Delayed Chlorosis in Arabidopsis Ecotype Dijon-G During Bacterial Infection and Dark-Induced Senescence
Funding: This work was supported by National Research Foundation of Korea (NRF), Ministry of Science and ICT (MSIT) of the Korea government. Grant Number: NRF-2010-0003285, NRF-2020R1A2C1101613.
ABSTRACT
Comparative evaluation of defence responses in different Arabidopsis ecotypes to pathogens is useful for understanding how plants acquire disease resistance and finding valuable genetic resources for disease resistance. In this study, leaf chlorosis was delayed in Arabidopsis ecotype Dijon-G (Di-G) in response to Xanthomonas campestris pv. campestris (Xcc) 8004 infection, as well as continuous darkness compared to the ecotype Columbia-0 (Col-0). However, Xcc bacterial proliferation within Di-G was slightly higher in Col-0. The Xcc infection led to lower expression of several pathogenesis-related genes (PDIOX, GLIP1 and PAD4) and senescence-related genes (DIN6 and SAG12) in Di-G. Dark-induced leaf senescence was delayed in detached Di-G leaves, showing a higher chlorophyll content than that of Col-0. Exogenous SA did not change the chlorophyll loss in the Xcc-inoculated Col-0 or Di-G leaves, but SA limited Xcc growth in Col-0 but not in Di-G. SA pretreatment compromised chlorophyll loss in Col-0 during dark-induced leaf senescence, but it remained unaltered in Di-G. These results show that Di-G may have more efficient machinery for attenuating chlorophyll degradation during Xcc bacterial infection and continuous darkness than Col-0. The different sensitivities to exogenous SA in Col-0 and Di-G suggest that the two ecotypes have adapted differently to their natural habitats in terms of plant immunity and leaf senescence.
1 Introduction
Xanthomonas campestris pv. campestris (Xcc) is a causal bacterium of black rot disease in a broad range of cruciferous plant species, including Arabidopsis, broccoli, Brussels sprouts, cabbage, cauliflower, kale, radish and turnip (Buell 2002). In nature, Xcc invades its host plants through hydathodes, stomata or wounds and colonises the vasculature for systemic invasion, giving rise to the symptoms of black rot. Different cultivars of cruciferous cabbage, cauliflower, and rapeseed have been screened to uncover genetic resources for black rot disease resistance (Kocks and Ruissen 1996; Lema et al. 2011; Rafael da Silva et al. 2015; Williams et al. 1971). Race-specific differential interactions have been found in turnip cultivars inoculated with different Xcc strains (Kamoun et al. 1992). Genetic and molecular investigations were conducted to identify disease resistance traits using different Xcc strains to infect various Arabidopsis ecotypes (Godard et al. 2000; Lummerzheim et al. 1993; Simpson and Johnson 1990; Tsuji et al. 1991). Bacterial effector protein AvrXccC originates from Xcc strain 8004, is delivered via type III secretion system (TTSS) and localised in the plant plasma membrane, and is required for full virulence in susceptible cabbage plants (Brassica oleracea) but for avirulence in resistant mustard (Brassica napiformis) plants (Wang et al. 2007). The AvrB_AvrC domain of the effector Xcc AvrXccC manipulates abscisic acid homeostasis in Arabidopsis and facilitates bacterial infection in the plant tissues (Ho et al. 2013). The RKS1 encoding resistance-related kinase 1 confers broad-spectrum disease resistance in Arabidopsis against Xcc as well as different pathovars of X. campestris (Delplace et al. 2020; Huard-Chauveau et al. 2013). However, limited information is known about the genetic resources for resistance to Xcc in Arabidopsis and the regulation of defence signalling triggered by Xcc or Xcc-derived molecules.
Leaf senescence signalling triggered by natural plant ageing or various exogenous stimuli, including continuous darkness, has been extensively analysed in Arabidopsis. Variations in the onset of leaf senescence among various Arabidopsis ecotypes from different natural habitats have also been valuable genetic resources for comprehending plant senescence regulation (Balazadeh et al. 2008; Levey and Wingler 2005). Many Arabidopsis mutants have shown altered senescing processes when grown in darkness (Liebsch and Keech 2016; Woo et al. 2013). Plant immunity against pathogen infection is sometimes associated with altered plant developmental processes such as abscission, senescence and early flowering (Patharkar et al. 2017; Zhou et al. 2015). However, limited information is available on the close relationship between Xcc infection and senescence in Arabidopsis, even though the exogenous biotic and abiotic stimuli trigger similar physiological changes in leaf chlorosis.
Salicylic acid (SA) is considered the most potent defence regulator in local and systemic acquired resistance for many plant species (Attaran and He 2012; Loake and Grant 2007). Arabidopsis mutants with reduced SA production or impaired SA perception have provided genetic clues about SA-mediated activation of plant disease resistance. The role of SA in plant immunity has been evaluated in Xcc-inoculated Arabidopsis. The SA levels in Arabidopsis leaf tissues were elevated upon Xcc inoculation, whereas attenuated SA signalling increased disease susceptibility, leading to more tissue damage and efficient Xcc bacterial proliferation (O'Donnell et al. 2003; Rong et al. 2010). Pretreatment with SA enhanced the disease resistance of Chinese cabbage (B. rapa) to Xcc (Lee et al. 2014). Interestingly, the disease resistance of six oilseed rape cultivars (Brassica napus) inoculated with Xcc was associated with the SA levels of the inoculated leaf tissues but also the ratio of SA to jasmonic acid, which was suppressed by Xcc inoculation (Islam et al. 2017). However, the role of SA on Xcc bacterial infection in different Arabidopsis ecotypes is rarely demonstrated.
The involvement of SA in plant senescence accompanying leaf chlorosis has been demonstrated. Endogenous SA was elevated in Arabidopsis leaves during senescence, and delayed leaf senescence and compromised senescence-associated gene expression were found in SA signalling mutants npr1 and pad4 (Morris et al. 2000). SA-inducible SA 3-hydrolase has been suggested to be a pivotal factor of negative feedback regulation for SA catabolism during leaf senescence in Arabidopsis (Zhang et al. 2013). Exogenously applied SA-induced the senescence of detached Arabidopsis leaves, but the SA-induced leaf senescence of the npr1 mutant was distinctly delayed (Chai et al. 2014).
Arabidopsis ecotype Dijon-G (Di-G) is a disease-susceptible ecotype to the necrotrophic fungus Alternaria brassicicola, which causes black spot disease, providing a compatible necrotrophic fungal interaction in Arabidopsis plants for research compared to the incompatible interaction of the Arabidopsis ecotype Col-0 (Mukherjee et al. 2009). The ecotype Di-G is also highly susceptible to other necrotrophic fungi, such as Botrytis cinerea, Sclerotinia sclerotiorum and Sclerotium rolfsii, whereas showing resistance to infection by the hemibiotrophic fungus Colletotrichum higginsianum (Lee et al. 2019). In this study, we evaluated the disease responses of the ecotype Di-G against infection by the hemibiotrophic bacterium Xcc compared to Col-0 regarding chlorotic symptom appearance and bacterial proliferation within the leaf tissues. The level of chlorosis in Col-0 and Di-G leaves under continuous darkness was also analysed to investigate the distinct correlation between bacterial infection- and senescence-triggered leaf chlorosis in the two different Arabidopsis ecotypes. In addition, the probable relatedness of SA with the Xcc infection and dark-induced senescence was elucidated.
2 Materials and Methods
2.1 Plant Growth and Environment
Arabidopsis thaliana ecotypes Columbia Col-0 (Col-0) and Dijon-G (Di-G) used in this study were obtained from the Arabidopsis Biological Resource Center. The seeds were sown on the commercial soil mixture ‘Fresh Bio No. 2’ (Gyeongju Chemicals Co. Ltd.) in one square pot (12 × 8 × 6 cm) and transferred to 4°C for 7 days. After seedling growth for 2 weeks in a walk-in growth room, four seedlings were transplanted in a square pot containing the same soil mixture. Arabidopsis plants were grown for 6 weeks under 12 h light/12 h dark conditions at 120 μmol/m2/s, 23°C ± 2°C, and 75% relative humidity until used (Lee et al. 2019). The environmental conditions in the plant growth room were tightly controlled in certain ranges of temperature, relative humidity and light period to acquire reliable and replicable data.
2.2 Bacterial Culture, Plant Inoculation, and In Planta Bacterial Enumeration
Black rot bacterium Xcc strain 8004 was cultured in nutrient broth (NB) (Becton, Dickinson and Company) supplemented with 100 μg/mL of antibiotic rifampicin at 30°C overnight. The bacterial cultures were centrifuged to harvest, and the bacterial suspensions prepared in sterile water were adjusted at OD600 = 0.10 with a population of 108 cfu/mL using a spectrophotometer. Fully expanded Arabidopsis rosette leaves were infiltrated at 10:00 with different doses of the bacterial suspension using a syringe without a needle. The Xcc-inoculated plants were placed in the same plant growth room under environmental conditions described above until used for further experimental analyses. The bacterial suspension (107 cfu/mL) was used for disease symptom observation and gene expression analyses. A low bacterial suspension (105 cfu/mL) was prepared to investigate bacterial proliferation within the leaf tissues. Bacterial proliferation within the inoculated leaves was monitored on nutrient agar (NA) supplemented with rifampicin (100 μg/mL) via serial dilution method (Lee and Hong 2015).
2.3 Dark-Induced Leaf Senescence
Fully expanded rosette leaves were detached from 6-week-old Col-0 and Di-G plants at 10.00 a.m. The leaves were placed on two-layered gauzes saturated with sterile distilled water in plastic boxes (13 × 9 × 4.5 cm), and tightly covered to maintain moisture. These boxes were incubated at 25°C under dark conditions. The detached leaves were harvested at different time points of incubation for leaf chlorophyll measurement and dark-regulated plant gene expression analysis.
2.4 Chemical Treatment
Sodium salicylate (sodium salt of salicylic acid, SA) (0.5 mM) was prepared in distilled water and evenly sprayed onto the plants. Water was foliar-sprayed on the same-aged plants as a mock treatment for SA.
2.5 Chlorophyll Quantification
The total chlorophyll content in the leaves was measured and calculated based on the formula previously described (Sung and Hong 2010). The total chlorophyll contents in the Col-0 and Di-G leaves treated with pathogen or darkness were expressed as relative chlorophyll (%) compared to the mock-treated controls of Arabidopsis ecotype Col-0.
2.6 Gene Expression Analyses
The total RNA from the samples was isolated from the leaves, and semi-quantitative RT-PCR was performed to analyse gene expression according to the methods of Lee et al. (2019, 2022). Two rosette leaves with similar developmental stages were harvested from one 6-week-old plant, and eight leaves from four plants were combined for one plant sample. The leaf tissues (0.1 g), macerated in a chilled mortar using a pestle and liquid nitrogen, were transferred to microtubes. RiboEx (GeneAll, GeneAll Biotechnology Co. Ltd.) (1 mL) was added to the frozen leaf powder and vigorously shaken. After incubation for 5 min at room temperature, the leaf samples were centrifuged at 12,000 × g for 10 min at 4°C. The supernatant was mixed with 0.2 mL of chloroform vigorously and incubated for 2 min at room temperature. Centrifugation was performed at 12,000 × g for 15 min at 4°C, and the supernatant was harvested and gently mixed with isopropyl alcohol by inversion. After overnight storage at −20°C, the total RNA was precipitated by centrifugation at 12,000 × g for 15 min at 4°C. The RNA pellets were washed with 75% ethanol (1 mL), harvested by centrifugation at 8000 × g for 5 min at 4°C, and dissolved in DEPC-treated distilled water.
First-strand cDNA synthesised from the total RNA (1 μg) using oligo (dT)17 and SuperScript III reverse transcriptase (Invitrogen) was used for semi-quantitative RT-PCR. Primer pairs to detect plant defence-associated genes are shown in Table 1. PCR was performed with an initial denaturation at 95°C for 2 min followed by 25–30 cycles of 95°C for 30 s, 52°C for 30 s and 72°C for 30 s, with a final extension at 72°C for 10 min. Arabidopsis ACTIN2 expression was evaluated as an internal control. PCR products underwent electrophoresis in agarose gels containing RedSafe Nucleotide Acid Staining Solution (iNtRON Biotechnology) and were visualised by UV-illumination. Three independent experiments were conducted for each gene expression, and the representative results were shown. The intensities of the PCR band were quantified using Image J and statistically analysed based on the three independent experiments.
| Gene name | Protein product/putative function | TAIR ID | Sequence (5′–3′) |
|---|---|---|---|
| PR1 | Pathogenesis-related protein 1 | At2g14610 | F: TCGTCTTTGTAGCTCTTGTAGGTG |
| R: TAGATTCTCGTAATCTCAGCTCT | |||
| PDIOX | Pathogen-inducible dioxygenase | At3g01420 | F: GTATGCGACGCCCTCAAGGATG |
| R: CCTTGAGACTCTCTGTAGTATTCACC | |||
| GLIP | GDSL-lipase | At5g40990 | F: CGATTGTGCACCAGCCTCATTGGTT |
| R: CAGCGCTTTGAGATTATAGGGTCC | |||
| EDS1 | Lipase-like protein | At3g48090 | F: TCGAAGGGGACATAGATTGG |
| R: CTTTTCATGTACGGCCCTGT | |||
| PAD4 | Lipase-like protein | At3g52430 | F: GGAAAGAAATGGATTTACGCATCT |
| R: CTAAGTCTCCATTGCGTCACT | |||
| NPR1 | Ankyrin-repeats transcription cofactor | At1g64280 | F: TCACTGGTACGAAGAGAACA |
| R: TGAGAGAGTTTACGGTTAGAC | |||
| DIN6 | Asparagine synthase | At3g47340 | F: AAGGTGCGGACGAGATCTTTGG |
| R: CGCGTTCGACATCATCTTGTCATTG | |||
| SAG12 | Cysteine protease | At5g45890 | F: CCACTCGACAATGAACTCATCATGC |
| R: CCGCCAGTCGCTTTTATATGCTC | |||
| CAB1 | Chlorophyll a/b-binding protein1 | At1g29930 | F: ACTACTCAACCTCAATGGCCG |
| R: ATGCTCTGAGCGTGAACCAA | |||
| ACTIN2 | Actin 2 | At3g18780 | F: TCACCACAACAGCAGAGCGGG |
| R: GGACCTGCCTCATCATACTCGG |
2.7 Statistical Analyses and Graph Drawing
All experiments, including bacterial inoculation and dark-induced senescence, were performed three or four times independently, with four replications for each experiment, as described in each Figure caption. Data were analysed for statistical significance by SAS (Statistical Analysis System, version 9.1). Analysis of variance (ANOVA) was used to determine the effects of Xcc bacterial inoculation on the total chlorophyll loss, in planta bacterial multiplication, and plant gene expression analyses, and the effect of dark treatment on the total chlorophyll loss. Means were compared using least significant difference tests. Graphs were drawn with SigmaPlot 10.0 (Systat Software Inc.).
3 Results
3.1 Chlorotic Symptom Development and In Planta Bacterial Proliferation in Col-0 and Di-G Leaves to Xcc Infection
Chlorotic symptoms and in planta bacterial growths were compared in Col-0 and Di-G leaves inoculated by Xcc (Figure 1). Chlorosis appearance in the Xcc-inoculated Di-G leaves was delayed compared to Col-0. The chlorosis in Col-0 and Di-G leaves was evaluated by inoculating different doses of Xcc bacterial suspensions (1 × 106, 5 × 106, 1 × 107, and 5 × 107 cfu/mL) and measuring the chlorophyll content at 48 and 72 h (Figure 1A). Increasing the Xcc bacterial inoculum density decreased the chlorophyll content in the leaf tissues of both ecotypes. Notably, the chlorophyll losses in Col-0 were significantly higher than Di-G for most bacterial inoculum doses. Distinct differences in chlorotic lesions were observed in the rosette leaves of both ecotypes inoculated with a high bacterial suspension (107 cfu/mL) (Figure 1B). Leaf chlorosis was apparent in Col-0 leaves infected with Xcc at 72 h, whereas the same dose of bacterial inoculum caused moderate chlorosis in Di-G. Higher chlorophyll level was found in the Di-G leaves inoculated by even a relatively low Xcc dose (105 cfu/mL) (Figure 1C). Chlorophyll contents in mock-inoculated Col-0 and Di-G were not different from uninoculated leaves at 10 days. Xcc (105 cfu/mL) inoculation significantly decreased chlorophyll contents in both ecotypes at 10 days compared to the mock inoculation, but the reduction ratio was much higher in Di-G leaves. Xcc bacterial growth in the inoculated leaves of both ecotypes was evaluated by inoculating bacterial suspension (105 cfu/mL) during bacterial infection (Figure 1D). The Xcc population in Col-0 and Di-G began to increase at 2 days after inoculation, but the bacterial growth in Di-G was slightly lower than in Col-0. The Xcc number increased in Col-0 and Di-G at 4 days, and no difference was found in bacterial multiplication within the Col-0 and Di-G leaves. The bacterial growth in Col-0 was steady-state at 7 and 10 days, whereas the bacterial growth in Di-G was more prominent and significantly higher.

3.2 Expression of Pathogenesis-Related Genes and Senescence-Induced Genes in Col-0 and Di-G Leaves Inoculated With Xcc
Transcriptional regulation of pathogenesis-related genes and senescence-induced genes was investigated in the Col-0 and Di-G leaves at 24 and 48 h after Xcc inoculation (Figure 2). The drastic chlorosis and slight wilting in the Xcc-inoculated Col-0 leaves at 72 h seem to cause improper RNA preparation and RT-PCR analysis. Representative results of the three independent semi-quantitative RT-PCR experiments and statistically analysed results were shown in Figure 2A and in Figure 2B, respectively.
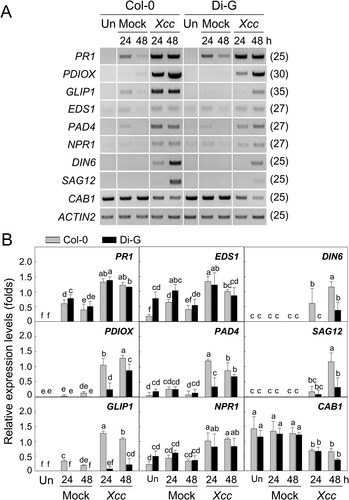
PR1 gene was expressed strongly in Col-0 by Xcc at 24 and 48 h compared to mock inoculation. PR1 gene expression was also induced in the Di-G leaves at the same time points. No difference was found in the Xcc-induced PR1 gene expression level between two ecotypes. PDIOX gene expression was highly activated in the Xcc-inoculated leaves of Col-0 at 24 h, and the expression increased at 48 h. Xcc inoculation also led to PDIOX induction in Di-G at 24 and 48 h, but the expression levels were much lower than Col-0 at each time point. GLIP1 expression level in Col-0 was elevated by Xcc inoculation at 24 h, and then slightly reduced at 48 h, whereas only a minute gene expression was induced in Di-G leaves by Xcc at 48 h. EDS1 gene expression was induced in Col-0 by Xcc inoculation at 24 h compared to the mock inoculation, and a bit decreased at 48 h. EDS1 gene expression was also activated in Di-G by Xcc at 24 and 48 h compared to the mock inoculation, but the inducible gene expression level in Di-G at each time point was not different from Col-0. PAD4 gene was expressed in Col-0 leaves by Xcc at 24 and somewhat reduced at 48 h. In the Xcc-inoculated Di-G leaves, PAD4 gene expression was unchanged at 24 and moderately increased at 48 h. There is no difference in the Xcc-mediated PAD4 induction between Col-0 and Di-G at 48 h. Xcc inoculation led to an increase in NPR1 gene expression in Col-0 at 24 and 48 h to the same levels. Xcc-induced NPR1 gene expression was found in Di-G with a similar level to that of Col-0, but the gene expression levels at 24 and 48 h remained at the same level as at 6 h.
DIN6 gene expression was induced in Col-0 by Xcc inoculation at 24 h and substantially increased at 48 h. Xcc-induced DIN6 gene expression did not occur in Di-G at 24 h compared to Col-0, and the expression increased a bit at 48 h. SAG12 gene expression was not detectable in Col-0 and Di-G leaves inoculated by Xcc at 24 h. Xcc-induced SAG12 expression in both ecotypes at 48 h, but the induction was much higher in Col-0 than in Di-G. CAB1 expression was reduced in Col-0 and Di-G by Xcc inoculation at 24 and 48 h, but the reduced gene expression level was the same in both ecotypes.
3.3 Different Chlorosis of Col-0 and Di-G Leaves Under Continuous Darkness
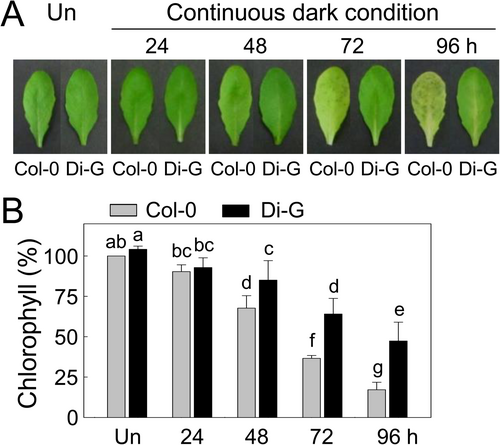
The temporal difference in chlorosis was observed in Col-0 and Di-G leaves during dark-induced leaf senescence (Figure 3A). We could not detect a visible difference in the leaf colour of Col-0 and Di-G under normal growth conditions. Continuous darkness-induced senescence resulted in chlorosis in the Col-0 leaves from 48 h, whereas chlorosis in the Di-G leaves was delayed, having relatively greener leaves under the same ambient conditions. The chlorosis differences in the Col-0 and Di-G leaves caused by dark-induced senescence were quantified by measuring their relative total chlorophyll contents compared to the untreated leaves from each ecotype (Figure 3B). Distinct decreases in the chlorophyll content of the Col-0 leaves occurred at 48–96 h in constant darkness, whereas the total chlorophyll content of the Di-G leaves under continuous darkness was significantly higher than that of Col-0 at each time point.
3.4 Effect of SA Pretreatment on Xcc Infection and Dark-Induced Senescence in Col-0 and Di-G Leaves
Disease responses and dark-induced senescence were investigated in Col-0 and Di-G leaves pretreated with SA before Xcc bacterial inoculation or continuous darkness (Figure 4). Bacterial invasion-mediated total chlorophyll loss and in planta bacterial proliferation were evaluated in the inoculated leaves of the two ecotypes pretreated with water and SA (Figure 4A,B). Xcc inoculation reduced chlorophyll contents significantly in the Col-0 and Di-G leaves pretreated with water at 48 h, compared to the mock inoculation. However, the total chlorophylls in the Xcc-inoculated Di-G leaves were higher than those of inoculated Col-0 leaves. Pretreated SA (0.5 mM) did not change the total chlorophyll levels in the Xcc-inoculated Col-0 and Di-G leaves (Figure 4A). The SA pretreatment decreased Xcc bacterial growth in Col-0 leaves at 96 h, but no difference was found in the bacterial growth in Di-G leaves with or without the SA pretreatment (Figure 4B).
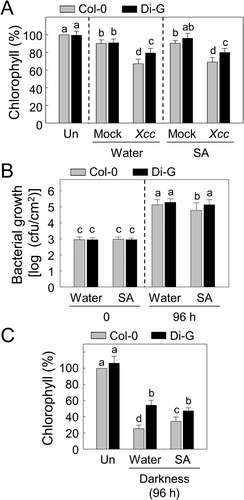
Col-0 and Di-G showed different responses to SA pretreatment during the dark-induced leaf senescence (Figure 4C). Pretreatment with SA attenuated dark-induced senescence in Col-0 leaves at 96 h of continuous darkness, shown by diminished chlorophyll losses compared to the water-pretreated leaves. However, the SA pretreatment did not change the dark-induced leaf senescence of Di-G. The chlorophyll levels were higher in Di-G leaves than in Col-0 under continuous darkness, regardless of the SA pretreatment.
4 Discussion
4.1 Uncoupled Chlorosis and In Planta Xcc Bacterial Growth in Col-0 and Di-G Leaves
Symptom development in Xcc-inoculated Arabidopsis leaves differs depending on the inoculation site and method (Meyer et al. 2005; Simpson and Johnson 1990; Tsuji et al. 1991). However, chlorosis and reduced chlorophyll content are common physiological responses in Xcc-inoculated leaves (Buell and Somerville 1995; Tsuji et al. 1991). In Arabidopsis mutants with impaired defence signalling, Xcc inoculation led to a changed symptom appearance compared to the wild-type (O'Donnell et al. 2003; Rong et al. 2010). Various Arabidopsis ecotypes from different habitats have shown slowed chlorosis in response to Xcc infection and have been useful genetic sources for disease resistance against Xcc (Buell and Somerville 1997; Carmo et al. 2007; Huard-Chauveau et al. 2013). However, despite the efforts to excavate the disease-resistant traits from these various ecotypes, the disease resistance phenotypes of Arabidopsis could still be overcome by diverse Xcc strains from different origins (Akimoto-Tomiyama et al. 2014; Guy et al. 2013). Interestingly, the disease responses of the Arabidopsis ecotype Di-G to necrotrophic and hemibiotrophic fungal inoculations were considerably different from those of the Col-0 ecotype (Lee et al. 2019; Mukherjee et al. 2009). In the current study, relatively less chlorosis occurred in Di-G leaves inoculated with hemibiotrophic Xcc compared to that observed in Col-0 leaves, which is similar to the delayed anthracnose symptom found in Di-G leaves inoculated with hemibiotrophic C. higginsianum (Lee et al. 2019). Di-G might have more efficient resistance to the hemibiotrophic fungal and bacterial pathogen invasion, but it conversely seems to lead to enhanced susceptibility to the necrotrophic pathogens. It will be interesting to investigate differential disease responses of Col-0 and Di-G to the hemibiotrophic Xcc and necrotrophic bacteria such as Pectobacterium carotovorum subsp. carotovorum (Hsiao et al. 2017; Kang et al. 2024). Unexpectedly, the Xcc bacterial proliferation within the inoculated Di-G leaves was higher than in the Col-0 leaves at the final infection stage despite delayed symptom development, suggesting uncoupled chlorosis and basal immunity to Xcc in the two ecotypes. This uncoupled symptom development and in planta bacterial growth was also discovered in two ecotypes, PrØ and Columbia, inoculated with Xcc (Tsuji et al. 1991). Ethylene production and signalling were suggested to be involved in the chlorosis appearance but not in bacterial growth in the Xcc-inoculated Arabidopsis leaves (O'Donnell et al. 2003). The altered ethylene production and signalling during the uncoupled chlorosis and in planta bacterial growth in the Xcc-inoculated Di-G leaves will be worth investigating further. Interestingly, decreased ethylene production and attenuated signalling may also be associated with delayed chlorophyll degradation in Di-G leaves during senescence induced by continuous darkness. Leaf senescence, accompanied by decreased chlorophyll content, was exhibited in Arabidopsis by exogenous application of ethylene, and dark-induced senescence of detached Arabidopsis leaves was accelerated by an ethylene precursor (Li et al. 2013; Liu and Wen 2012).
4.2 Differential Gene Expressions in Col-0 and Di-G Leaves Under Xcc Bacterial Infection
Gene expression of the two ecotypes, Col-0 and Di-G, was found to be different in response to Alternaria brassicicola infection, oxalic acid and SA (Mukherjee et al. 2009; Lee et al. 2019). A. brassicicola infection strongly activated PR1 expression in Di-G, but PDIOX and GLIP1 expression was lower. In this study, PR1 expression was not different in Col-0 and Di-G against Xcc infection, whereas Xcc-inducible PDIOX and GLIP1 expression in Col-0 was significantly suppressed in Di-G. These indicate that PDIOX and GLIP1 expression may be down-regulated in Di-G by fungal A. brassicicola and bacterial Xcc infection via the same defence signalling pathways. PDIOX initiates the generation of lipid-derived oxylipins in Arabidopsis to deal with fungal Colletotrichum higginsianum and bacterial Pseudomonas syringae pv. tomato invasion (de León et al. 2002; Shimada et al. 2014). GLIP1 also enhanced immunity in Arabidopsis to fungal A. brassicicola and bacterial Erwinia carotovora (Oh et al. 2005; Kwon et al. 2009). Interestingly, both PDIOX and GLIP1 are associated with metabolic changes in the lipid signalling of plants under pathogen attack. Deciphering PDIOX and GLIP1 expression regulation in Di-G can reveal fine-tuning of plant defence via lipid-mediated signalling to A. brassicicola and Xcc infection.
Expression of SA-responsive genes, such as PR1, EDS1 and PAD4, was higher in Di-G leaves than in Col-0 leaves by exogenous SA, but NPR1 gene expression was not different in the two ecotypes (Lee et al. 2019). Xcc infection might activate SA-dependent defence signalling in Arabidopsis, evidenced by enhanced chlorosis and bacterial proliferation in the sid2 mutant (Rong et al. 2010). SA-responsive PR1, EDS1 and NPR1 did not show different Xcc-induced expression in Col-0 and Di-G, but early expression of PAD4 in the Xcc-inoculated Col-0 leaves was distinctly compromised in Di-G. PAD4 is required for Arabidopsis immunity against fungal Erysiphe cichoracearum, oomycete Peronospora parasitica and bacterial P. syringae pv. maculicola, but not to fungal Botrytis cinerea (Glazebrook et al. 1997; Jirage et al. 1999; Ferrari et al. 2003; Xiao et al. 2005). These indicate that PAD4 is a regulatory component that is pivotal for SA defence signalling, but not for jasmonic acid and ethylene pathways. The attenuated PAD4 expression can be one of the reasons for enhanced Xcc proliferation in Di-G. PAD4 can physically interact with EDS1 to form a heterodimer in certain circumstances as a co-regulator of plant defences (Fey et al. 2001; Rietz et al. 2011). EDS1 is transcriptionally not lessened in the Xcc-inoculated Di-G, but the decreased PAD4 in Di-G may lead to functional defects in the EDS1-mediated SA signalling to Xcc infection (Dongus and Parker 2021). These results suggest that Di-G has different sensitivities to exogenous SA and Xcc-mediated endogenous SA signalling compared to Col-0. In addition, other SA-dependent genes can be differentially regulated in Col-0 and Di-G during the Xcc bacterial infection. Enhanced ABA signalling can be one of the reasons for the increased Xcc growth in Di-G under SA-mediated defence activations. This is supported by exogenous ABA increasing Xcc bacterial growth within the Arabidopsis leaf tissues (Ho et al. 2013).
We investigated the expression of senescence-related genes in Col-0 and Di-G to decipher the relation of Xcc infection with leaf senescence. DIN6 and SAG12 distinct expression in Col-0 indicates Xcc infection accelerates leaf senescence, but reduced DIN6 and SAG12 expression in Di-G was closely associated with delayed chlorosis development. DIN6 and SAG12 encode an asparagine synthase and a cysteine protease, respectively, and both gene expressions during the Arabidopsis leaf senescence were involved in remobilisation and reallocation of nitrogen (Fujiki et al. 2001; Fernández-Calvino et al. 2016; James et al. 2018, 2019). Highly damaged Col-0 leaves by Xcc infection may need to reuse nitrogen sources efficiently compared to Di-G through DIN6 and SAG12 activation.
4.3 Delayed Dark-Induced Senescence in Di-G Leaves
Eight Arabidopsis ecotypes were classified into two groups in terms of early senescence behaviours (Bur-0, St-0, Col-0, Lip-0, and Cvi-0) and late senescence ones (N13, Bl-1, and Gre-0) (Balazadeh et al. 2008). We found that the leaf senescence-related dark-induced genes DIN6 and SAG12 were also associated with the chlorosis caused by Xcc bacterial infection. Delayed chlorosis in Di-G leaves during the dark-induced senescence seems to relate to the lower expression of DIN6 and SAG12 at the later stages of the Xcc-induced leaf chlorosis. DIN6 and SAG12 gene expression during the different dark-induced senescence remains to be demonstrated in further studies. These results indicate that Xcc infection and dark-induced senescence may share signal pathways for Arabidopsis leaf chlorosis, at least in part. Di-G seems to develop a retardation system to keep the leaves green during infection and senescence singalling. Interestingly, we could not observe a distinct difference in leaf senescence and flowering time of Col-0 and Di-G under the natural growth conditions. Exogenous stimuli, including dark conditions, are likely to mediate the difference in leaf senescence of Col-0 and Di-G. In addition to the light limitation, the two Arabidopsis genotypes might also have shown differential leaf senescence by other external and internal cues induced by phytohormones and nutritional status, and further investigation using various stimuli will provide valuable information on the leaf senescence of Col-0 and Di-G.
4.4 Differential Roles of Exogenous SA in Leaf Chlorosis Caused by Xcc Infection and Dark-Induced Senescence
SA signalling pathways have been shown to affect plant immunity as well as senescence. As the plants grow old, the SA level gradually increases in Arabidopsis leaves, and the expressions of defence-related PR1 and senescence-related SAG12 genes concurrently become induced in the senescent leaves (Morris et al. 2000). Arabidopsis mutants s3h and rse1, which have elevated SA levels and PR1 expression, demonstrated accelerated leaf senescence, indicating that overproduced SA could promote leaf senescence (Lee et al. 2020; Zhang et al. 2013). Exogenous SA application induced senescence in the detached Arabidopsis leaves, in which MPK6 and NPR1 were associated with the SA-promoting senescence (Chai et al. 2014). Despite increasing evidence that SA-mediated signalling overlaps with plant immunity and senescence (Guo et al. 2017; Wang et al. 2020), Arabidopsis ecotypes having both SA-dependent alterations in plant immunity and senescence have yet to be thoroughly investigated. In the present study, the involvement of SA in Xcc infection and dark-induced senescence was investigated in Col-0 and Di-G by SA pretreatment. SA pretreatment did not alter the different chlorophyll degradations observed in Col-0 and Di-G leaves inoculated with Xcc, suggesting that SA signalling was not directly related to the delayed leaf chlorosis of the Xcc-inoculated Di-G. However, Di-G may be less sensitive to SA signalling to limit in planta Xcc bacterial proliferation compared to Col-0 because the Xcc growth was reduced in the SA-pretreated Col-0 leaves but was unchanged in the SA-pretreated Di-G. Interestingly, SA pretreatment mitigated dark-induced leaf chlorosis only in Col-0, not Di-G. This suggests that pre-elevated SA can efficiently delay chlorophyll degradation in Col-0 leaves under continuous darkness. However, Di-G may be less sensitive to SA or have deficient SA-induced tolerance to dark-triggered chlorosis. More recently, it was suggested that SA promoted leaf senescence in Arabidopsis through coordination with ethylene (Yu et al. 2021). The signalling interaction with ethylene may be involved in the different chlorosis phenotypes of SA-mediated responses to Xcc infection and dark-induced senescence in Di-G.
5 Conclusions
Arabidopsis ecotype Di-G has shown delayed leaf chlorosis during Xcc infection and dark-induced senescence compared to Col-0. However, in planta Xcc bacterial proliferation was higher in the inoculated Di-G leaves, showing an uncoupled symptom appearance and disease resistance. Several defence-associated and senescence-related genes were distinctly expressed in the Xcc-inoculated Col-0 leaves during the bacterial infection, but some inducible gene expressions were attenuated in Di-G leaves. Exogenous SA could not induce plant immunity against Xcc infection and ameliorate dark-induced chlorosis in Di-G, although plant immunity and dark-induced senescence were improved in Col-0 by SA. This study provides a basis for the uncoupling of symptom development and plant immunity in Col-0 and Di-G, as well as the attractive features of Di-G concerning delayed chlorosis during Xcc infection and dark-induced senescence.
Author Contributions
Jeum Kyu Hong: conceptualisation of experimental design, writing and critical review. Young Hee Lee, Yun Jeong Kim: performing experiments and figure preparation. Hee Jin Park, Byung-Wook Yun: data analysis, critical review and editing.
Acknowledgments
This work was supported by National Research Foundation of Korea (NRF) grant funded by Ministry of Science and ICT (MSIT) of the Korea government (No. NRF-2010-0003285, NRF-2020R1A2C1101613).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




