Integrated Physiological and Transcriptomic Analysis Reveals Transcription Factors Are Crucial for Melatonin-Mediated Drought Tolerance in E. ulmoides
Minmin He and Zhanchao Yang contributed equally to this work.
Funding: This work was supported by the National Key Research and Development Program of China (No. 2021YFA1300401), the Innovation Center for Academicians of Hainan Province and the Specific Research Fund of the Innovation Platform for Academicians of Hainan Province (No. YSPTZX202309), the Key Technology Research and Development Program for Rubber-producing Plants of Guizhou Province (No. BQW2024-005), the Special Fund of Guizhou University (LJ2023-03), the Guizhou Provincial Basic Research Program (Natural Science) (No. QianKeHeJiChu-ZK[2024]YiBan 041) and the Cultivation Project of Guizhou University (No. 2020-54).
ABSTRACT
Eucommia ulmoides is a Chinese herbal medicine, and much attention has been paid to its tolerance mechanism under stress conditions. Among them, drought is a severe stress that affects the plant's growth and development. Here, we assessed the protective efficiency of different concentrations of melatonin in the leaves of Eucommia ulmoides under drought stress. Our study revealed how exogenous melatonin enhanced drought tolerance in E. ulmoides through integrated physiological and molecular mechanisms. Melatonin preserved photosynthetic capacity by upregulating genes like PsbS, stabilizing PSII, scavenging ROS, and activating antioxidant enzymes. Moreover, melatonin promoted the reconstruction of redox homeostasis by mediating MAPK and ABA signal transduction and endogenous hormone crosstalk signals to balance ROS homeostasis and stress gene expression. It also differentially regulated MYC transcription factors (e.g., EuMYC2/8), redirected jasmonic acid signaling from root growth to stress adaptation, and optimized carbon metabolism by promoting starch-to-sugar conversion and enhanced the phenylpropanoid flux, reinforcing cell walls and antioxidant defenses. Our results provide new insights into the morphological, physiological, and transcriptional responses in the leaves of E. ulmoides for drought stress and reveal the molecular mechanism of exogenous melatonin in improving the drought resistance ability in E. ulmoides.
1 Introduction
Eucommia ulmoides (E. ulmoides), commonly known as “dù-zhòng” in Chinese, is a tertiary relict tree species endemic to central China. Renowned for its rich reservoir of bioactive secondary metabolites, it has been documented in classical Chinese pharmacopeias such as Shen Nong Ben Cao Jing and Ben Cao Gang Mu for over two millennia (Zhu and Sun 2018). Today, its cultivation spans multiple Chinese provinces, for example, Neimeng, Guizhou, and East Asian countries like Japan and Korea, predominantly at elevations of 300–1300 m. Phytochemical studies have identified 138 distinct compounds in its bark and leaves, including lignans, iridoids, and flavonoids, which confer multifaceted pharmacological properties (Zhang et al. 2013; Wang et al. 2019). These bioactive constituents exhibit (1) cardiovascular benefits in the form of antihypertensive and anti-atherosclerotic effects; (2) immunomodulatory activity by enhancing macrophage phagocytosis and lymphocyte proliferation; (3) bone health promotion with inhibiting osteoclastogenesis via RANKL signaling suppression; and (4) antioxidant capacity by scavenging free radicals, for example, DPPH, ABTS+, and protecting biomolecules (DNA, deoxyribose) from oxidative damage. Such broad-spectrum bioactivities position E. ulmoides as a promising candidate for nutraceutical and pharmaceutical development.
Water is crucial for plant growth and development. However, due to uneven precipitation distribution and poor soil drainage systems, drought stress has become a major cause of global crop loss. It limits agricultural production by over one-third in arable areas and accounts for nearly 70% of worldwide crop yield loss (Zhu et al. 2023). Drought stress is particularly damaging during the reproductive stage of plants, causing irreversible physiological, metabolic, and molecular changes (Cohen et al. 2020). Under drought conditions, plant cell elongation is hindered due to disrupted water transport from the xylem to elongating cells (Kanwal et al. 2017). This leads to reduced leaf areas, plant height, and overall crop development, as mitosis, cell expansion, and elongation are impaired (Dubey et al. 2021). Plants respond to drought stress by rapidly synthesizing abscisic acid in roots, which triggers downstream signaling pathways to close stomata. The resulting reduced CO2 uptake decreases photosynthetic efficiency (Kim et al. 2022). The primary injury to plants under drought stress is membrane lipid peroxidation, causing cell membrane damage and disintegration (Liu et al. 2015; Hu et al. 2016). Excessive reactive oxygen species (ROS) accumulation can impair cellular functions, leading to lipid peroxidation, protein denaturation, photoinhibition, stomatal closure, and altered enzyme activity. To counteract these effects, plants have evolved mechanisms to maintain cellular balance and reduce oxidative damage. Under drought stress, plants accumulate more solutes and ROS-detoxifying proteins in the cytosol and extracellular matrix, and regulate antioxidant enzyme activities (Li et al. 2018; Xie et al. 2018; Razi and Muneer 2021). Superoxide dismutase (SOD) converts superoxide radicals to oxygen and hydrogen peroxide (H2O2), while catalase (CAT) and peroxidase (POD) break down H2O2 into water and oxygen (Huang et al. 2019; Catalá 2007). POD also removes H2O2, protects enzyme proteins, and promotes lignin formation. To maintain an appropriate cellular water potential balance under stress, plants accumulate solutes like proline, glycine betaine, soluble sugars, and spermine in cells, which help maintain the cell osmotic potential and enhance the water absorption capacity (Ozturk et al. 2021; Li et al. 2024).
Melatonin (N-acetyl-5-methoxytryptamine), a widely occurring natural biomolecule, is crucial for plant health. As a unique biological stimulant and plant growth regulator, exogenous melatonin can boost crop yield and quality by inducing gene expression and helping plants resist biotic and abiotic stresses (Moustafa-Farag et al. 2020; Bargunam et al. 2025). Melatonin safeguards photosynthetic systems, regulates stomatal function, and influences enzymes in amino acid, carbohydrate, lipid, and nitrogen metabolism (Karumannil et al. 2023). It can also enhance drought resistance and delay leaf senescence in various plant species under stress (Guo et al. 2023; Zia et al. 2019). Research showed that drought stress negatively impacts maize seedlings, reducing biomass, photosynthetic pigments, and increasing malondialdehyde and ROS (Ahmad, Ali, et al. 2021; Ahmad, Muhammad, et al. 2021). However, melatonin application could mitigate ROS-induced oxidative damage by enhancing photosynthetic pigments, antioxidant enzyme activities, relative water content, and osmoprotectants in maize seedlings. In olive seedlings subjected to salt stress, melatonin application restored growth parameters such as stem height, root length, and biomass (Zahedi et al. 2021). Exogenous melatonin also alleviates ionizing radiation-induced damage in wheat seedlings by modulating growth, osmotic adjustments, and photosynthetic capacity (Kurt-Celebi et al. 2022). Li, Huan, et al. (2022) and Li, Wang, et al. (2022) found that melatonin treatment significantly inhibits gray mold development and induces ROS outbreaks in cherry tomato fruits, enhancing their disease resistance. Under salt and drought stress, exogenous melatonin upregulated stress-responsive genes like OsSOS, OsNHX, OsHSF, and OsDREB in rice (Zhang et al. 2013). In drought-stressed soybeans, melatonin enhanced tolerance by improving photosynthetic rates and reducing abscisic acid, while increasing jasmonic acid and salicylic acid levels in roots (Imran et al. 2021). At the molecular level, melatonin enhanced plant drought tolerance by regulating the MAPK pathway, photosynthetic system, flavonoid biosynthesis, ascorbate-glutathione cycle, hormone metabolism, and carbohydrate metabolism (Wang et al. 2025; Menhas et al. 2025).
In this study, we examined the mechanisms of exogenous melatonin-mediated drought tolerance in the seedlings of Eucommia ulmoides by integrating morphological, physio-biochemical, and transcriptomic analyses. Our results provide deeper insights into the molecular responses associated with the melatonin-mediated alleviation of drought in E. ulmoides seedlings.
2 Material and Methods
2.1 Plant Materials and Treatments
E. ulmoides seeds were cultivated in a culture room at the College of Life Sciences, Guizhou University. After low-temperature (10°C) vernalization, the seeds were germinated on filter paper and transplanted into pots for soil culture (soil ratio example, sand:peat soil:perlite:vermiculite was 3:3:1:1), with five seedlings planted at uniform intervals in each pot. E. ulmoides seedlings were cultivated in a climate-controlled LED incubator (Model: HGZ-H250, Shanghai Yuejin Medical Instrument Co.) under the following conditions: temperature maintained at 22°C±1°C, relative humidity at 50%±5%, photosynthetic photon flux density at 160 ± 20μmolm−2 s−1, and a 14/10 h light/dark photoperiod. After 1 month of growth, the seedlings were transplanted, and one seedling was planted into each pot. After 3 months, uniform seedlings were selected for further study. All pots were fully watered until the relative water content (RWC) of the soil reached 100%, with the RWC calculated by weighing the soil. Drought treatment began when the soil's RWC decreased to 70%. The soil RWC in the normal water group (Control) was maintained at approximately 85%, while the soil RWC in the drought treatment groups was kept at approximately 50%. Melatonin solutions of different concentrations were applied via foliar spray for seven consecutive days.
In this experiment, five treatment groups were established as follows: (1) Control: seedlings were cultivated under normal water conditions, with the soil RWC maintained at 85%. The leaves were sprayed with distilled water daily. (2) Drought: seedlings were subjected to drought conditions, with the soil RWC maintained at 50%±5%. The leaves were treated with distilled water daily. (3) Drought+50μmol L−1 melatonin (D + MT50): under drought stress (soil RWC at 50%±5%), seedlings were treated with a 50 μmol L−1 melatonin solution applied to the leaves daily. (4) Drought+100 μmol L−1 melatonin (D + MT100): under drought stress (soil RWC at 50% ±5%), seedlings were treated with a 100μmol L−1 melatonin solution applied to the leaves daily. (5) Drought+200 μmol L−1 melatonin (D + MT200): under drought stress (soil RWC at 50% ± 5%), seedlings were treated with a 200 μmol L−1 melatonin solution applied to the leaves daily. Each treatment group consisted of six replicates, with 30 selected seedlings per replicate.
2.2 Phenotypic Index and Chlorophyll Content Determination
On the 7th day after drought and exogenous melatonin treatment, the following relevant phenotypic indexes were measured. The leaf inclination angle was measured by an electronic angle ruler, and the angle between the ventral normal of the first leaf and the zenith direction (stem) of each seedling was recorded as the leaf inclination angle, with nine repeats in each treatment. The length and width of the leaves were measured with a Vernier caliper. Three plants were randomly selected for each treatment, and the second leaf from the top down was selected to be recorded.
Visual detection of cell death was evaluated by staining of trypan blue as a method of Ahmad, Ali, et al. (2021) and Ahmad, Muhammad, et al. (2021). For each treatment, intact leaves were immersed in 20 mL of 0.0625% (w/v) trypan blue solution and incubated at 25°C for 24 h. Subsequently, samples were moved from the staining solution and placed in a de-staining solution (2.5 g mL−1 chloral hydrate) for 12 h. This procedure was repeated in three independent biological replicates.
Chlorophyll a (Chla) and chlorophyll b (Chlb) were extracted with 96% ethanol, expressed as mg g−1, and measured at wavelengths of 470, 665, and 649 nm following a previously published method (Khan et al. 2020).
2.3 Determination of Cell Membrane Lipid Permeability and Antioxidant Enzyme Activity of E. ulmoides
Membrane lipid peroxidation is a common phenomenon in organs under stress or during senescence (He et al. 2022). The stability of membrane lipids and the degree of peroxidation are typically evaluated by measuring the malondialdehyde (MDA) levels. Leaf tissues (0.5 g fresh weight) were homogenized in 5 mL of 5% (w/v) trichloroacetic acid and centrifuged at 10,000 × g for 15 min at 4°C. The supernatant (2 mL) was mixed with 2 mL of 0.67% (w/v) thiobarbituric acid (TBA) in a 95°C water bath for 30 min. After rapid cooling on ice, the reaction mixture was centrifuged at 8000 × g for 10 min. The absorbance of the supernatant (MDA-TBA adduct) was determined at 450, 532, and 600 nm using a spectrophotometer (UV-2000, UNICO Instruments Co. Ltd.), with MDA concentration calculated according to Shi et al. (2015).
The nitro-blue tetrazolium (NBT) staining method, which is based on the principle of O2·− reducing yellow NBT to dark blue insoluble formaldehyde (He et al. 2022), was used to measure superoxide radicals. Enzyme extracts were prepared according to the procedures of Rahman et al. (2019). The activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) were determined following the protocols of He et al. (2022). Three biological replicates were performed for each treatment.
2.4 Determination of the Osmotic Adjustment Substance Content of E. ulmoides
Soluble sugars, soluble proteins, and free proline act as osmotic regulators, helping plants tolerate oxidative stress. They maintain the cellular osmotic balance by lowering cell water osmotic pressure under stress (Ahmad, Ali, et al. 2021; Ahmad, Muhammad, et al. 2021), thus ensuring plant growth. Soluble sugar content was measured using an anthrone colorimetric method by Boxboi Kit (Beijing Boxbio Science & Technology Co. Ltd.). Soluble protein content was measured using Coomassie brilliant staining (Geng et al. 2020). Free proline content was determined by the method described by Wehner et al. (2016). The free proline content was extracted with toluene, and the absorbance was read at 520 nm. Three biological replicates were performed for each treatment.
2.5 The Comprehensive Evaluation of Drought Tolerance of E. ulmoides
| Treatment | Leaf inclination | Leaf length | Leaf width | Total chl | Soluble protein | Soluble sugar | Free proline | MDA | SOD | CAT | POD | Score | Rank |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.96 | 0.86 | 0.86 | 1 | 0 | 0 | 0 | 1 | 0.62 | 1 | 1 | 0.71 | 2 |
| Drought | 0 | 0.25 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0.25 | 5 |
| D + MT50 | 0.49 | 0.65 | 0.56 | 0.27 | 0.93 | 0.78 | 0.52 | 0.35 | 0.62 | 0.3 | 0.19 | 0.48 | 3 |
| D + MT100 | 1 | 1 | 1 | 0.63 | 0.6 | 0.63 | 0.32 | 0.76 | 0.95 | 0.52 | 0.87 | 0.73 | 1 |
| D + MT200 | 0.32 | 0 | 0.93 | 0.29 | 0.95 | 0.38 | 0.43 | 0.15 | 1 | 0.56 | 0.44 | 0.46 | 4 |
2.6 RNA Extraction, Illumina Sequencing, and Transcriptome Data Analysis
After foliar spraying of the melatonin for 7 days, the leaf samples were transported on dry ice to BENAGEN Co. Ltd. for RNA extraction, library construction, and NovASeq 6000 sequencing. High-throughput sequencing platforms generate a large amount of raw data from cDNA library sequencing in FASTQ format. The trimmomatic software was used to process the raw reads, remove low-quality reads, and finally obtain high-quality clean reads. The resulting high-quality sequences were compared with the reference genome of E. ulmoides using the hisat2 software. The resulting alignment files were subjected to quality assessment. Blast comparison was performed on the Plant TFDB database for the identification and classification of plant transcription factors (TFs). The screened readings were compared with the reference genome of E. ulmoides by Star (version: 2.7.9a). According to the expression matrix, the samples were analyzed by PCA. Gene expression levels were analyzed by FPKM values (fragments per kilobase of exon model per million mapped reads). Differentially expressed genes (DEGs) were identified by the conditions of |log2 (fold change)| > 1 and p < 0.05. GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment analyses were performed to determine the biological role and function of differentially expressed genes (Table S1–S7).
2.7 Quantitative Real-Time PCR Analysis of MYC Gene Family
Total RNAs of the different treatments were used to perform quantitative real-time PCR (qRT-PCR). Total RNAs were reverse-transcribed and qRT-PCR analyses were performed using the RT Kit with gDNA remover (AT311, Trans-Gen Biotech). Based on the selected MYC gene family (Table S8), the Batch g-PCR Primer Design function in TBtools was used to design primers. qRT-PCR was performed using the ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech) on a LightCycler 96 Real-Time PCR System (Roche Diagnostics Co. Ltd.) with programs: 95°C for 30 s, 40 cycles for 10 s at 95°C, and 30 s at 60°C. The relative gene transcript levels were calculated with the EuUBCE2 gene as an internal control gene, using the 2−ΔΔCt method.
2.8 Statistical Analysis
The experiment used a randomized complete block design. The data are expressed as mean and standard error (SE). All data were subjected to analysis of variance according to the completely randomized design model using the SPSS 22.0 software (SPSS Inc.). Differences among means of the treatments were detected using Duncan's test. A p value < 0.05 was considered significant. The figures were done with Origin 2021 (Origin Lab Corporation). The correlated heatmap was completed by TBtools.
3 Results
3.1 Effects of Exogenous Melatonin on Growth and Leaf Chlorophyll Content Under Drought Stress in E. ulmoides
Drought stress significantly inhibits overall plant growth, but the application of melatonin significantly increased the growth characteristics of E. ulmoides seedlings. Compared with the control, both the leaf wilting and the leaf inclination were serious under drought stress (Figure 1A). Combined with the leaf inclination angle measurements (Figure 1B), melatonin application significantly mitigated drought-induced leaf water loss and wilting. When melatonin concentration was increased to 100 μmol L−1, the leaf inclination was the smallest, and there was no significant difference between D + MT100 and the control plants. After 7 days of drought treatment (Figure 1C), leaf length and leaf width decreased 2.35 and 8.3 mm. However, the leaf length and leaf width decreased the least in the D + MT100 treatment, which were 0.53 and 0.73 mm, respectively. The leaf width after spraying 100 and 200 μmol L−1 melatonin was significantly higher than that in drought treatment plants.
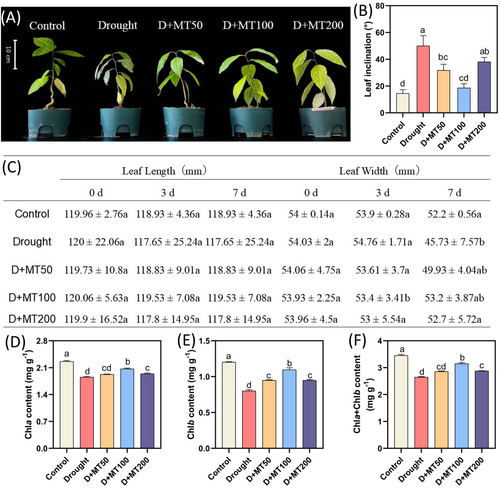
Compared with the control, drought treatment significantly decreased the content of Chla and Chlb in leaves. Under the same drought stress conditions, Chla, Chlb, and total Chl increased at first and then decreased significantly with the increase in the concentration of melatonin solution sprayed on the leaves. Chla (Figure 1D) reached the maximum of 2.069 mg g−1 FW after spraying 100 μmol L−1 melatonin, which was increased by 11.66% compared with drought treatment and significantly different from the other melatonin concentration treatments. At the same time, compared with drought treatment, the Chlb content was significantly increased by spraying 50–200 μmol L−1 melatonin. The change trend of total chlorophyll (Chla + Chlb) content was consistent with that of Chla. To sum up, 100 μmol L−1 melatonin significantly increased the photosynthetic pigment content of E. ulmoides seedlings. Taken together, those results suggested that exogenous melatonin could improve the growth characteristic of E. ulmoides under drought stress.
3.2 Effects of Exogenous Melatonin on Malondialdehyde Content, O2·− Levels, and Antioxidant Enzyme Activity in the Leaves Under Drought Stress
Malondialdehyde (MDA) content is usually used to reflect lipid peroxidation and oxidative injury of plants subjected to adverse environments. Here, we noticed that drought stress significantly increased MDA content in E. ulmoides leaves (Figure 2A) while spraying 50–100 μmol L−1 melatonin significantly decreased MDA accumulation. There was no significant difference in MDA content between drought treatment and melatonin concentration of 200 μmol L−1. Cell death phenotypic verification showed that drought stress significantly increased the blue patch area of leaves (Trypanblue positive rate: 80% increase compared with control), indicating impairment of cell membrane integrity and activation of programmed death (Figure S2A). Exogenous melatonin treatment (D + MT100) reduced the distribution of the blue patched area by 60%, confirming that melatonin alleviates drought-induced cell death by protecting cell membrane structure.
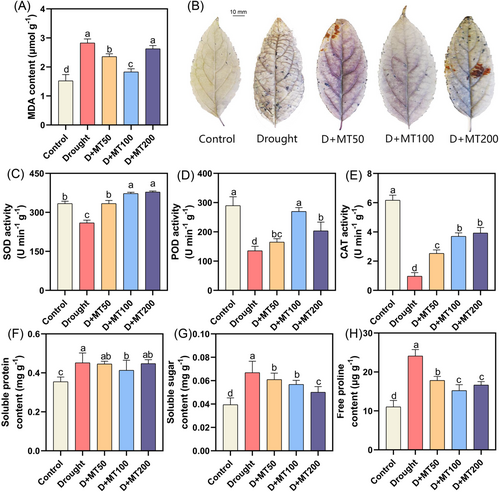
The O2·− level and its distribution were detected in leaves by NBT staining. Compared to the control, the NBT assay revealed a higher accumulation of dark blue colored pigment, indicating a higher level of O2·− under drought stress, which was widely distributed throughout the leaves. Under drought stress, melatonin spray reduced O2·− accumulation. The stained color of the leaves was the lightest at 100 μmol L−1, which was similar to the control (Figure 2B). The activity of SOD, POD, and CAT was markedly enhanced after the application of melatonin in comparison with untreated E. ulmoides seedlings subjected to drought stress (Figure 2C,D). Interestingly, different melatonin treatments showed quite different patterns for improving the activities of different antioxidant enzymes. For instance, SOD and CAT activities increased significantly by 28.75%–75.66% and 161.37%–306.21% with the increase of melatonin concentration and reached the maximum value at 200 μmol L−1. The POD activity increased first and then decreased with the increase in melatonin concentration, reaching the maximum at 100 μmol L−1 melatonin.
Our results showed that drought stress caused lipid damage and promoted the production of a large amount of ROS. Exogenous melatonin effectively reduced the accumulation of O2·− and increased the activity of antioxidant enzymes, thus delaying the oxidative damage caused by drought stress in E. ulmoides leaves.
3.3 Effects of Spraying Melatonin on Soluble Protein, Soluble Sugar, and Free Proline Contents in E. ulmoides Under Drought Stress
Drought stress causes the excessive accumulation of osmotic substances. However, melatonin could improve this phenomenon (Figure 2F–H). In this study, spraying different concentrations of melatonin significantly reduced the accumulation of osmotic regulatory substances. Under drought stress, the contents of soluble protein, soluble sugar, and free proline increased by 27.32%, 69.56%, and 118.72% compared to the control. The spraying of melatonin reduced the contents of three osmotic regulating substances to different degrees. Compared with drought treatment, the contents of soluble sugar and proline decreased significantly after spraying melatonin by 8.85%–25.07% and 26.01%–36.85%, respectively.
3.4 Correlation Analysis of Physiological Indexes and the Comprehensive Evaluation of Drought Tolerance
PCA and correlation analyses were conducted to examine the principal factors influencing E. ulmoides growth under different treatments (Figure S1A,B). PCA and correlation heat maps separated the different sample groups based on the assignment of the 11 growth and physiological indicators. The scores of PC1 and PC2 were 80.6% and 11.5% under drought stress. Leaf inclination angle, SOD, POD, CAT, and chlorophyll index were positively correlated.
As shown in Table 1, the membership function method of fuzzy mathematics was used to analyze the physiological and biochemical indexes such as leaf length, leaf width, leaf inclination angle, chlorophyll content, soluble protein, soluble sugar, proline, MDA content, SOD, CAT, and POD of E. ulmoides seedlings under various treatments, and the comprehensive evaluation of tolerance was calculated. The results showed that the drought tolerance of E. ulmoides seedlings under different treatments was ranked (from strong to weak) as D + MT100 > Control > D + MT50 > D + MT200 > Drought. Combined with the analysis of the above physiological data and tolerance comprehensive evaluation, we selected Control, Drought, and D + MT100 (100 μmol L−1 melatonin under drought stress) treatments for transcriptomic determination and differential gene expression analysis.
3.5 RNA Sequence Analysis
The PCA analysis of the transcriptome data (Figure S2B) showed that drought treatment caused 32%–38% global transcriptional reprogramming (PC1 dominated), while melatonin shifted the transcriptional profile back towards the control (D + MT100 lies between Control and Drought).
In this study, RNA-seq of the nine cDNA libraries produced 63.8 GB high-quality clean reads (Q30 bases larger than 95.4%), meeting the requirements for in-depth sequencing. A total number of 4358 DEGs were screened (Figure S2C). In Control versus Drought, 6065 DEGs showed drought-induced stress responses (e.g., SOD/APX) and growth suppression. In Control versus D + MT100, 8228 DEGs indicated melatonin-mediated circadian preadaptation. In Drought versus D + MT100, melatonin specifically reversed drought-induced DEGs. Venn analysis (Figure S2D) revealed 237 core genes enriched in signaling and antioxidant enzymes. Melatonin-induced transcriptional reprogramming would explain the reduction in trypan blue uptake under drought (Figure S2A).
3.6 GO Term and KEGG Pathway Annotation and Enrichment Analysis
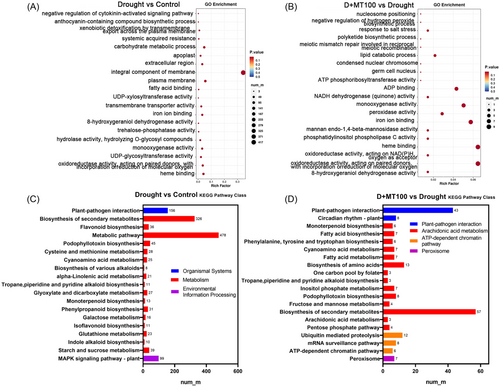
GO annotation analysis showed that the determined DEGs were classified into three ontologies, including biological process, cellular component, and molecular function for two comparison treatments (Figure 3A,B). Compared with the Control, there were 454 DEGs enriched in the integral component of the membrane in drought treatment (Figure 3A). Exogenous melatonin resulted in more enrichment of DEGs in molecular function (Figure 3B). In addition, compared to the Control treatment, KEGG pathway analysis presented that these DEGs were mainly classified in metabolism, environmental information processing, and organismal systems under drought stress (Figure 3C). Among them, DEGs were significantly enriched in metabolic pathway and biosynthesis of secondary metabolites. Under drought stress (Figure 3D), DEGs were more enriched in metabolism, genetic information processes, cellular processes, and organismal systems in melatonin spray treatment (D + MT100). Among them, 57 DEGs were enriched in the biosynthesis of secondary metabolism.
3.7 DEGs Involved in Photosynthesis
In our study, we focused on the common DEGs that could reflect the expression levels mediated by exogenous melatonin under drought stress. According to the transcriptome data (ko00195), drought stress caused significant downregulation of the PsbS (novel1283, novel1281) and PsbC protein (evm. TU. Chr2.1769) regulatory genes (Figure S3). Under drought stress, the PsbS protein regulatory gene (evm. TU. Chr12.1448) was upregulated in E. ulmoides leaves treated with 100 μmol L−1 melatonin.
3.8 DEGs Involved in Starch and Sucrose Metabolism
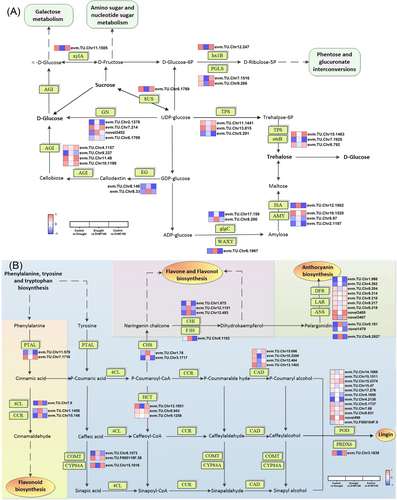
Starch and sucrose metabolism (ko00500) play an important role in carbon source allocation, and their biological functions vary throughout plant development and in response to environmental stimuli. In the starch and sucrose metabolism pathways (Figure 4A), drought stress significantly upregulated the expression levels of GN (glucan endo-1,3-beta-glucosidase), ISA (isoamylase), AMY (alpha-amylase), glgC (glucose-1-phosphate adenylyltransferase), and WAXY (granule-bound starch synthase), and downregulated xyIA (xylose isomerase), hx1B (6-phospho-3-hexuloisomerase), PGLS (6-phosphogluconolactonase), SUS (sucrose synthase), TPS (trehalose 6-phosphate synthase), otsB (trehalose 6-phosphate phosphatase), and AGI (beta-glucosidase). Exogenous melatonin significantly upregulated the expression levels of xyIA, hx1B, PGLS, and SUS, and downregulated WAXY, ISA, and AGI.
3.9 DEGs Involved in Phenylpropanoid Metabolism
Phenylpropanoid biosynthesis (ko00360, ko00940), flavone and flavonol biosynthesis (ko00941) were significantly enriched pathways in E. ulmoides under drought stress (Figure 4B). The genes encoding PTAL (phenylalanine/tyrosine ammonia-lyase), 4CL (4-coumarate–CoA ligase), CCR (cinnamoyl-CoA reductase), CHS (chalcone synthase), CHI (chalcone isomerase), LAR (leucoanthocyanidin reductase), and ANS (anthocyanidin synthase) were up-regulated by drought, but down-regulated by melatonin treatment in the leaves. In contrast, the genes encoding CAD (cinnamyl-alcohol dehydrogenase), DFR (bifunctional dihydroflavonol 4-reductase/flavanone 4-reductase), POD (peroxidase), CYP84A (ferulate-5-hydroxylase), HCT (shikimate O-hydroxycinnamoyltransferase), and PRDX6 (peroxiredoxin 6) were down-regulated in drought stress, but up-regulated by melatonin treatment.
3.10 DEGs Involved in MAPK Signaling Pathway
Mitogen-activated protein kinase (MAPK) can transfer signals activated by abiotic stress to regulate multiple metabolic processes. The MAPK signaling pathway (ko04016) was significantly enriched in melatonin (D + MT100) spray treatment (Figure 5A). FLS2/BAK1-related genes were upregulated by melatonin under drought conditions. Drought stress also significantly upregulated the respiratory burst oxidase homologous protein D (RbohD) gene, while excessive H2O2 further regulated serine/threonine protein kinase (OXI1) gene transduction. Spraying melatonin on E. ulmoides leaves downregulated the RbohD gene. The related genes to abscisic acid such as PYR/PYL (abscisic acid receptor PYR/PYL family), PP2C (protein phosphatase 2C), SnRK2 (serine/threonine-protein kinase SRK2) and MAPKKK17/18 (mitogen-activated protein kinase kinase kinase 17/18) were upregulated by melatonin under drought conditions.
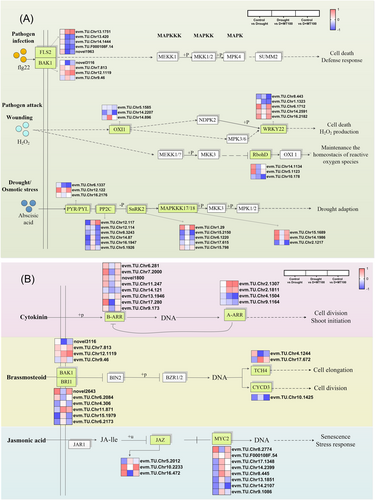
3.11 DEGs Involved in Plant Hormone Signal Transduction
KEGG pathway enrichment analysis showed that plant hormone signal transduction (ko04075) was significantly enriched (Figure 5B). During the cytokinin signaling transduction process, B-ARR (GARP family transcription factor, ARR-B subfamily) and A-ARR (type-A Arabidopsis response regulator) related genes are downregulated under drought stress, while exogenous melatonin increases the expression levels of these genes. In the brassinosteroid and jasmonic acid signaling pathways, BAK1 (brassinosteroid insensitive 1-associated receptor kinase 1), BRI1 (protein brassinosteroid insensitive 1), TCH4 (xyloglucan: xyloglucosyl transferase TCH4), CYCD3 (cyclin D3), JAZ (jasmonate ZIM domain-containing protein), and MYC2 (transcription factor MYC2) also respond to exogenous melatonin regulation under drought stress.
3.12 Effects of Exogenous Melatonin on Transcription Factors and EuMYC Gene Expression Under Drought Stress
Our transcriptome data showed that most members of the transcription factors like MYB, bHLH, NAC, AP2/ERF, WRKY, and bZIP showed up-regulated and down-regulated patterns under different drought treatments. Transcription factors played pivotal roles in regulating E. ulmoides drought tolerance through orchestrating stress-responsive transcriptional networks. Myelocytomatosis oncogene proteins (MYCs) have been an important research object of plant stress and hormone response in recent years. MYC transcription factors are a subgroup of the bHLH family members. In the transcriptome data of E. ulmoides seedlings treated with melatonin, we found that the MYC2 gene was significantly up-regulated and down-regulated before and after spraying melatonin, indicating that this gene plays an important role in the response to drought stress (Figure 6).
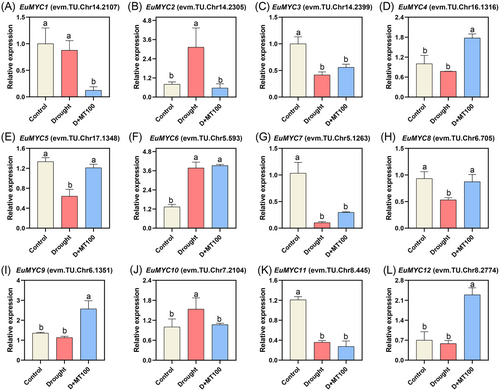
After drought stress, most members of the MYC gene family of E. ulmoides were induced to varying degrees, among which three MYC genes were significantly down-regulated, namely EuMYC3, EuMYC7, and EuMYC11, down-regulated by 58.3%, 89.7%, and 64.2%, respectively. Drought treatment induced 3.12-fold and 3.77-fold up-regulation of EuMYC2 and EuMYC6 respectively versus controls. After spraying melatonin, the expression of two members in the MYC gene family, named EuMYC1 and EuMYC2, were found to be down-regulated by 611.5% and 450.7% compared with the drought stressed group. The two genes EuMYC4 and EuMYC12 were significantly up-regulated by 129.2% and 293.6% after spraying melatonin, respectively. The expression levels of EuMYC2 and EuMYC10 were significantly increased by drought stress, while melatonin treatment significantly reduced their expression levels. The expression levels of EuMYC5 and EuMYC8 were oppositely significantly reduced by drought stress, while melatonin treatment significantly increased their expression levels. Many members of the MYC gene family showed significant changes in expression levels in response to drought stress and spraying melatonin, suggesting that they were involved in the defense of E. ulmoides seedlings against drought stress.
4 Discussion
According to the Food and Agriculture Organization of the United Nations, climate change generates considerable uncertainty about future water availability in many regions (Yang et al. 2023). Wang et al. (2021) found that retarded growth and severe cell death were observed in flooded soybeans, but these phenotypes were ameliorated by the application of exogenous melatonin, which improves tolerance to water deficit by promoting cuticle formation in tomato plants (Yang, Bu, et al. 2022; Yang, Le, et al. 2022). In this study, drought stress caused E. ulmoides leaves to lose water and wilt, leading to the peroxidation of membrane lipids, chlorophyll degradation, and DNA damage, eventually limiting crop growth. Melatonin plays an important role in improving drought tolerance by regulating various physiological and molecular processes in plants (Szafrańska et al. 2017). The core objective of this discussion is to elucidate the underlying mechanisms by which exogenous melatonin confers drought resilience in E. ulmoides, integrating our transcriptomic findings with key biochemical and physiological observations.
4.1 Exogenous Melatonin Improved Photosynthetic Pigments and Photosynthetic Capacity Protecting the Photosynthetic Machinery
Photosynthesis is crucial for converting solar energy into chemical energy in chloroplasts, directly influencing biomass accumulation and crop yield (Wang et al. 2025). In higher plants, chlorophyll, a key component of the thylakoid membrane protein complex, exists as Chla and Chlb (Dong et al. 2022). A decline in those photosynthetic pigments impairs light energy capture, thus affecting photosynthesis (Xu et al. 2025). During senescence or stress, rapid degradation of free chlorophyll and its metabolites is essential to prevent photooxidation toxicity (Hörtensteiner 2004). Drought stress significantly induces an increase in chlorophyll catabolic enzyme activity (Xie et al. 2022). Accumulated ROS in chloroplasts inhibit the D1 protein (encoded by PsbA), disrupting PSII function (Liu et al. 2023). The PsbS protein in the thylakoid membrane regulates energy-dependent, non-photochemical quenching of excess chlorophyll excitation, serving as a short-term stress-resistance mechanism (Kurt-Celebi et al. 2022). The role of PsbS in qE generation has been confirmed in various vascular plants, including rice PsbS knockout mutants (Zulfugarov et al. 2014) and Populus PsbS RNAi lines (Yang, Bu, et al. 2022; Yang, Le, et al. 2022).
In this study, exogenous melatonin significantly improved the growth and photosynthetic capacity of E. ulmoides under drought stress (Figure 1). While drought stress typically inhibits plant growth, causing leaf wilting and reducing chlorophyll content, melatonin application, especially at 100 μmol L−1, effectively alleviates these adverse effects by reducing water loss. Mechanistically, this improvement can be attributed to melatonin's multifaceted action on the photosynthetic apparatus: (1) reduced photooxidative damage. By mitigating water loss and maintaining leaf structure (Figure 1), melatonin likely reduces the overall stress load, limiting the activation of chlorophyll degradation pathways, for example, via reduced pheophorbide a oxygenase activity. (2) enhanced photoprotection. Our transcriptome analysis reveals that melatonin specifically upregulates key photosynthetic genes like PsbS (Figure S3). This molecular upregulation provides a direct mechanistic link to the observed preservation of chlorophyll content and photosynthetic function. By enhancing PsbS levels, melatonin likely boosts the capacity for qE, thereby dissipating excess light energy as heat more efficiently. This protects the PSII reaction center (particularly the D1 protein) from ROS-induced damage during drought, explaining the improved photosynthetic performance observed biochemically on chlorophyll levels and physiologically by growth maintenance.
In photosynthetic eukaryotes, the Calvin cycle operates in the plastid stroma to assimilate CO2 using assimilatory power generated by the light reactions. This carbon fixation pathway provides precursors for starch and sucrose biosynthesis (Elsayed et al. 2022). Sucrose and starch, as primary non-structural carbohydrates in plants, serve dual roles as carbon/energy carriers and signaling molecules critical for stress adaptation (Wang et al. 2025). Under drought, starch degradation was prioritized (e.g., upregulation of AMY, ISA) to release soluble sugars for osmotic adjustment and energy supply, while sucrose utilization was reduced (downregulation of SUS, TPS). This shift aligns with the known strategy of mobilizing stored carbon for immediate stress mitigation. Exogenous melatonin further optimized this carbon partitioning response. Melatonin enhanced sugar signaling (xyIA, hx1B upregulation), potentiating the osmotic and energy-supplying roles of the released soluble sugars. Concurrently, it curbed excessive starch synthesis (WAXY downregulation), preventing the unnecessary redirection of carbon towards storage under stress conditions where rapid mobilization is key. Collectively, these transcriptomic changes in carbohydrate metabolism genes (Figure 4A) provide a molecular basis for the observed biochemical improvement in carbohydrate conversion and transport efficiency under melatonin treatment. By fine-tuning the expression of these key enzymes, melatonin ensures carbon resources are optimally allocated towards drought defense mechanisms like osmoprotection and energy supply.
4.2 Melatonin-Mediated Multitarget Regulation in Drought Resistance: Coordinated Antioxidant Defense, Osmotic Homeostasis, and Phenylpropanoid Pathway Reprogramming
Drought stress disrupts plant homeostasis by inducing oxidative damage and osmotic imbalance (Wang, Sun, et al. 2023; Wang, Zhou, et al. 2023), as evidenced by malondialdehyde (MDA) accumulation, a key biomarker of lipid peroxidation and membrane injury (Chen et al. 2010; Cherono et al. 2021; Xiong et al. 2022; Zhang et al. 2022). In E. ulmoides, melatonin treatment significantly reduced MDA levels (Figure 2A), demonstrating its effectiveness in preserving membrane integrity. It boosted the activities of antioxidant enzymes (SOD, POD, CAT), directly enhancing the cellular capacity to detoxify ROS. Melatonin itself acts as a potent direct scavenger of ROS and helps stabilize ion homeostasis. This combination of direct scavenging and induction of antioxidant enzyme activity in defense mode provides a strong explanation for the observed reduction in oxidative damage. Soluble proteins and sugars act as osmotic regulators and energy sources, while free proline stabilizes membranes and regulates stress-responsive genes (Siddiqui et al. 2019; Ferchichi et al. 2018). Melatonin balanced osmotic regulation by modulating drought-induced accumulations of soluble proteins, sugars, and proline (Figure 2F–H). This biochemical modulation of osmoprotectants complements the antioxidant effects by helping maintain cellular turgor and function under water deficit. The coordinated elevation of these compatible solutes, coupled with reduced oxidative stress, underscores melatonin's systemic capacity to concurrently alleviate oxidative damage and maintain cellular homeostasis under drought (Jin et al. 2022; Kurt-Celebi et al. 2022).
At the transcription level, melatonin orchestrated the phenylpropanoid pathway reprogramming to reinforce structural and antioxidant defenses (Figure 4B). Drought upregulated early biosynthetic genes (PTAL, 4CL, CHS, ANS) initiating lignin and flavonoid synthesis. However, critical downstream genes such as CAD and DFR remained uninduced, creating a metabolic bottleneck that limited the production of mature defense compounds. Reactivating CAD promotes the final steps of lignin biosynthesis, expanding the pool of non-enzymatic antioxidants beyond those produced by early pathway steps. This strategy was further amplified through redox enzyme induction (POD, PRDX6). This pathway redirection is further supported by melatonin-induced carbon reallocation (starch-to-sugar conversion), ensuring substrate availability for these energy-demanding defense syntheses. Therefore, the transcriptomic data reveal how melatonin overcomes a drought-induced metabolic bottleneck in the phenylpropanoid pathway, enabling the production of a broader spectrum of structural and antioxidant compounds, which biochemically contribute to the observed reductions in oxidative damage (MDA) and wilting.
4.3 Melatonin Regulated Drought Resistance in E. ulmoides Leaves: Oxidation-Hormone Balance via MAPK-ROS and ABA Signaling Synergy
The MAPK cascade, an evolutionarily conserved signaling module in plants, serves as a central regulator of stress-responsive processes encompassing growth modulation, cellular differentiation, and phytohormone cross-talk (Singh and Jwa 2013). Drought stress activates MAPK pathways, leading to ROS production and oxidative damage. Our investigation revealed that drought stress pronouncedly upregulated RbohD expression (Figure 5A), correlating with the observed oxidative burst. Notably, melatonin treatment effectively suppressed RbohD expression. This transcriptional downregulation provides a direct molecular mechanism for melatonin's ability to maintain ROS homeostasis: by reducing the primary source of stress-induced ROS (RbohD/NADPH oxidase activity) while simultaneously enhancing ROS scavenging capacity (as shown in Section 4.2). Concurrently, melatonin influenced the ABA-dependent pathway, a key regulator of plant responses to abiotic stress (Wang, Sun, et al. 2023; Wang, Zhou, et al. 2023). Under drought, melatonin reconfigured ABA signaling networks by modulating core components of the phosphorylation cascade: it enhanced PYR/PYL receptor availability, inhibited PP2C phosphatases, and amplified SnRK2 kinase activity, collectively promoting ABA sensitivity and drought-responsive gene expression. This dual-channel regulation, spanning MAPK-mediated oxidative control (RbohD suppression) and ABA-dependent signaling potentiation, establishes an integrated stress-response framework (Liu et al. 2025). These coordinated transcriptional changes like increased receptors, decreased repressors, and increased activators strongly suggest that melatonin potentiates ABA signaling efficacy. This would collectively promote ABA sensitivity and the transcriptional activation of downstream drought-resilience genes, for example, those involved in stomatal closure, osmoprotectant synthesis, and LEA proteins. Melatonin works by both reducing the generation of ROS and enhancing scavenging, as well as increasing ABA signaling sensitivity. Through this combined approach, melatonin coordinates a comprehensive adaptive strategy that supports the observed physiological and biochemical improvements, such as reduced damage, maintained turgor, and induced defenses.
4.4 Melatonin Regulated the Crosstalk of Plant Hormone Signals in E. ulmoides Leaves
The intricate interplay between phytohormones underpins plant developmental plasticity and stress adaptation. Auxin and cytokinin, two pivotal regulators of organogenesis, exhibit antagonistic yet coordinated roles in apical dominance and root-shoot patterning, with their dynamic equilibrium dictating in vitro morphogenic outcomes (Nordström et al. 2004). Brassinosteroid (BR) signaling, mediated by the receptor complexes (BRI1/BRL1-BAK1), orchestrates growth-stress trade-offs (Jaillais and Vert 2016). Jasmonate (JA) is involved in plant growth, development, nutrient uptake, and defense, impacting photosynthetic pigments and source-to-sink (Waheed et al. 2022). JA can enhance photosynthetic efficiency under drought, often acting through the central transcriptional regulator MYC2 (Asensio et al. 2012). Our transcriptomic data reveals that melatonin acts as a hormonal integrator under drought, strategically recalibrating multiple hormone pathways to favor defense. Melatonin suppressed drought-induced BAK1 and BRI1 expression (Figure 5B), potentially attenuating BR-driven growth prioritization to reallocate resources toward stress mitigation. It also suppressed cytokinin-activated B-ARR genes and auxin-responsive A-ARR, further dampening growth-promoting signals. While drought robustly upregulates the JA-responsive transcription factor MYC2 expansion (Figures 5B and 6)—typically promoting stress responses like root expansion—exogenous melatonin counterintuitively down-regulated MYC2 expression. This suggests a nuanced, strategic shift orchestrated by melatonin: rather than amplifying the general JA/MYC2 stress response, which can be energy-intensive, for example, for root growth, melatonin appears to redirect resources toward core biochemical defenses like antioxidants, osmoprotectants, and cell wall reinforcement, while potentially mitigating excessive growth-inhibitory aspects of JA signaling under severe stress. This aligns with the suppression of growth-promoting hormones (BR, CK, Auxin).
Such pleiotropic regulation positions melatonin as a meta-regulator, effectively decoupling stress-induced hormonal hyperactivity from energy-intensive growth processes. This rebalancing of the auxin-cytokinin-BR-JA nexus towards stress acclimation optimizes resource partitioning for drought survival, providing a hormonal explanation for the observed growth maintenance under stress when melatonin is applied (Figure 1).
4.5 Melatonin Induced MYC Family Gene Expression to Improve Plant Drought Tolerance
Transcription factors are key controllers of gene expression, activated by their molecular environment (Maassen et al. 2025). MYC transcription factors, particularly MYC2, are central hubs in the JA signaling pathway, regulating diverse processes through the COI1-JAZ-MYC complex and participating in hormone cross-talk (Song et al. 2022). MYC genes influence cytokinin metabolism and development (Dong et al. 2021). In Arabidopsis thaliana, AtMYC2, AtMYC3, and AtMYC4 work together to regulate leaf development, protein accumulation, and seed yield. Among them, MYC2 can bind to the promoter of its target gene ERD1 and activate its expression, thus activating the JA-induced dehydration stress response (Li et al. 2019). At the same time, there is a synergistic effect between JA and auxin to regulate thermal morphogenesis (Agrawal et al. 2022) in A. thaliana. In tomato, MYC2 can activate the transcription of ethylene response factor SIERF.B8, thus enhancing JA signal transduction and tomato tolerance to low-temperature stress (Ding et al. 2022). In this study, we observed that different EuMYC members exhibited distinct responses to melatonin and drought stress (Figure 6). EuMYC5 and EuMYC8 expression decreased significantly under drought but increased significantly after melatonin application. EuMYC2 expression increased significantly after drought stress but decreased significantly after melatonin spraying. These differential expression patterns provide crucial molecular evidence for the complex role of the MYC family in drought adaptation and melatonin response. The upregulation of EuMYC2 by drought parallels its established role as a key regulator of JA and abiotic stress responses, similar to the well-studied AtMYC2. Melatonin's downregulation of EuMYC2 (as noted in Section 4.4) suggests a mechanism for tempering certain aspects of the canonical JA stress response, potentially preventing its growth-inhibitory costs or redirecting resources, consistent with the hormonal rebalancing observed. EuMYC5 and EuMYC8 were specifically induced by melatonin under drought conditions, which represents a novel finding. While their precise functions require further study, their melatonin-dependent upregulation suggests they may mediate unique aspects of melatonin-induced drought tolerance, potentially distinct from or complementary to the MYC2 pathway. This could involve regulating specific subsets of defense genes or participating in alternative signaling modules activated by melatonin. Collectively, the specific modulation of different MYC TFs by melatonin, suppressing the general stress responder MYC2 while inducing other MYC members like MYC5/8, offers a sophisticated transcriptional mechanism underlying melatonin's ability to fine-tune the plant's drought response program. This adds a crucial layer of molecular detail to the hormonal crosstalk discussed in Section 4.4 and highlights MYC factors as important targets in melatonin-mediated drought resilience.
5 Conclusion
This study demonstrates that melatonin enhances Eucommia ulmoides drought tolerance through integrated physiological and molecular reprogramming (Figure 7). Melatonin stabilizes photosynthesis by upregulating PsbS to protect PS II and optimizing carbon allocation via starch-to-sugar conversion. It mitigates oxidative damage by suppressing RbohD-driven ROS bursts while activating enzymatic (SOD/POD/CAT) and non-enzymatic (flavonoids) antioxidant systems, coupled with osmotic adjustments through proline and soluble sugar regulation. Melatonin coordinates the ABA-MAPK signaling crosstalk, enhancing ABA sensitivity via PYR/PYL-PP2C-SnRK2 rebalancing and suppressing MAPK-mediated oxidative stress. Crucially, it reprograms hormonal networks by attenuating growth-promoting pathways like BR signaling via BAK1/BRI1 and MYC2-driven root expansion to prioritize stress adaptation. Isoform-specific MYC regulation (EuMYC2 downregulation vs. EuMYC5/8 induction) reveals its precision in redirecting JA signaling from development to antioxidant defense. These mechanisms position melatonin as a meta-regulator that hierarchically resolves growth-stress trade-offs via transcriptional specificity and metabolic reallocation. Our findings provide a blueprint for melatonin-based biostimulants to enhance crop resilience, warranting further exploration of MYC spatiotemporal dynamics and field validation.
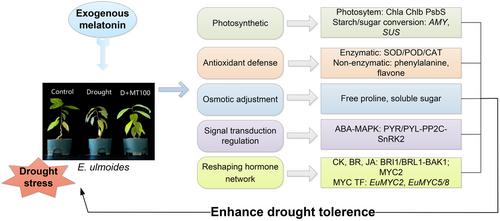
Future studies should dissect the upstream regulators of MYC TFs and their functional divergence using gene-editing tools. The conservation of melatonin-induced MAPK and hormonal networks across species requires validation, while multi-omics integration of proteomics and metabolomics could unravel post-translational modifications and metabolite dynamics. Field trials are essential to optimize melatonin application protocols and assess ecological impacts. Furthermore, evolutionary analyses of melatonin-responsive pathways may inform synthetic biology strategies for climate-resilient crops. Collectively, this work advances melatonin as a sustainable agrochemical candidate, offering a molecular blueprint for enhancing drought tolerance in woody perennials and beyond.
Author Contributions
X.W. designed the experiments. M.H., Z.Y., L.Y., F.F., and X.C. conducted the experiments. M.H. and Z.Y. analyzed the data. M.H., Z.Y., J.W., S.H., X.W., G.A., and M.Y. provided guidance on the experiment methods. X.W., J.W., S.H., M.H., and Z.Y. supervised the experiments and revised the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors confirmed data supporting the results of this study are available in the article and supporting information. The raw transcriptome sequencing data are available from the Sequence Read Archive (SRA) database of NCBI (accession number PRJNA1272224).




