Diesel Tolerance in the Antarctic Grass Deschampsia antarctica: From Laboratory to Field in Extreme Conditions
Funding: This work was supported by Agencia I+D+i in Argentina (PICT 2020 0440 and PIP 3193/21) and the Argentine Antarctic Institute (IAA). C.B.D. was supported by a Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) doctoral fellowship, PIP3193/21, 14120210300380CO01.
ABSTRACT
Diesel spills represent a significant challenge to Antarctic ecosystems, particularly in ice-free areas where stations and wildlife co-occur. Taking into consideration the Protocol on Environmental Protection to the Antarctic Treaty, the use of native species emerges as a suitable solution for this problem. Here, we evaluate the tolerance and potential of the native grass Deschampsia antarctica for phytoremediation of diesel-contaminated soils, combining in vitro and field assays at Carlini Research Station. Using a dose–response approach, we measured biometric parameters, photosynthetic pigments, and antioxidant enzyme activities under varying diesel concentrations. In vitro experiments suggested high half-maximal inhibitory dose (ID50) values: 3741, 5709 and 8425 mg kg−1 for root growth, chlorophyll content, and total biomass, respectively. Field experiments showed a 14.5%, 47.9%, and 27.5% reduction in biomass, root growth and chlorophyll content at the highest diesel concentration (40,000 mg kg−1), suggesting that root growth is the most sensitive parameter. Antioxidant enzyme activities, including guaiacol peroxidase (GPX, EC 1.11.1.7) and superoxide dismutase (SOD, EC 1.15.1.1), presented contrasting trends between in vitro and field conditions, underscoring the influence of environmental factors on stress responses. These results propose root growth as an indicator of diesel-induced stress, contributing to optimizing phytoremediation strategies. Overall, our findings highlight the plant's tolerance to high contaminant levels, even under conditions of maximum bioavailability, and demonstrate its potential for phytoremediation in extreme environments, supporting the development of sustainable remediation strategies for Antarctic soils.
1 Introduction
Human activity requires energy sources for comfort and work (Tamïr 2021). This is especially true in Antarctica, where the extreme environmental conditions are constantly challenging. To date, diesel stands as the primary energy source on the Antarctic continent, providing reliability but also carrying the risk of mismanagement and spills (Martínez Álvarez et al. 2025; Ruberto et al. 2020). According to the Council of Managers of National Antarctic Programs (COMNAP 2024), there are 76 active stations on the Antarctic continent. Fuel spills are recognized as one of the biggest environmental threats, particularly in ice-free areas where research stations and wildlife coexist (Lim et al. 2021). These areas are hotspots for fuel transportation and are critical locations where aircraft, land vehicles, and vessels are refuelled. Developing solutions to incidental spills is challenging, as a consequence of the isolation of the Antarctic continent and environmental factors such as freezing temperatures, extensive ice-covered areas, and the freeze–thaw cycle (Raymond et al. 2017). In this context, bioremediation, broadly defined as the use of biological tools for cleaning up contaminated environments, stands out as a suitable approach (Margesin and Schinner 2001; Sun et al. 2024).
Successful experiences in bioremediating hydrocarbon-contaminated soils in the Antarctic continent have been reported (de Jesus et al. 2015; Delille et al. 2008; Martínez Álvarez et al. 2017; McWatters et al. 2016; Ruberto et al. 2009), most of which rely on the catabolic capabilities of microorganisms. However, bioremediation, which depends only on microorganisms, presents limitations, for instance, when the hydrocarbon mixture contains recalcitrant compounds such as aromatic hydrocarbons or when the availability of the contaminant is limited (Hussain et al. 2018; Smułek et al. 2020). In such cases, phytoremediation—a technique involving plants to remediate contaminated soils—emerges as a promising approach to overcome these limitations. This technology has been reported as an effective method to target a large number of pollutants, including diesel among them (Gerhardt et al. 2017; Thijs et al. 2017).
In alignment with the Protocol on Environmental Protection to the Antarctic Treaty (1991), the use of native species is critical for the development of biological solutions in this region. Furthermore, beyond the Antarctic context, there is abundant evidence that phytoremediation using native species is often more effective and efficient than its non-native counterparts (Futughe et al. 2021; Pandey and Bajpai 2019). Deschampsia antarctica É. Desv. (Poaceae), one of the two vascular plants native to the Antarctic Peninsula, stands out as a promising candidate due to its extensive coverage in ice-free areas (Cavieres et al. 2018; Vera et al. 2013) and the demonstrated hydrocarbon tolerance of species from the same genus in subpolar regions (Macoustra et al. 2015).
To assess the potential of D. antarctica for phytoremediation, we conducted tolerance bioassays to characterize the plant's physiological and biochemical responses to diesel. The physiological and biochemical parameters presented in this work were selected because they are easily measurable in field conditions, making them particularly suitable given the logistical constraints of Antarctic research. This practicality ensures their applicability in the challenging Antarctic environments, while also providing a robust foundation for future studies on advanced physiological mechanisms in D. antarctica.
These bioassays were performed under in vitro conditions, where the contaminant is the major stress factor and at its maximum bioavailability. In vitro setups offer several practical advantages: they allow plants to grow under controlled laboratory conditions, independent of external weather fluctuations, and they often promote faster plant development compared to plant–soil systems (Reynoso-Cuevas et al. 2008). Additionally, in vitro systems ensure that the observed effects are solely attributable to the plant itself, excluding potential interference from microbial communities in the rhizosphere.
The main purpose of conducting in vitro tolerance assays is to establish a controlled environment that allows the determination of key parameters, such as the ID50 (inhibition dose 50), which refers to the diesel dose at which a 50% reduction in a given parameter is observed compared to the control without diesel. According to King et al. (2024), modeled toxicity data, including parameters like the ID50—also referred to as the effective dose 50 (ED50) or inhibition concentration 50 (IC50) (Eze et al. 2021; Macoustra et al. 2015)—are preferable to hypothesis-driven metrics, such as the no-observable-effect concentration (NOEC) and lowest-observable-effect concentration (LOEC). Establishing the ID50 provides a clear threshold of diesel concentration that D. antarctica can tolerate while maintaining its functionality, regardless of the substrate. This is essential when proposing a robust biotechnological tool that can be confidently applied to a variety of contaminated soils across Antarctic stations. With numerous stations and a wide range of contamination levels, these data ensure that treatment strategies can be tailored effectively to the specific hydrocarbon concentrations reported at each site.
In addition, field assays were carried out at Carlini Research Station, located on 25 de Mayo (King George) Island, South Shetlands, Antarctica (Figure 1), to assess the plant's performance in real, complex and variable environmental conditions, such as extreme temperatures, nutrient limitations, and interactions with native microbial communities. These assays are critical to determine the extent to which in vitro data can be extrapolated to field conditions (Merini et al. 2011; Zuzolo et al. 2021). The natural Antarctic environment introduces additional factors that can influence plant responses, potentially altering the sensitivity and dynamics of specific parameters. By integrating data from both in vitro and field experiments, we aimed to comprehensively understand the plant's potential for hydrocarbon remediation in Antarctic soils.
Here, we present results on the tolerance and potential application of D. antarctica in phytoremediation strategies for Antarctic soils contaminated with diesel. Section 2 describes the experimental design and methods used for both in vitro and field assays. Section 3 details the findings, including dose–response modeling and performance evaluations under different environmental conditions. Finally, Section 4 discusses the implications of these results for developing sustainable and effective phytoremediation strategies in Antarctica.
2 Materials and Methods
2.1 Experimental Setups
2.1.1 In Vitro Assay
2.1.1.1 Plant Material and In Vitro Growth Conditions
Wild specimens of D. antarctica collected from Carlini station were washed 3 times with tap water to eliminate remaining soil and other debris, and then immersed in alcohol 96° for 20 s followed by a sodium hypochlorite treatment (4% active chloride) for 20 min. The disinfectant was eliminated by washing 3 times with sterile distilled water. Explants were then transferred to sterile glass tubes containing 25 mL of Murashige and Skoog (MS) medium (Murashige and Skoog 1962) with 3% (w/v) sucrose and 8 g L−1 micropropagation grade agar (PhytoTechnology Laboratories). Cultures were incubated in a growth chamber (Ingelab, model I-317PFH) at 15°C with a 16/8 h photoperiod and a photon flux density of 120 μmol m−2 s−1 at 20 cm.
45-day-old specimens were subcultured to fresh medium with increasing concentrations of diesel (0, 100, 1000, 2500, 5000, 10,000 mg kg−1) (Figure 2A). Eight replicates of each treatment were kept in the growth chamber for 90 days until samples were harvested to measure biometric, photosynthetic, and antioxidant parameters.

As previously mentioned, an in vitro assay was designed to evaluate the tolerance of D. antarctica to diesel under conditions of maximum bioavailability, where the contaminant is fully accessible to the plant. Based on the results from this assay, concentrations were identified at which plant viability was not compromised. Subsequently, a field experiment was conducted (Figure 1B), with increased diesel levels (see Section 2.1.2), considering that in soil, bioavailability is lower due to factors such as soil structure, organic matter adsorption, and microbial activity. This increase in diesel concentrations aimed to replicate more realistic environmental conditions while still challenging the plant's tolerance to hydrocarbon contamination.
2.1.2 Field Assay
Based on the results of the in vitro assay, a field experiment was performed under Carlini Station's environmental conditions (Figure 2B). Axenic specimens of D. antarctica coming from the same subculture mentioned above were planted in 7-L pots containing increasing concentrations of diesel (0, 2500, 5000, 10,000, 20,000, 40,000 mg kg−1). Five replicates were used per treatment. Morphological homogeneity between the specimens was considered. The soil used for this experiment was obtained from a human impact-free area in the surroundings of the Station. This soil was characterized by a total organic carbon (TOC) content of 1.22%, organic matter (OM) content of 2.22%, and phosphorus (P) levels of 19.91 mg kg−1. The soil was sieved using a 5 mm mesh to remove stones and unwanted large particles. Hydrocarbon levels were generated from serial dilutions of diesel-spiked soil. All generated pots were left under Antarctic environmental conditions to settle for a week. Pots were watered once a week for 90 days during the entire Antarctic summer campaign (2023–2024). After this period, plant specimens were harvested to measure the same biometric, photosynthetic and antioxidant parameters as in the in vitro assay.
2.2 Biometric Parameters
Each treatment was harvested, and the shoots and roots were washed under tap water and oven-dried at 98°C for 48 h. Then, dry biomass was weighed, and root growth was measured using the ImageJ image analysis software (Schneider et al. 2012) with the SmartRoot plugin (Lobet et al. 2011).
2.3 Photosynthetic Pigments
2.4 Determination of ID50
The half-maximal inhibitory dose (ID50) was determined for total biomass, root growth, and total chlorophyll content. The ID50 represents the diesel dose at which a 50% reduction in a given parameter is observed compared to the control without diesel. Statistical models were used to estimate the ID50 values for each parameter, following the methodology described at Section 2.6.
2.5 Antioxidant Enzyme Activity
Fresh plant material (0.100 g) was homogenized with 1 mL of buffer (50 mM potassium phosphate buffer pH 7.4 with 1 mM EDTA, 0.5% v/v triton, and PVP 1% w/v). The mixture was centrifuged at 17,200 g at 4°C for 30 min, and the supernatant was used for the determinations. The concentration of the soluble proteins was determined according to Bradford's methodology (Bradford 1976) using bovine serum albumin (BSA) for the standard curve. This extract is referred to as the protein extract for the following enzyme activity assays.
2.5.1 Superoxide Dismutase (SOD) (EC 1.15.1.1)
Superoxide dismutase activity was determined by inhibiting the photochemical reduction of nitrobluetetrazolium (NBT), as described by Giannopolitis and Ries (1977). The reaction mixture contained 160 μL of an O2˙− generating solution (14.3 mM methionine, 82.5 μM NBT, and riboflavin 2.2 μM) and increasing volumes of the plant extract in a range of 0 to 40 μL and completed to a final volume of 200 μL with extraction buffer in a 96-well plate. The absorbance of the solutions was measured in a plate reader at 560 nm (FlexStation 3 Multi-Mode Microplate) at time 0 and 10 min after incubation in light at 25°C. Similar mixtures, kept in the dark, were used as reaction blanks. One unit of SOD is defined as the volume of extract necessary to inhibit 50% of NBT photoreduction. The specific activity of SOD was expressed as units per mg of protein.
2.5.2 Guaiacol Peroxidase (GPX) (EC 1.11.1.7)
Guaiacol peroxidase activity was determined by following the increase in absorbance at 470 nm, due to the formation of tetraguaiacol using a Shimadzu UV-1800 spectrophotometer. The reaction mixture contained 50 mM phosphate buffer (pH 7.4), 1 mM guaiacol, 0.1 mM H₂O₂, and 0.1 mL of the extract, in a final volume of 1 mL (extinction coefficient ε 470 nm = 26.6 mM−1 cm−1), following the method of Maehly (1954).
2.6 Statistical Analysis and Modeling
Bayesian Generalized Linear Models were fitted to evaluate the effect of increasing diesel concentration on each measured parameter (total biomass, root growth, chlorophyll content, GPX and SOD activities). Each parameter was modeled following a Gamma distribution with a log link function, except for the chlorophyll content, which was modeled using a Skew Normal distribution (identity link). The diesel concentration, the condition (in vitro vs. field), and the interaction between them were included as fixed covariates. Moreover, the dispersion parameters of the distributions were modeled as functions of the condition to account for heteroscedasticity. All models were fitted with the brms package (Bürkner 2017) using R software version 4.2.0.1 (R Core Team 2021). Flat priors were implemented (brms default priors), and five chains of 3000 iterations each were run, leaving 1000 to warm up. The minimum mean effective sample size was 3832 –for the GPX model–, and all R̂ values were equal to 1.00, indicating convergence (Vehtari et al. 2021). Posterior predictive checks showed that the models accurately fit the data (Supporting Information). We reported response curves to diesel concentration for all the studied parameters and the estimated ID50 for total biomass, root growth, and total chlorophyll content.
To compare the effect size of diesel concentration among parameters and assays, we calculated quotients between the posterior distributions of the estimated means for each parameter under different diesel concentrations. Specifically, we calculated the quotients between the estimated means in the absence of diesel and at 10,000 mg kg−1 in field conditions and under in vitro conditions (Qfield-10,000 and Qinvitro-10,000) and the quotients between the estimated means in the absence of diesel and at 40,000 mg kg−1 in field conditions (Qfield-40,00). For all quotients, the higher estimated mean was used as the numerator, allowing for reporting how many times the parameter increased or decreased with changes in diesel concentration. These quotients, as well as all estimates, were summarized by their posterior distribution means and their 95% equal-tailed credible intervals (presented in brackets).
3 Results
3.1 Effect of Diesel Exposure on Biometric Parameters
3.1.1 Total Biomass
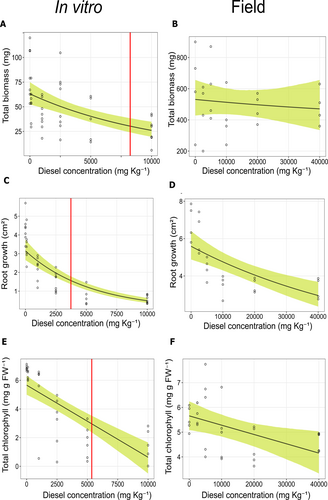
Total biomass decreased by 36.7 mg [20.1, 53.8] (59.7%) when diesel concentration increased from 0 to 10,000 mg kg−1 (Figure 3A) in the in vitro assay. Specifically, biomass dropped from 63 mg [51.6, 76.8] at 0 mg kg−1 diesel to 26.3 mg [18.9, 36.3] at 10,000 mg kg−1. Contrastingly, total biomass did not exhibit a significant change in the field assay, decreasing only by 55 [−173, 265] mg (14.5%) from 531 mg [428, 656] at 0 mg kg−1 to 476 mg [322, 656] at 40,000 mg kg−1 (Figure 3B). The Bayesian R2 (Gelman et al. 2019) for the biomass model was 0.8 [0.73, 0.84], indicating a good fit of the model to the data.
3.1.2 Root Growth
Root growth declined by 2.66 [2.14, 3.29] cm2 (84.7%) as diesel concentration increased from 0 to 10,000 mg kg−1 in the in vitro assay (Figure 3C). This reduction represents a decrease from an initial value of 3.15 cm2 [2.66, 3.75] to 0.49 cm2 [0.37, 0.64]. In the field, root growth showed a smaller decline of 2.63 [1.52, 3.75] cm2 (47.9%) across diesel concentrations tested (Figure 3D), with values dropping from 5.59 cm2 [4.88, 6.40] to 2.95 cm2 [2.36, 3.68] at 40,000 mg kg−1. The Bayesian R2 for the root growth model was 0.72 [0.65, 0.79], supporting a good explanatory power for the observed variation (Table 1).
| Parameter | ID50 (mg kg−1) | CI 2.5% | CI 97.5% | R 2 | CI 2.5% | CI 97.5% |
|---|---|---|---|---|---|---|
| Total biomass | 8425 | 5360 | 15,105 | 0.80 | 0.73 | 0.84 |
| Root growth | 3741 | 3144 | 4541 | 0.72 | 0.65 | 0.79 |
| Total chlorophyll | 5709 | 4617 | 7169 | 0.53 | 0.40 | 0.63 |
- Note: CI 2.5% and CI 97.5% indicate the lower and upper bounds of the 95% credible interval for the ID50 and R2 values.
3.1.3 Total Chlorophyll Content
Total chlorophyll content showed a pronounced decline in the in vitro experiment, with chlorophyll levels decreasing by 5.20 [3.73, 6.51] mg g−1 FW (92.5%) when diesel concentration increased from 0 to 10,000 mg kg−1 (Figure 3E). Initial levels of chlorophyll were 5.83 [4.99, 6.32] mg g−1 FW, which dropped to 0.63 [−0.5, 1.66] mg g−1 FW. In contrast, field chlorophyll levels only decreased by 1.51 [0.34, 2.59] mg g−1 FW (27.5%) (Figure 3F), with values ranging from 5.66 [5.09, 6.24] mg g−1 FW to 4.15 [3.33, 5.03] mg g−1 FW across diesel concentrations. The Bayesian R2 for the chlorophyll content model was 0.54 [0.4, 0.63], indicating that the model explains a substantial proportion of the variance (Table 1); however, some dispersion remains, especially for in vitro assay data (Figure 3E).
3.1.4 Inhibition Dose 50 (ID50)
The estimated diesel concentration required to inhibit 50% of the response (ID50) varied across the measured parameters in the in vitro assay (Table 1). For total biomass (Figure 3A), the ID50 was 8425 mg kg−1 [5360, 15,105], whereas for root growth the ID50 was 3741 mg kg−1 [3144, 4541] (Figure 3C). Finally, for total chlorophyll content the ID50 was 5709 mg kg−1 [4617, 7169] (Figure 3E). ID50 values estimated for the field assay are not shown as they exceed the range of diesel concentrations tested.
3.2 Effect of Diesel Exposure on Enzyme Antioxidant Activity
Guaiacol peroxidase (GPX) enzymatic activity showed an upward trend across diesel concentrations in the in vitro assay, starting from 8.35 [7.34, 9.42] mmol min−1 mg−1 protein in the absence of diesel and reaching 11.6 [9.28, 14.27] mmol min−1 mg−1 protein at 10,000 mg kg−1 of diesel concentration (Figure 4A). Superoxide dismutase (SOD) activity also displayed a slight increase with diesel concentration, beginning at 30.7 [27.7, 33.9] U SOD mg−1 protein in the control and reaching 31.1 [28.4, 34] U SOD mg−1 protein at the highest concentration (Figure 4A).
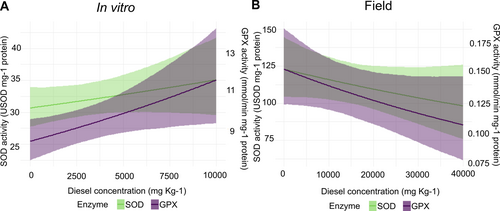
In contrast, both GPX and SOD activities exhibited a decreasing pattern in the field conditions (Figure 4B). GPX activity showed a downward trend with increasing diesel concentrations, starting at 0.153 [0.125, 0.186] mmol min−1 mg protein in absence of diesel and declining to 0.107 [0.077, 0.147] mmol min−1 mg protein at the highest concentration tested. SOD activity followed a similar decreasing trend, beginning at 123 [104, 144] U SOD mg−1 protein and reducing to 98 [75, 125] U SOD mg−1 protein as diesel concentration increases up to 40,000 mg kg−1. The Bayesian R2 values for the GPX and SOD models were 0.85 [0.82, 0.87] and 0.71 [0.63, 0.76], respectively.
3.3 Comparing Responses of D. antarctica to Diesel Exposure
Total biomass and root development showed the most pronounced response under in vitro conditions and were considerably higher than the effect size for SOD and GPX activities. Specifically, estimated total biomass was reduced 2.48 [1.58, 3.65] (Qinvitro-10,000) times when increasing diesel concentration from 0 to 10,000 mg kg−1 diesel concentration, and estimated root development decreased 6.55 [4.6, 9.07] times when facing the same change (Figure 5). However, GPX activity only increased 1.39 [1.05, 1.8] times, and estimated SOD activity almost did not change (Qinvitro-10,000 = 1.01, [0.99, 1.04]) across those diesel concentrations.
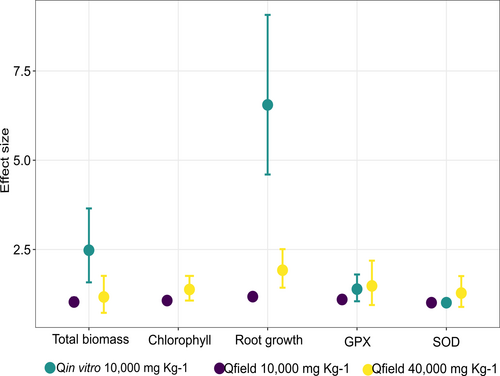
Estimated Qfield-10,000 ranged from 1.01 (for SOD activity) to 1.18 [1.09, 1.26] (for root development), meaning almost no effect was observed on either of the parameters when diesel concentration reached 10,000 mg kg−1 in field conditions. In general, Qfield-10,000 were slightly lower than Qfield-40,000 (Figure 5), suggesting little effect of diesel concentration in the field.
Qinvitro-10,000 values for biomass and root development were higher than estimated Qfield-40,000, which were equal to 1.17 [0.73, 1.76] and 1.92 [1.43, 2.51], respectively. This means that the effect size of diesel on these parameters was also higher under in vitro conditions when compared to 4 times higher diesel concentrations in the field. Contrastingly, Qinvitro-10,000 values for GPX and SOD activities were lower than Qfield-40,000 (1.48 [0.95, 2.19] and 1.28 [0.89, 1.76], respectively), and importantly, the trend of the response reversed since both activities in field conditions showed not an increase as under the in vitro condition but a reduction (Figure 4).
When comparing all the parameters, root growth showed the most pronounced response under both in vitro and field conditions (Qinvitro-10,000 and Qfield-40,000; Figure 5).
4 Discussion
4.1 Evaluating Deschampsia antarctica Potential for Phytoremediation
Our findings characterized D. antarctica potential for phytoremediation applications in polar soils contaminated with diesel, the most used petroleum hydrocarbon derivative in Antarctica. Grasses are frequently selected for phytoremediation due to their fast growth, strong colonization abilities, and extensively branched root systems, which provide a substantial surface area for microbial colonization and plant-microbe interactions (Glick 2003; Hutchinson et al. 2001). These characteristics, also observed in D. antarctica, enhance its potential as a phytoremediation agent, particularly in the extreme conditions of polar regions where few species can thrive.
D. antarctica has proven to be a robust candidate for phytoremediation in polar soils contaminated with hydrocarbons, with ID50 values exceeding 3741 mg kg−1 in vitro (Table 1) and the ability to maintain functionality at contamination levels of up to 40,000 mg kg−1 in field conditions (Figure 3). This information is critical not only for establishing concentration thresholds that guide remediation strategies and experimental designs but also for confirming the suitability of this species for hydrocarbon remediation and identifying key response parameters necessary to develop effective laboratory-based remediation processes.
4.2 Key Response Parameters and Stress Tolerance Mechanisms
Root growth emerged as the most sensitive parameter to diesel exposure, followed by total chlorophyll content and total biomass (Table 1). Effect size analysis further confirmed root growth as the most affected parameter under both in vitro and field conditions (Figure 5). Similarly, Tang et al. (2011) reported that root elongation was the most sensitive parameter in maize exposed to hydrocarbons, with an EC50% (effective concentration 50%) of 10,100 mg kg−1 (Total Petroleum Hydrocarbons) in an artificial soil experiment. Consistently, in the sub-Antarctic species Deschampsia chapmanii, root growth was also identified as the most sensitive parameter, with an IC50 greater than 2400 mg kg−1 in an artificial soil experiment with low nutrient content (Macoustra et al. 2015). Highlighting root growth as a reliable indicator of diesel-induced stress, our study shows its utility in quantifying the impact of contaminants, facilitating cross-experimental comparisons while reducing the complexity and time required to measure multiple parameters.
Our results suggest that D. antarctica possesses an intrinsic capacity to endure significant contamination levels before experiencing a 50% reduction in these key growth parameters. While the lowest ID50 for D. antarctica was shown to be equal to 3741 mg kg−1, grasses like Echinochloa crus-galli and Rhynchelytrum repens reported lethal doses of 3000 mg kg−1 hydrocarbon contamination also in in vitro conditions (Reynoso-Cuevas et al. 2008). This capacity to tolerate diesel might be supported by its antioxidant enzyme activities, as in vitro assays revealed an active antioxidant response, with increased SOD and GPX activity correlating with diesel concentration (Figure 4A). However, under field conditions, both SOD and GPX activities decreased as diesel concentration increased (Figure 4B). This contrast can be partially attributed to the absence of nutrient limitations in vitro, whereas the Antarctic soils used in this study, characterized by low levels of total organic carbon (1.22%), organic matter (2.22%), and phosphorus (19.91 mg kg−1), impose significant constraints on nutrient availability. This divergence aligns with findings from Zuzolo et al. (2021), who observed a significant decrease in SOD and GPX activities in four different species exposed to hydrocarbon contamination under field conditions over time, highlighting the dynamic nature of antioxidant responses to prolonged stress in natural environments. Similarly, Gago et al. (2023) suggested that D. antarctica employs different photosynthetic and stress tolerance mechanisms depending on soil nutrient availability, adapting its metabolic strategies to optimize resource use under nutrient-limited conditions.
4.3 Practical Implications and Long-Term Perspectives
Combining in vitro and field experiments proved crucial for an integrative understanding of the stress response and diesel tolerance of D. antarctica. Notably, D. antarctica showed considerable tolerance to diesel under in vitro conditions, demonstrating that, regardless of identifying the environmental factors contributing to its lower response under field conditions, this species has the potential to tolerate highly effective diesel concentrations. This underscores the importance of combining both experimental approaches to guide phytoremediation strategies.
The reduced effect size under field conditions compared to in vitro assays (Figure 5) highlights the natural tolerance of D. antarctica in its natural habitat. The reduced sensitivity observed under field conditions, particularly in root growth and chlorophyll content, may suggest a degree of acclimation of D. antarctica to diesel exposure in its natural environment. Acclimation refers to the ability of plants to adjust their physiological performance in response to prolonged environmental stress, often reducing sensitivity and enhancing survival (Atkin and Tjoelker 2003). This response could involve metabolic regulation or interactions with native microbial communities and merits further investigation.
As recently highlighted by Pertierra et al. (2025), research on terrestrial Antarctic organisms remains limited, especially in terms of their ecological roles and responses to abiotic challenges. Particularly, the scarcity of long-term field studies investigating native species for phytoremediation in polar regions represents a significant gap in the literature. This study addresses this issue by demonstrating the necessity of extended, real-world experiments to capture the dynamic and multifaceted nature of plant responses to hydrocarbons in Antarctic soils. By identifying critical diesel tolerance thresholds through in vitro assays, we ensured that field trials replicated realistic contamination scenarios within the plant's viability range. Field results validated these findings, confirming D. antarctica as a resistant candidate for ecological restoration in diesel-contaminated polar soils. These results underscore the potential of D. antarctica as a native species for remediation in Antarctic environments characterized by freezing temperatures, poor soil quality, and extreme environmental stressors.
5 Conclusions
This study suggests D. antarctica is highly tolerant to diesel in its natural habitat, representing a foundational step in understanding the potential of this species for phytoremediation in Antarctic hydrocarbon-contaminated soils. We established laboratory-derived thresholds that can be extrapolated to any condition in Antarctic research stations and validated these findings through field experiments. By aligning with the conservation priorities established by the Madrid Protocol, this work contributes to the development of ecologically responsible strategies for mitigating diesel contamination in Antarctica and sets the stage for future research, including plant-microorganism interactions and practical applications.
Author Contributions
C.B.D.: writing – original draft, investigation, methodology, data curation. F.M. and L.R.: conceptualization, supervision, writing – original draft, resources. M.P.: writing – original draft, methodology. L.R.: methodology, writing – review and editing. M.V.R.: methodology. W.M.C.: review and editing, resources.
Acknowledgments
We would especially like to thank the Carlini Station crew (2023–2024), the dedicated logistics team of the National Antarctic Directorate (DNA), the COCOANTAR program, and the Argentine Antarctic Institute (IAA) for their invaluable support during this research. We are also grateful to Lucas Borque, Maximiliano Sosa, and Marcos Esteso for their assistance with sample processing.
Open Research
Data Availability Statement
Data will be made available upon request.




