M. Chan (University of Wisconsin-Whitewater) for kindly providing the cngc2-2 seeds.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data will be available on request.




