Drought Stress Modifies the Source-Sink Dynamics of Nitrogen-Fixing Soybean Plants Prioritizing Roots and Nodules
Funding: This work was supported by grants PID2021-122740OB-I00 funded by MCIN/AEI/10.13039/501100011033, “ERDF: A way of making Europe” and TED2021-130111B-I00, “Next Generation funding”. Open access funding was provided by the Public University of Navarra.
ABSTRACT
Soybean plants are one of the most cultivated legume crops worldwide. Their ability to establish nitrogen-fixing symbiosis with rhizobium bacteria allows the reduction of molecular nitrogen to ammonium, contributing to a reduction in the dependence on nitrogen fertilizers. However, nitrogen fixation is highly sensitive to environmental stresses, such as water deficit, and the regulatory mechanisms underlying this inhibition remain debatable. In the current study, we analyzed carbon (C) allocation dynamics in drought-stressed soybean plants following the application of [U-13C]-sucrose to source leaves. Three sets of plants were analyzed: well-watered plants, mild drought, and severe drought-stressed plants. 13C distribution was monitored for up to 6 h post-application. Under optimal water conditions, 13C was mainly allocated to young (sink) leaves. During drought stress, transport trends changed, prioritizing C allocation primarily to the roots and nodules to a lesser extent. Metabolite profiling identified drought- and tissue-specific variations in the levels of the major C and N compounds.
1 Introduction
Globally valued for its nutritional benefits to both humans and animals, soybean plants (Glycine max L. Merr.) are a widely cultivated crop with an established international market. Soybean provides approximately 70% of the total legume grains produced worldwide (Peoples et al. 2019). Although soybean areas in Europe are limited by climatic constraints, the development of new varieties that are better adapted to cool weather is promising (Zimmer et al. 2016). In 2022, global soybean production was approximately 349 tons, of which 124 tons were produced in Europe (FAOStat 2024). As a model organism for agronomic, physiological, and molecular research, soybean has become one of the most extensively investigated legumes due to its economic significance. Other relevant ecological features of soybeans and legumes in general are their ability to establish a symbiotic relationship with microorganisms, generally called rhizobia (Oldroyd 2013). This symbiosis leads to the development of a specialized organ in the roots, the nodule, where rhizobium bacteria reduce molecular N to ammonia, which can be readily incorporated into the plant metabolism (Udvardi and Poole 2013). This reaction is catalyzed by the nitrogenase complex at the expense of large quantities of ATP (estimated to be 16 mol per mole of reduced nitrogen). The required ATP is produced by active bacterial respiration using C substrates, mostly malate, which are provided by the plant (White et al. 2007). This source of reduced N reduces the dependency on nitrogen fertilizers in agrosystems and, therefore, its negative impact on the environment (Ciampitti et al. 2021).
Despite the many advantages of the use of legumes, symbiotic nitrogen fixation is very sensitive to environmental stresses such as extreme temperatures, defoliation, salinity, drought, acidity, and nutrient limitation (Araújo et al. 2015). With an expected 40% increase in the world population, understanding the consequences of climate change on crop production is a challenge to ensure the required 70%–100% increase in food supply estimated by 2050 (Bruinsma 2009; Shanker et al. 2014). In particular, water deficit is one of the natural hazards that most significantly affects plant growth and crop yield in agricultural systems (Cohen et al. 2021). Due to the importance of legume crops in the global diet, research aimed at understanding the physiological basis of drought stress responses is of great relevance (Khatun et al. 2021; Dollete et al. 2024).
In plants subjected to progressive water stress, the photosynthetic rate decreases as stomatal conductance decreases (Lawlor and Tezara 2009). This has led to the assumption that drought-induced inhibition of nitrogen fixation may be due to a shortage of photosynthates in the nodules. Nevertheless, Durand et al. (1987) showed that under mild drought stress, water deficit causes an inhibition of nitrogenase-linked respiration that occurs before a decline in net photosynthesis. This observation focused on a direct effect on nodules, and several regulatory mechanisms have been proposed: (i) alterations in internal oxygen concentration in nodules due to changes in the permeability of the oxygen diffusion barrier (Witty et al. 1987; Hunt and Layzell 1993); (ii) feedback regulation of nitrogenase by plant N status (Serraj et al. 1999; King and Purcell 2005); and (iii) limitation of the C respiratory substrate in nodules due to a decrease in sucrose synthase activity (González et al. 1995; Arrese-Igor et al. 1999 and references therein). In soybean, the hypothesis of oxygen-mediated regulation and C limitation due to a reduction in sucrose synthase activity and transcription levels has been experimentally proven (González et al. 1995; Gordon et al. 1997). Subsequent work proposed a role for N feedback mechanisms involving shoot N status (Vadez and Sinclair 2001; King and Purcell 2005). However, a comparison of two soybean cultivars with contrasting tolerance to water deficit suggested that the drought-induced decline in nitrogen fixation could be mediated by an accumulation of ureides in nodules, but not in leaves (Ladrera et al. 2007). Indeed, the operation of a local regulatory mechanism was later corroborated in studies using split-root systems (Gil-Quintana, Larrainzar, Seminario, et al. 2013).
Interconnected with the regulatory mechanisms described above, the strength of nodules as C sinks in plants plays a relevant role in the regulation of nitrogen fixation. N2-fixing root nodules are strong sink tissues for photoassimilates and compete for C with other sink tissues, such as flowers and pods, at the reproductive stage (Waters et al. 1980). In faba bean (Vicia faba), drought has been shown to affect the long-distance transport of C from leaves to nodules, reducing nodule sink strength as a consequence (Parvin et al. 2020). However, most analyses exploring C source-sink relationships in drought-stressed plants have used labeled 13CO2 to trace C allocation. In leaves, assimilation of the lighter 12CO2 molecule is favored over that of the heavier 13CO2 isotope (Farquhar et al. 1989). Nevertheless, under drought stress, stomatal conductance decreases, leading to reduced 13C/12C (δ13C) ratios. This is a widely used approach for estimating plant drought dynamics in ecology (Peters et al. 2018). In contrast, for the analysis of C allocation dynamics, this approach may present a bias associated with differential stomatal conductance in well-watered vs. drought-stressed plants, and it may not accurately reflect transport rates.
In this study, we propose foliar application of 13C-labeled sucrose as an alternative strategy to analyze source-sink relations in soybean plants subjected to a progressive water deficit. Three sets of plants were analyzed: well-watered (control), mild drought, and severe drought-stressed plants. To understand C allocation dynamics, the youngest fully expanded leaf in each set of plants (considered a “source” leaf) was labeled with [U-13C]-sucrose. Samples corresponding to other source leaves, younger leaves (sink leaves), stems, roots, and nodules were collected at the start of the experiment (time = 0 h, corresponding to 2 h after the beginning of the photoperiod), and at times 3 and 6 h after the labeled sucrose application. This sample timing allowed for quantitative estimation of sucrose transport across plants. We performed a physiological characterization of the water deficit imposed and analyzed the major C and N metabolites in these tissues at different time points to consider diurnal metabolite variations. This experimental setup allowed us to test the following hypotheses: (1) water deficit alters the C allocation dynamics in the plant, and there is competition for C in the different sink tissues (young leaves vs. roots vs. nodules); (2) although drought is likely to reduce sucrose transport to nodules, sucrose availability is not the limiting factor regulating symbiotic nitrogen fixation; and (3) metabolite profiling allows for the discrimination of drought-associated and tissue-specific changes in major C and N compounds.
2 Materials and Methods
2.1 Plant Growth Conditions
Soybean seeds (Glycine max L. Merr. var. Sumatra) were surface sterilized in 1% NaClO (v/v) and 0.01% SDS (w/v) for 40 min, washed with deionized water, and then placed in 0.01 N HCl for 10 min (Labhilili et al. 1995). Seeds were germinated and sown in 1 L pots containing a mixture of vermiculite: perlite (2:5, v/v). Seedlings were inoculated with 1 mL of a hup− Bradyrhizobium diazoefficiens (previously named japonicum) strain UPM792 on days 0 and 3 after sowing. Plants were grown for 1 month under controlled conditions (24°C/18°C day/night temperature, 60%/70% day/night relative humidity, 16 h photoperiod and 450 μmol m−2 s−1 light intensity) and watered to field capacity twice a week with a N-free nutrient solution (Rigaud and Puppo 1975).
2.2 Experimental Design and Stable Isotope Labeling Method
A total of 72 soybean plants were grown for 4 weeks. The plants were separated into three groups. Two groups were subjected to a gradual water deficit by stopping irrigation, while the third group maintained a full water supply. Based on leaf water potential values (Ψleaf), we identified three categories: control, mild drought, and severe drought conditions. To facilitate harvesting at various times throughout the day, each category was further divided into three subgroups comprising eight plants each, with samples taken 2, 5, and 8 h after the start of the photoperiod. This corresponded to samples at t = 0, t = 3, and t = 6 h following sucrose application. Because sample collection is a destructive procedure, a separate set of plants was used for each time point.
To determine the effect of drought on 13C allocation, half of the plants were labeled with stable isotope-labeled sucrose, [U-13C]-sucrose (Sercon Ltd.), while the other half were treated with unlabeled sucrose to determine natural 13C abundance. Upon reaching the two levels of drought stress, both control and stressed plants were labeled (time = 0 h), corresponding to 2 h after the beginning of the photoperiod, following established protocols (Fondy and Geiger 1977). Briefly, the adaxial surface of the youngest fully expanded leaf was gently abraded with a fine-grained sandpaper. Next, 25 μL of a solution containing 20 mM [U-13C]-sucrose buffered in 5 mM potassium phosphate buffer (pH 6.5) was applied to the abraded area. Uptake of the sucrose solution was allowed to continue for 3 or 6 h. The following tissues were collected at these time points: labeled leaf, sink leaf (approximately 26% of full lamina length), other source leaves, stems, roots, and nodules. The samples were weighed, and one set of aliquots was immediately frozen in liquid nitrogen and stored at −80°C for analytical determination. The other set was dried at 70°C for 72 h, ground to a fine powder, and weighed into tin capsules for elemental analysis-isotope ratio mass spectrometry (EA-IRMS) and dry weight (DW) measurements. The same procedure was performed for the non-labeled plants.
2.3 Plant Physiological Characterization
To establish the effect of water stress on plants, non-destructive parameters including stomatal conductance, net photosynthesis, Ψleaf, and apparent nitrogenase activity (ANA) were measured. These parameters were measured 2 h after the beginning of the photoperiod in the youngest fully expanded leaf.
Stomatal conductance and net photosynthesis were measured in a portable open system mode (model LCpro+; ADC BioScientific Ltd.) using an ADC PLC-7504 leaf chamber. Ψleaf was measured using a pressure chamber (Scholander et al. 1965). Nitrogen fixation was measured as ANA (Witty and Minchin 1998) following the H2 evolution of intact plants in an open flow-through system under N2/O2 (79%/21%) using an electrochemical H2 sensor (Qubit Systems). The H2 sensor was calibrated with high-purity gases using a gas mixer at the same rate as that of the sampling system (500 mL min−1).
2.4 Carbon Isotope Analyses
The percentages of C and δ13C were determined using EA-IRMS in a DeltaPlus (ThermoQuest) coupled to an elemental analyser NC2500 (CarloErba, Italy). The results of the C isotope ratio analyses were expressed in delta notation. According to Farquhar et al. (1982), the C isotope composition with respect to Vienna Pee Dee Belemnite (VPDB) is δ13C (‰) = [(Rs/RVPDB)−1] × 1000, where R is the 13C/12C ratio of the sample and standard, respectively. These results were used to obtain 13C (mg) transported to each tissue from the labeled source leaf. First, the calculations to obtain the % of 13C were performed according to Ariz et al. (2011) but using the RVPDB standard. Subtracting the %13C of the tissues from labeled plants from that of the unlabeled plants and multiplying the %C and biomass of each tissue, the transported 13C (mg) was finally obtained as previously described (Parvin et al. 2020).
2.5 Metabolite Extraction and Determination
For the determination of soluble carbohydrates, 0.1 g aliquots of fresh tissue (including sink leaves, source leaves, stems, roots, and nodules) were extracted, as previously described (Marino et al. 2006). Carbohydrates were determined by ion-exchange chromatography in a 940 Professional IC Vario with an amperometric detector (Metrohm AG, Switzerland) equipped with a Metrosep Carb 2 Guard/4.0 + Metrosep Carb 2−150/4.0 columns. The samples were eluted with 300 mM NaOH/1 mM C2H3NaO2 at 30°C.
To determine the organic acid content, 0.1 g aliquots of fresh tissue (including sink leaves, source leaves, stems, roots, and nodules) were extracted, as described by Gálvez et al. (2005). Anions and organic acids were determined by ion-exchange chromatography in a 940 Professional IC Vario with a conductivity detector (Metrohm AG) by gradient separation with a Metrosep A Supp16 150/4.0 column. Samples were eluted with solvent A (deionized water) and solvent B (20 mM Na2CO3 + 2.5 mM NaOH) at 55°C according to the manufacturer's instructions.
Lastly, the same extract used for the measurement of soluble carbohydrates was used to determine N compounds. High-performance capillary electrophoresis (P/ACE 5500; Beckman Coulter Instruments) was used to measure the content of ureides and asparagine according to the method described by Sato et al. (1998). A solution of 0.1 M Na2B4O7 · 10H2O (pH 9.2) in 25 mL L−1 OFM-Anion BT (Waters Corp.) was used as the electrolyte in a fused-silica capillary tube 60 cm in length. The levels of ureides and asparagine were detected at an optical density of 190 nm.
2.6 Statistical Analysis
Data are presented as the mean ± SE of ≥ 3 biological replicates. Significance of the physiological parameters was analyzed by one-way ANOVA. The Shapiro–Wilk test and Levene's test were used to verify the sample's normal distribution and homogeneity of variances, respectively. In the case of no normality or homogeneity, a log transformation was applied.
Comparisons between each treatment and time point were performed using the least significant difference (LSD) or Dunnett's T3 test at p ≤ 0.05. Metabolite analysis and δ13C, total 13C, and 13C distributions were analyzed by two-way ANOVA. Comparisons between each treatment and time were performed using the LSD or Dunnett's T3 test. Differences were considered significant at p ≤ 0.05. The Perseus software (version 1.6.5.0; Tyanova et al. 2016) was used to represent the heatmap and cluster aggregation. Metabolite levels underwent a base-2 logarithmic transformation, followed by standardization using Z-scores. R was used to perform principal component analysis (PCA) using the prcomp() function on the dataset of metabolite content and 13C values for each tissue and drought level.
3 Results
3.1 Water Deficit Effects on the Physiological Status of Soybean Plants
Plants were subjected to water deprivation for 5–7 days, and leaf water potential was monitored to establish two levels of drought stress: mild drought (Ψleaf = −0.44 ± 0.03 MPa) and severe drought (Ψleaf = −0.85 ± 0.08 MPa). Well-watered plants were taken as the control group (Ψleaf of −0.24 ± 0.05 MPa; Table 1). During the experiment, stomatal conductance remained relatively constant in control plants (0.31 ± 0.01 mol m−2 s−1), while drought stress provoked an 81% and 93.2% decline under mild and severe water deficit, respectively (Table 1). Net photosynthetic rates declined by 59% under mild drought stress conditions, whereas under more severe conditions, a 91.5% decline was observed (Table 1). The effect of water withholding on nitrogen fixation was measured by hydrogen evolution (ANA) in intact soybean plants. Under mild water stress, ANA was more negatively affected than photosynthesis or Ψleaf, declining by approximately 71.4% compared with control plants. Under severe water deficit conditions, almost complete inhibition of nitrogen fixation was observed, with an 88.8% decline (Table 1). These results are in line with those of previous studies in which nitrogen fixation was more negatively affected by mild drought stress than other physiological parameters.
| Parameter (units) | Control | Drought | |
|---|---|---|---|
| Mild | Severe | ||
| Leaf water potential (MPa) | −0.24 ± 0.05a | −0.44 ± 0.03b | −0.85 ± 0.08c |
| Stomatal conductance (mol m−2 s−1) | 0.31 ± 0.01a | 0.06 ± 0.02b | 0.02 ± 0.00b |
| Photosynthesis (μmol CO2 m−2 s−1) | 7.91 ± 0.29a | 3.24 ± 0.54b | 0.67 ± 0.20c |
| ANA (μmol H2 g−1 NDW min−1) | 1.16 ± 0.02a | 0.33 ± 0.04b | 0.13 ± 0.02c |
- Note: Values represent mean ± SE (n biological replicates ≥ 4). Different letters within a row indicate significant differences from control plants (one-way ANOVA with posthoc LSD/T3 Dunnet test; p ≤ 0.05).
- Abbreviation: NDW, nodule dry weight.
3.2 Drought Stress Reduces Natural C Isotopic Composition in Source and Sink Leaves
In order to set a baseline for natural variation of C isotopic composition in soybean leaves, we analyzed (1) whether sampling at various time points during the day had an effect on δ13C values, and (2) whether plants subjected to different water regimes presented significant variations in δ13C values. Regarding the first variable, time-dependent variations in δ13C values were observed only under control conditions, leading to more negative values in the source leaves and roots at the end of the experiment (Figure 1A,D). No time effects were observed for the drought stress treatments (Figure 1). Regarding the effect of water status, in control plants, source leaves had more negative δ13C values than sink leaves, stems, nodules, or root samples, indicating 13C enrichment in non-photosynthetic tissues (Figure 1). Water withholding affects the C isotopic composition, resulting in less negative δ13C values in leaves and roots (Figure 1). In the source leaves, there was no significant variation in δ13C during the 6 h of the experiment under drought stress. In contrast, in control plants, δ13C changed towards more negative values, indicating a 13C loss in this tissue (Figure 1A). The effect of water stress on δ13C was more significant in sink leaves than in the source leaves. Moreover, the δ13C of sink leaves under severe stress became less negative over time, unlike the observed trend in severely stressed source leaves (Figure 1A,B). In the root samples, δ13C of drought-stressed plants remained constant over the course of the experiment, whereas δ13C of control roots declined over time (Figure 1D). In contrast, there was no significant effect of drought on the δ13C in stems and nodules (Figure 1C,E).
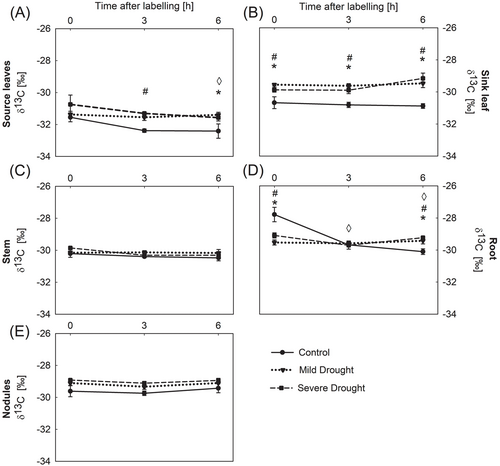
3.3 Water Deficit Changes C Allocation Among Source and Sink Tissues
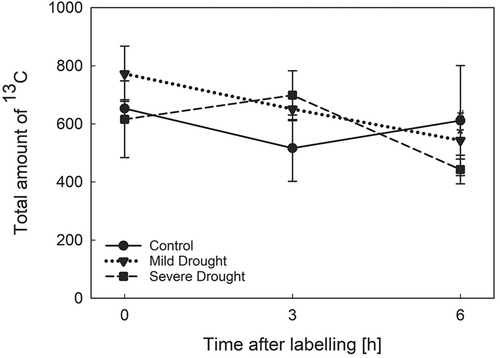
Drought stress is a condition that has an impact on plant metabolism and physiology at several levels. To employ labeled sucrose as a proxy to estimate C allocation dynamics, it was necessary to test whether the total amount of 13C did not present variations due to water deficit or time of sampling. To do so, we measured the total amount of 13C, calculated as the sum of the 13C values in each tissue (Figure 2; Table S1). Neither water deficit nor time produced significant changes in the total amount of 13C in the plants during the experiment.
We then calculated the rate of transport of labeled sucrose applied to the source leaf across the plant. Figure 3 shows the percentage of 13C fixed in each tissue as a proportion of the total amount of 13C found in the soybean plants at each time point. In control plants, approximately 16.2% of 13C was transported to other tissues at 3 h post labeling, while 22.4% was distributed at 6 h (Figure 3A). During the first 3 h, the major fate of 13C transported was the roots (5.5%), followed by the sink leaves (5.4%); the third and fourth main locations were the nodules (3.9%) and stems (1.9%; Figure 3A). After 6 h of labeling, the strongest sink for 13C was found to be the sink leaves (15%), while the 13C transported to the remaining tissues was relatively lower compared to the previous time point (Figure 3A).
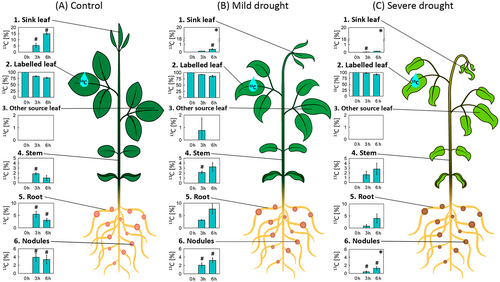
However, in plants subjected to mild water deficit, there was a reduction in the amount of 13C transported from the labeled leaves. At time 3 h, only 8.3% of the total 13C was transported, which increased to 14.5% after 6 h of labeling (Figure 3B2). At 3 h post labeling, the allocation of 13C was concentrated in the root (3.2%), followed by the stems (2.1%) and nodules (2.1%; Figure 3B). Transport to sink leaves was significantly reduced compared with the control conditions (only 0.1% of the transported 13C). The same trend was observed 6 h after labeling. Thus, the largest decline in transported 13C was observed in sink leaves, dropping from 5.4% to 0.1% of allocated C at 3 h and from 15% to 0.4% at 6 h after labeling (Figure 3A1,B1). In both roots and nodules, the amount of 13C transported under mild drought was slightly delayed but not significantly changed during stress (Figure 3A,B).
In plants under severe drought stress, the export of 13C was further limited and only 2.9% of the C was exported at 3 h, reaching a maximum of 8.2% of 13C transported at 6 h after labeling (Figure 3C2). As in mild stress, the amount of 13C transported to sink leaves was close to zero, while the sink strength in other tissues was maintained (Figure 3C). However, in nodules, there was a significant reduction in the amount of 13C allocated at 6 h post-labeling compared to that in control plants (Figure 3A6,C6).
Taken together, these results show that under drought stress, roots and, to a lesser extent, nodules maintain their relative sink capacity, whereas there is a reduction in the amount of C allocated to young, growing leaves.
3.4 The Concentration and Distribution of Plant Metabolites Were Affected by Both Drought Conditions and Type of Tissue
To obtain an overview of the main metabolic changes occurring in soybeans subjected to a progressive water deficit, we analyzed the levels of major C and N metabolic compounds in different plant tissues during the sampling period. Due to the complexity of the dataset, we ran a PCA to identify the variables most strongly associated with the drought treatment and different tissues (Figure 4A). PCA differentiated the data into four groups: source leaves, sink leaves, and nodules clustered largely independently, whereas stem and root samples were grouped together. PCA showed that drought treatment predominantly influenced the distribution of metabolites and 13C allocation in different tissues, with sampling time having a lesser impact. The first PCA component accounted for 29.2% of the variance and was responsible for the differentiation between the sink leaves and root/stem groups. PCA1 was influenced most by starch and raffinose, strongly correlated with the stem/root group and polyols and organic acids on the side of the sink leaves. PCA2, which accounted for 20.8% of the variance, separated the source leaves from nodules. In this separation, the distribution of hexoses during drought was correlated with the source leaves, whereas trehalose, ureides, and 13C allocation were associated with nodule samples.
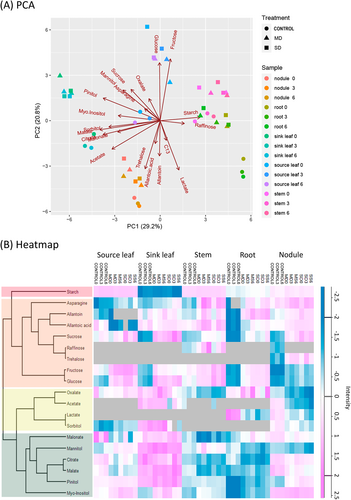
To visualize and establish a hierarchy of the main metabolic changes occurring across the plant under different drought conditions, we plotted the normalized (log2) levels of the metabolites in a heatmap (Figure 4B). The levels of starch showed a unique profile (red cluster), showing relatively high but stable levels (not affected by water deficit) in source leaves, stems, and, to a lesser extent, nodules, while the levels in roots and particularly sink leaves were relatively lower. The second cluster (orange) included mono- and disaccharides such as sucrose, glucose, and fructose, as well as the main N compounds exported in soybean, allantoin, and allantoic acid. Drought caused a progressive accumulation of these compounds in practically all the tissues tested, with the exception of allantoic acid in the source leaves, whose levels were reduced under water deficit. Interestingly, raffinose was detected mostly in the stems, whereas trehalose was found only in the nodule tissue. Metabolites in the yellow cluster included organic acids, such as oxalate, acetate, and lactate, all of which showed a drought-induced decrease, particularly in nodules. Lastly, the green cluster grouped metabolites whose distribution depended to a greater extent on the plant tissue tested, such as organic acids and polyols. This representation also showed that the effect of drought stress was greater than the effect of harvesting time on the distribution of the detected metabolites. After this outline, to expand the analysis, the most relevant changes for each tissue are summarized below. The values corresponding to each metabolite are provided as Tables S2–S6.
Drought caused a general increase in carbohydrates detected in the source leaves, with a higher accumulation in hexose content (Table S2). In the case of sucrose, its content remained stable under mild drought but increased under more severe stress. Regarding nitrogen compounds, drought caused a decrease in ureide concentrations, and allantoin was not detected under severe drought (Table S2). Instead, asparagine concentration increased with time and drought stress, reaching values of 8.4 ± 2.0 and 13.0 ± 3.2 μmol g−1 DW at 6 h under mild and severe stress, respectively. The organic acid content decreased, with malonate being the most negatively affected, with a decrease of approximately 85% and 70% under mild and severe stress at time 0 h, respectively (Table S2). Under control conditions, the malate content increased during the experiment with 33.31 ± 3.4 μmol g−1 DW at 6 h, but its concentration decreased due to drought. Regarding polyols, water deficit increased the myo-inositol content (Table S2).
In sink leaves, both levels of drought and time also caused an increase in carbohydrates, except for starch, whose concentration was almost negligible (Table S3). Under mild drought conditions, glucose and sucrose levels decreased during the experiment, while severe drought changed the trend towards the highest values (Table S3). The ureide content varied with harvesting time and drought level. In contrast, asparagine concentration increased as drought stress increased (Table S3). Regarding organic acids, unlike the trend observed in source leaves, drought increased malate content in sink leaves, presenting values 4.6- and 7.5-fold higher than in source leaves under mild and severe stress at 6 h, respectively. Moreover, the highest pinitol content was found in sink leaves. Drought induced the accumulation of polyols, except sorbitol, the content of which decreased under severe stress. The greatest increase was observed in the myo-inositol concentrations under both drought conditions (Table S3).
In the stem, hexose concentration increased under severe drought conditions (Table S4). Raffinose, which was only found in stems and nodules in this study (Tables S4 and S6), and sucrose accumulated due to drought, and this stress caused their accumulation during the experiment. This trend was also observed for ureide content (Table S4). Asparagine reached values more than 6-fold under both drought conditions, depending on time (Table S4). Within the organic acids group, there was a general decrease in their content under mild or severe stress (Table S4). Regarding polyols, only the myo-inositol content increased under mild or severe stress (Table S4).
In the roots, there was a general increase in the concentration of carbohydrates due to drought, with hexoses showing the largest relative increase (Table S5). Only the starch content remained stable. The concentration of N compounds was almost negligible in the control roots, but there was a large increase under drought (Table S5). A different trend was observed in organic acids, where water deficit increased the content of malonate and oxalate, and the contents of citrate and lactate were reduced (Table S5). Drought increased the concentrations of myo-inositol and pinitol (Table S5).
In general, drought increased the concentration of all detected carbohydrates in nodules, except for raffinose, which was only detected under control conditions (Table S6). The concentration of ureides increased as drought increased, although a decrease in allantoin content was observed under severe stress conditions at 3 and 6 h (Table S6). The asparagine content was higher than that in the other tissues and increased due to the water deficit (Table S6). Regarding organic acids, drought stress caused a general decrease, with the effect on malate content being more significant, which decreased around 50% under both mild and severe stress (Table S6). As for polyols, nodules had the highest content of myo-inositol, although drought did not have any effect on their concentration. In contrast, there was an increase in pinitol content, while sorbitol content decreased (Table S6).
4 Discussion
4.1 Soybean Reduces C Allocation to Sink Leaves, Prioritizing Roots and Nodules Under Water Deficit Conditions
Progressive drought stress imposed on nodulated soybean plants caused changes in the long-distance transport of C. In well-watered plants, sink leaves were the dominant sink tissue, receiving 15% of the total translocated 13C (Figure 3A). In contrast, under drought stress, translocation towards this tissue was almost completely inhibited (Figure 3B,C), while 13C allocation to stems and roots was maintained (Figure 3C). It is well known that during a water deficit period, plants arrest their growth (Schubert et al. 1995; Lemoine et al. 2013), allocating C resources to the root system to ultimately improve the plant capacity to acquire water (Geiger et al. 1996; Palta and Gregory 1997; Arndt and Wanek 2002). This C allocation trend was also observed in the metabolic profiling of roots and nodules, in which carbohydrate accumulation was detected (Figure 4B; Table S5). Similar results were obtained by Purcell et al. (1997), who reported a higher tolerance to drought associated with continued allocation of photosynthates to nodules. However, under severe drought, 13C translocation to the nodules decreased (Figure 3C). Similarly, in faba bean, the sink activity of nodules was higher than that of other tissues, although this did not lead to increased 13C incorporation under drought conditions (Parvin et al. 2020).
4.2 Drought-Stressed Soybean Nodules Show a Progressive Accumulation of Sucrose While Malate Levels Are Reduced
Metabolite analysis showed increased levels of carbohydrates (sucrose, fructose, and glucose) in the nodules and sink leaves under water deficit conditions (Figure 4B). In the case of nodules, based on 13C measurements, the transport of sucrose was maintained despite the water deficit, although to a lesser extent than in the control plants. This observation, together with the fact that malate, the final C product used for bacteroid respiration, accumulates during drought stress in nodules, supports the hypothesis that the regulation of nitrogen fixation by C is associated with a decline in sucrose synthase activity (González et al. 1995; Gálvez et al. 2005). Indeed, the 13C content was found to be inversely correlated with hexose and sucrose contents in the PCA analysis (Figure 4A), whereas it was positively correlated with the levels of ureides and lactate, which are relevant metabolites in nodules.
In legumes, the accumulation of N compounds has been suggested to play a role in the feedback inhibition of nitrogen fixation, including that of ureides (Atkins et al. 1992; Serraj et al. 1999; Vadez et al. 2000), and asparagine (Bacanamwo and Harper 1997). In this study, drought caused the accumulation of ureides and asparagine in both roots and nodules (Figure 4B; Tables S5 and S6). Instead, in the source leaves, the concentration of ureides declined until they were undetectable under severe stress (Table S2). These results suggest that the observed inhibition of nitrogen fixation is likely not related to the accumulation of ureides in leaves, in line with previous studies (King and Purcell 2005; Ladrera et al. 2007). The role of asparagine in the inhibition of nitrogen fixation has also been questioned, as a general increase in single amino acid content observed in both nodules and leaves suggests more complex N signal regulation (Gil-Quintana, Larrainzar, Arrese-Igor, et al. 2013). Interestingly, in sink leaves, ureide content remained stable under severe stress (Figure 4B; Table S3). These results suggest that different urea catabolism pathways act in the various tissues analyzed rather than impairing long-distance transport from nodules to shoots. Gil-Quintana, Larrainzar, Seminario, et al. (2013) showed that urea catabolism is more negatively affected than de novo synthesis under water-deficit conditions. Thus, our results support the co-existence of both malate limitation and N compound accumulation regulatory mechanisms in soybean.
4.3 Drought Stress Alters the Levels of Organic Acids and Polyols in Different Tissues Allowing to Establish a Metabolic Fingerprint
Organic acids play a major role in providing redox equilibrium in plants (for a review, see Igamberdiev and Eprintsev 2016). In our study, drought stress, a type of oxidative stress, provoked differential variation in the levels of organic acids across plants. In contrast to the above-mentioned decline in malate in nodules, both in the sink and source leaves, malate was found to accumulate. Interestingly, malate accumulation in the cytosol or vacuoles of guard cells is related to the regulation of stomatal movement (Hedrich and Marten 1993). In contrast to the well-established role of malate in nodules, malonate is described as a poor C source for bacteroids (Karunakaran et al. 2013), and its function in this symbiotic organ remains unknown (Booth et al. 2021). Li and Copeland (2000) suggested that its metabolism is not related to abiotic stress, but rather a consequential effect related to malate metabolism. The variation in the contents of these two organic acids observed in the current study seems to agree with this suggestion (Figure 4B; Table S6). Trinchant and Rigaud (1996) suggested that oxalate oxidation is important in nodules, preventing the production of ROS near bacteroids, which could explain the decline in oxalate content in drought-stressed nodules (Table S6).
Several studies have highlighted the role of polyols in osmotic regulation under drought conditions (Noiraud et al. 2001; Streeter et al. 2001; Dumschott et al. 2019). Although sorbitol synthesis has also been described in nodules, polyol synthesis occurs mainly in source leaves (Colebatch et al. 2004). Thus, its occurrence in sink tissues indicates its likely translocation (Noiraud et al. 2001; Streeter et al. 2001). In the current study, we observed that drought altered polyol levels differently, depending on the tissue analyzed. For instance, the concentrations of mannitol and sorbitol in sink leaves showed an effect of drought prior to this in source leaves, indicating both lower assimilation in sink leaves and transport impairment under severe stress (Figure 4B; Tables S2 and S3). The PCA results also highlighted the association of polyol levels as part of the drought response of sink leaves. The biosynthesis of pinitol from glucose-6-phosphate includes myo-inositol biosynthesis (Dumschott et al. 2019). In the source leaves and roots, there was an accumulation of myo-inositol, but no effect on pinitol, whereas in nodules, there was no effect on myo-inositol, although the pinitol content increased. This suggests that pinitol synthesis is regulated during drought, or that its translocation to sink tissues increases.
5 Conclusions
In conclusion, the results presented here highlight the complexity of source-sink relationships and metabolic changes in drought-stressed soybean plants. The combination of leaf [U-13C]-sucrose labeling and analysis of natural 13C abundance allowed the monitoring of C allocation throughout the plant under water deficit conditions. In well-watered plants, C allocation is directed mainly toward shoot growth. However, under water deficit stress, C allocation to the roots was prioritized, likely to promote root growth. Metabolite analysis and C transport data showed that under drought stress, nodules are not limited to photoassimilates, supporting the hypothesis of a limitation of respiratory substrates for bacteroids in the form of malate. Finally, our results support the hypothesis that the drought-induced inhibition of nitrogen fixation in soybean is not related to the accumulation of ureides in leaves.
Taken together, these results suggest that increasing root and nodule sink strength through the modulation of key enzymes in C and N metabolism is a promising strategy to improve plant performance under drought stress (Kunert et al. 2016). This has been elegantly demonstrated by Thu and Tegeder (2025) using soybean plants overexpressing a nodule-specific ureide permease (UPS1; Collier and Tegeder 2012; Carter and Tegeder 2016). These plants showed enhanced N allocation to leaves, maintained higher photosynthetic rates, and thus increased plant biomass. This improved growth allowed greater nodule C-sink strength and nitrogen fixation rates, not only under high water availability but also under water-deficit conditions. Future work will require both the identification of key candidates for enhanced root and nodule sink strength strategies and extending the trials to experiments under field conditions.
Author Contributions
M.I.R. designed, performed the experiments, and analyzed the data. E.L. and C.A.-I. contributed to the original idea, collaborated on the experimental design, data representation, and interpretation, and supervised the project. All the authors contributed to the discussion and writing of the manuscript.
Acknowledgments
We would also like to thank Gustavo Garijo and Regina Galarza for technical assistance and Inma Molina for her help in the design of Figure 3. Bradyrhizobium diazoefficiens UPM792 was kindly donated by Professor Tomás Ruiz Argüeso (Polytechnic University of Madrid).
Open Research
Data Availability Statement
The data supporting these findings are available in the Supporting Information of this article.




