EjLAC12 is involved in postharvest chilling injury in loquat fruits by regulating lignin polymerization
Abstract
Loquat fruits are susceptible to chilling injury at low temperatures following harvest, and heat shock treatment (HT) effectively mitigates chilling-induced lignification in these fruits. Laccase (LAC) is involved in lignin deposition. In this study, 43 laccase genes were identified based on the loquat genome, and the expression patterns of laccases were analyzed during fruit development and postharvest storage. The expression pattern analysis and subcellular localization revealed that EjLAC12 is involved in the regulation of lignin biosynthesis. Furthermore, the overexpression of EjLAC12 in tomato plants resulted in increased lignin and flavonoid contents. Overall, EjLAC12 promotes the accumulation of lignin during low-temperature storage, whereas HT treatment can alleviate its transcription, thereby mitigating postharvest chilling-induced lignification in loquat fruits.
1 INTRODUCTION
Low-temperature storage can extend the storage period of fleshy fruits and decrease deterioration, such as fruit decay, which is currently the main way to slow senescence and maintain fruit quality. However, some fruits, including peach, mango, banana, kiwifruit, and loquat, are sensitive to chilling, leading to quality deterioration due to chilling injuries (Liu et al., 2023; Zhang et al., 2023; Ma et al., 2022; Zheng et al., 2022). In loquat fruits, lignification is induced by low temperatures, leading to increased fruit firmness, increased lignin content, weakened flavor, roughness and decreased juiciness, which strongly affects the quality of the fruit (Zhang et al., 2023; Liu et al., 2019). Previous studies have indicated that heat shock (HT) treatment can effectively retard chilling-induced lignification in postharvest red-fleshed loquat fruits (Xu et al., 2019). However, the precise regulatory mechanism involved remains elusive. Lignin is a phenylpropanoid-derived polymer. Previous studies on lignin biosynthesis have focused mainly on the vegetative tissues of plants such as Arabidopsis thaliana, poplar, loblolly pine, and switchgrass (Han et al., 2022; Dixon et al., 2019). The upstream process of lignin biosynthesis predominantly involves the synthesis of lignin monomers, which include a series of enzymes, including phenylalanine ammonia lyase (PAL), cinnamate 4-hydroxylase (C4H), 4-coumarate CoA ligase (4CL), cinnamyl alcohol dehydrogenase (CAD), and several others. Lignin monomers are subsequently synthesized and transported to the apoplast, where they are catalyzed by monomer polymerization genes, namely, peroxidase (PRX) and laccase (LAC), to form the macromolecular lignin. However, research on these monomer polymerization genes (PRX and LAC) remains relatively scarce (Vanholme et al., 2019; Zhao et al., 2016). For an extended period, the enzyme involved in lignin monomer polymerization was believed to be PRX. It was not until Sterjiade et al. (1992) isolated the LAC gene from the higher plant Acer truncatum that its biological function in catalyzing lignin monomer polymerization in vitro was demonstrated. LACs belong to the copper protein family and are abundant in bacteria, plants, and insects. These laccases can be categorized into plant laccases and fungal laccases on the basis of their source. Currently, numerous studies have focused on fungal laccases, which are extensively employed in papermaking, industrial pollutant detoxification, and other applications (Abdi Dezfouli et al., 2024). Moreover, plant laccases are reported to facilitate the oxidation of phenolic substances, the conversion of phenolic acids into less toxic oligomers, and the polymerization of lignin monomers (Braunschmid et al., 2018). Recently, the roles of plant laccase genes have been characterized and studied in a variety of species, including Arabidopsis (Yonekura Sakakibara et al., 2021), citrus (Jin et al., 2016), rice (Liu et al., 2017), sorghum (Wang et al., 2017), pear (Xue et al., 2019), tea plants (Camellia sinensis (L.) O. Kuntze; Zhu et al., 2023), and poplar (Liao et al., 2023). Subsequent studies have shown that certain LAC genes play crucial roles in lignin biosynthesis. Wang et al. (2015) reported that the mutation of BdLAC5 in Brachypodium distachyon led to a decrease in the lignin content in the stem and an increase in saccharification efficiency. Wang et al. (2020) identified the laccase-encoding gene ChLAC8 in Cleome hassleriana, which specifically catalyzes the polymerization of C-type lignin. Zhuang et al. (2020) demonstrated that Arabidopsis AtLAC3 is involved in lignin biosynthesis in the Casparian strip.
In this study, the loquat LAC gene family was systematically identified on the basis of the genome. Transcriptomic and metabolomic analyses, along with subcellular localization studies, suggested the role of EjLAC12 in lignin deposition. Furthermore, lignin content assays and broad-target metabolome analyses were conducted to analyze the biological functions of EjLAC12-OE tomatoes in lignin metabolism, and the results validated the potential role of EjLAC12-OE tomatoes in flavonoid metabolism.
2 MATERIALS AND METHODS
2.1 Identification of the LAC gene family
The loquat genome sequence (Genome Warehouse (https://ngdc.cncb.ac.cn/gwh/) under accession number GWHBOTF00000000) was downloaded, the gene sequences of the genome were extracted via TBTOOLs, and the mRNA sequences were converted into protein sequences. The Simple HMM search plugin was used to identify the members of the laccase (PF07731) and set the sequence score > 1e-05. The obtained sequences were reconfirmed via the National Center for Biotechnology Information CDD database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) to identify the conserved domain as the laccase superfamily.
2.2 Gene expression analysis
The raw transcriptome data of loquat fruits stored at 0°C and subjected to HT treatment after harvest were downloaded from the NCBI SRA database (SRP128075), Raw RNA-Seq data were first used to remove adapters and low-quality sequences using Trimmomatic, followed by de novo assembly of contigs using Trinity, removal of redundancy in Trinity-assembled contigs using iAssembler, and finally the normalization of raw counts of contigs to RPKM using Bowtie. The method for transcriptome analysis was described by Liu et al. (2019). The expression of LACs and known lignin synthesis genes in Supplementary Figure S1 was plotted via Origin 8.
The Heatmap plugin of TBTOLs was used to display the expression patterns of laccases in loquat fruits of different varieties and postharvest treatments, and the corresponding transcriptome data of different varieties were downloaded from the supplementary table in the study of Jing et al. (2023); Supplementary Table S1. The specific parameters used were as follows: log scale, column scale, cluster rows, and original value.
2.3 Subcellular localization
The full-length CDS of EjLAC12 was inserted into the pCAMBIA1300-35S-eGFP vector via a fusion connection. After the fusion vector was successfully constructed, it was transformed into competent Agrobacterium GV3101, and the empty vector was used as a control. Agrobacterium with EjLAC12-GFP or the empty GFP vector were spread on LB medium with Kan and Get, and a small amount of bacteria was clipped from the medium to a new LB medium after two days. On the third day, Agrobacterium was diluted to 0.75–1.0 at 600 nm using an infestation buffer (pH 5.6 100 mM phosphate buffer containing 10 mM MES, 10 mM MgCl2, 150 mM acetosyringone, 0.01% Silweet-77, 42 mM D-glucose). Agrobacterium was injected into the onion with a syringe and the area of infestation was labeled. After 24–48 h of dark incubation at 28°C, the inner epidermal cells of the infested area were torn off and immersed in 5 μg ml−1 FM4-64 solution for 20 min and rinsed once using 0.9% NaCl. A confocal scanning microscope system (A1+ CellManipulator Plus, Nikon) was used to excite GFP fluorescence at 488 nm laser and FM4-64 fluorescence at 546 nm laser. For plasmodesmata, changes in fluorescence were observed using 10% NaCl drops on onion inner epidermal cells. Prediction of the structure of the EjLAC12 protein was performed using DeepTMHMM (https://dtu.biolib.com/DeepTMHMM).
2.4 Phylogenetic analysis
The loquat laccase genes specifically expressed in fruit (FPKM>5), and the laccase genes of Arabidopsis thaliana, Zea mays, and some lignin-related laccase genes downloaded from the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/) were used for phylogenetic analysis. The protein sequences were imported into MEGA11, aligned in MUSCLE mode, and a neighbor-joining tree was constructed with default parameters.
2.5 Over-expression of EjLAC12 in tomato
The full-length sequence of EjLAC12 was inserted into the pCAMBIA-1301 vector via fusion. The Ailsa Craig (AC) tomato was used for genetic transformation by Wuxi Dimod Bio-Seed Technology Co. The seeds were sterilized and grown in MS solid media for two weeks, after which the cotyledons were subjected to genetic transformation. Transgenic lines were screened via PCR using extracted DNA as a template. The RNA of the transgenic and wild-type (WT) tomato plants was extracted via the Easy Plant (Polysaccharide and Polyphenols) RNA Extraction Kit (Easy-Do), and the transcript of EjLAC12 was performed by TransScript® II Green One-Step qRT-PCR SuperMix (Transgene). The primers used are listed in Supplementary Table S3.
2.6 Histochemical analysis of lignin and lignin quantification in tomato
The visualization of lignin content in tomato stem sections was performed via the hydrochloric acid-phloroglucinol staining method. Stem tissues of the same thickness (approximately 8 mm) near the base were collected for cryomicrotomy. Staining was performed by soaking in 1% pyrogallol or 12% hydrochloric acid solution, and the samples were observed under an optical microscope after 5 min. The lignin content of the EjLAC12-OE tomato stems was determined as described by Xu et al. (2019), and 0.5 g of fresh tomato stems were used for lignin quantification. Lignin content analysis of the EjLAC12-OE fruit was performed via a lignin content analysis kit (ultraviolet spectrophotometry, BC4200; Solarbio) according to the instructions, and 0.08 g of fruit tissue without seeds and juice was used.
2.7 Widely targeted metabolome analysis for EjLAC12-OE tomato
At least three leaves of 40-day-old seedlings of three different lines of EjLAC12-OE and WT tomato plants were collected, rapidly frozen in liquid nitrogen and transported to Biomarker Technologies Co., Ltd., for widely targeted metabolome sequencing. Metabolome profiling was performed via a Waters Acquity I-Class PLUS ultrahigh-performance liquid chromatography tandem AB Sciex Qtrap 6500+ high-sensitivity mass spectrometer. The metabolome data analysis was performed via the BMKCloud of Biomarker Technologies (www.biocloud.net).
2.8 Determination of flavonoid contents
Ground leaves (0.1 g) were added to 2 mL of anhydrous ethanol, shaken in a shaker at high speed for 1 h and centrifuged at 10 000 × g for 5 min. Then, 100 μL of the supernatant was transferred to a new 2 mL centrifuge tube. Thirty microlitres of 5% sodium nitrite was added, mixed thoroughly and incubated for 5 min. Then, 30 μL of 10% aluminum nitrate was added, mixed thoroughly and incubated for 5 min. A total of 400 μL of NaOH was added to the solution, which was subsequently diluted to 1 mL with methanol, mixed thoroughly, allowed to stand for 10 min, and the absorbance at 510 nm was determined via a microplate system (ReadMax 1200, Flash). The standard curve was generated via the use of rutin to convert the absorbance to the flavonoid content.
2.9 Phenoloxidase enzyme activity assay
Protein extraction from plants was referred to Wang et al. (2004) with slight modifications. Leaves (0.2 g) were ground in 100 mM pH = 7.0 PBS buffer and centrifuged at high speed to extract the supernatant as crude protein. The enzyme reaction mixture consisted of 200 μL of 100 mM pH = 7.0 PBS buffer containing 10 μL of H2O2, 5 μL of guaiacol and 20 μL of crude protein, and heat-inactivated crude protein was used as a negative control. The kinetic curve of enzyme activity at 480 nm was obtained via the kinetic mode of a microplate system (ReadMax 1200, Flash), and the reaction temperature was set to 37°C.
2.10 Statistical analysis
The statistical significance of differences was calculated via the independent-sample t-test in SPSS (version 27). The least significant difference (LSD) at the 5% level was calculated via DPS7.05 (Zhejiang University).
3 RESULTS
3.1 Identification of the loquat LAC gene family
On the basis of the loquat genome database (2n = 34), the loquat LAC gene family was identified via the Pfam database and reconfirmed via the NCBI CDD database. A total of 43 LAC gene family members were identified. These genes were distributed mainly on chromosome LG04, LG09, LG10, LG16, and LG17, and were named EjLAC1–EjLAC43 (Figure 1 & Supplementary Table S2). The collinearity analysis results revealed 29 pairs of laccase-related collinear regions, such as EjLAC12 (LG16), EjLAC13 (LG10), EjLAC1 (LG14) and EjLAC2 (LG06; Figure 1).
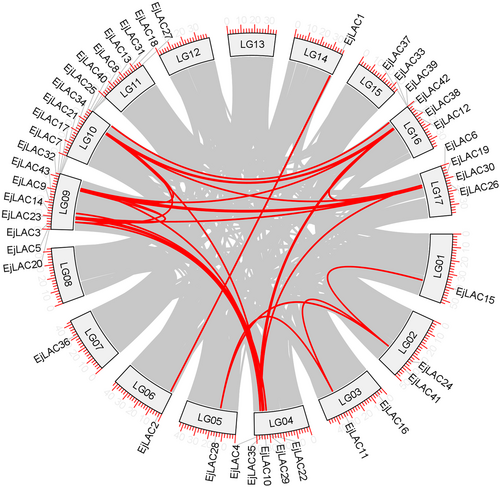
3.2 Expression patterns of EjLACs and subcellular localization of EjLAC12
To analyze the function of LACs in loquat fruits, gene expression analysis was performed via the transcriptome data of different loquat varieties (cultivated and wild) according to Jing et al. (2023) and ‘Luoyangqing’ loquat postharvest treatments according to Liu et al. (2019). To ensure the accuracy of the data, the genes with an FPKM expression level of less than 5 were filtered out.
As shown in Figure 2A, the gene expression of loquat LACs in different varieties during the development stage revealed that, with the exception of EjLAC6, EjLAC12, EjLAC17, and EjLAC25, the expression levels of most laccase genes were very low during fruit development. Overall, the laccase genes were divided into three expression patterns, namely, gene expression was significantly upregulated in the green fruit stage (GF), color turning stage (CT) or fruit ripening stage (FR). EjLAC6, EjLAC25, EjLAC32, EjLAC15, EjLAC26, and EjLAC28 presented relatively high expression levels in the early fruit development stage (GF) and almost no expression in the CT and GF stages, and the expression of these genes in the wild type was significantly greater than that in the cultivated variety, indicating that the function of these genes weakened during long-term domestication. The expression of EjLAC12, EjLAC17, and EjLAC21 was upregulated in the late fruit development stage FR, and the expression levels in cultivated varieties were significantly greater than those in wild varieties, indicating that the functions of these genes were strengthened during long-term domestication and selection. The expression of EjLAC19 was significantly upregulated in the FR stage of the cultivated variety, but almost no expression was detected in the wild type.
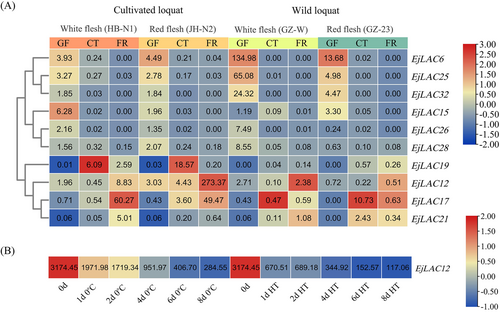
Furthermore, the expression levels and expression patterns of EjLACs during postharvest treatment were determined. Among the 43 EjLACs, four EjLACs were expressed in fruits (Supplementary Figure S1), and EjLAC12 was the most highly expressed laccase in loquat fruits during postharvest storage (Figure 2B). HT treatment effectively retarded the postharvest chilling-induced lignification of loquat fruits, and the abundance of EjLAC12 transcript significantly decreased after HT treatment, which was positively correlated with the change in lignin content reported by Liu et al. (2019).
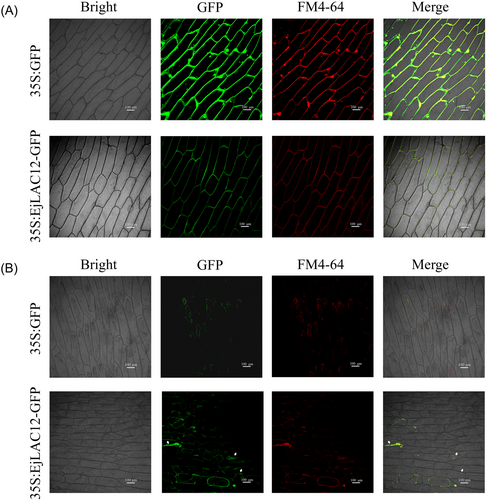
Phylogenetic tree analysis was performed by clustering the loquat fruit-expressed EjLACs with the Arabidopsis thaliana and Zea mays LAC genes. The results revealed that loquat EjLAC12 belongs to the third subfamily and is clustered together with Arabidopsis AtLAC5 and poplar TrLAC90 (Supplementary Figure S2). The subcellular localization of EjLAC12 was performed in onion epidermal cells, and FM4-64 was used as a membrane staining marker in red. The localization results revealed that EjLAC12 was located on the cell membrane, and the empty GFP protein was located on the cell membrane and nucleus (Figure 3A). After cell plasmolysis, the fluorescence of EjLAC12 was observed in the cell membrane, plasmolysis gap and cell wall (Figure 3B).
3.3 Phenotypic and broad targeted metabolomic analysis of EjLAC12-overexpressing tomato plants
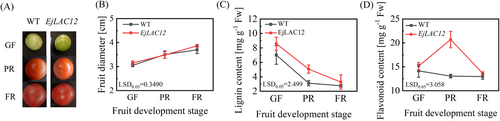
Flavonoids, lignin, and neolignin are the main secondary metabolites of plants and are derived mainly from the phenylalanine metabolic pathway. To explore the function of EjLAC12, we generated 13 EjLAC12-overexpressing (EjLAC12-OE) transgenic tomato plants in three separate lines. Compared with the wild-type lines, EjLAC12 was significantly overexpressed at the RNA level, and the expression levels of the separate lines are shown in Supplementary Figure S3. To dynamically analyze the effects of overexpressing EjLAC12 on the development of fruits, the plants of EjLAC12-OE 1 (#1) were chosen to investigate its changes on fruit size, flavonoids and lignin. Compared with those of WT fruits, the appearance and size of EjLAC12-OE fruits did not change noticeably during fruit development (Figure 4A and B). The lignin content in the pulp continued to decrease, but overall, the pericarp lignin content of EjLAC12-OE tomatoes was consistently higher than that of the WT, and the greatest difference was observed during the PR period (Figure 4C). Moreover, the flavonoid content of the EjLAC12-OE tomato fruits was higher than that of the WT fruits as a whole, particularly at the PR stage (Figure 4D).
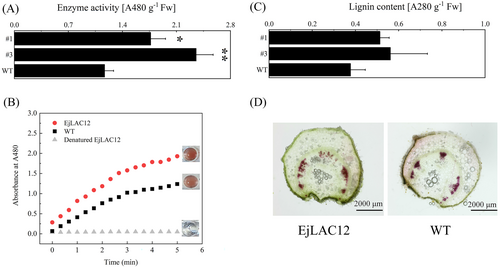
In addition to fruits, stems from two Lines were used for lignin content analysis and visualization to further enhance the reliability of the results. Guaiacol was used as a common substrate to determine the oxidase activities such as laccase and peroxidase and was used to analyze whether the laccase EjLAC12 is overexpressed at the protein level in EjLAC12-OE tomatoes. As shown in Figure 5A, the oxidase activity of EjLAC12-OE in the stems was higher than that of WT. The kinetic analysis of enzyme activity indicated that overexpressing EjLAC12 accelerated the rate of the oxidative polymerization reaction of a brownish-red polymer (Figure 5B). The analysis of lignin content in the stems revealed that overexpressing EjLAC12 increased the lignin content (Figure 5C), which is consistent with the results of lignin staining (Figure 5D). All these data indicated the function of EjLAC12 in monolignol polymerization.
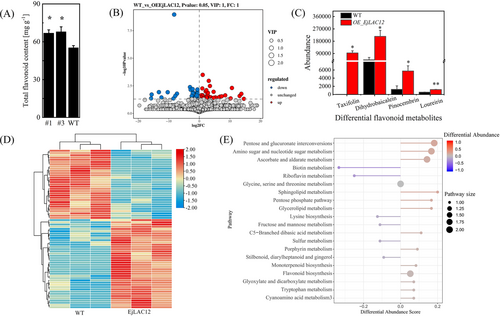
Flavonoids were enhanced in EjLAC12-OE tomato fruits. To clarify the different profiles of flavonoids, leaves were used for flavonoid content analysis and broad-targeted metabolome sequencing analysis, which is more abundant in metabolism than fruits. As shown in Figure 6A, the flavonoid content of EjLAC12-OE tomato leaves was much higher than that in fruits. Using the LC-QTRAP platform for broad-targeted metabolomic profiling of EjLAC12-OE and WT tomato leaves, a total of 3537 metabolites were detected (Supplementary Table S4). Using the multivariate OPLS-DA model, the differentially abundant metabolites between EjLAC12-OE and WT tomato leaves were preliminarily screened (VIP≥1, FC≥1, p ≥ 0.05), and 40 significantly upregulated and 31 significantly downregulated differentially abundant metabolites were detected (Supplementary Table S5, Figure 6B). Among these flavonoid metabolites, taxifolin, dihydrobioticein, pinocembrin and loureirin were the most significantly increased in EjLAC12-OE tomato leaves compared with WT leaves (Figure 6C). The differentially abundant metabolites were divided into different groups according to the metabolite content and are shown in Figure 6D. The clusterprofiler (Figure 6D) was used to perform enrichment analysis on the KEGG annotation of the differentially abundant metabolites via the hypergeometric test method. The results revealed that metabolites such as pentose and glucuronate interconversions, amino sugar and nucleotide sugar metabolism, and flavonoid biosynthesis were upregulated, and metabolites involved in pathways such as biotin biosynthesis and riboflavin metabolism were down-regulated (Figure 6E).
4 DISCUSSION
Low temperature is widely used for the transportation and storage of harvested fruits, significantly extending their shelf-life. However, many fruits, particularly chilling-sensitive ones such as loquats, are susceptible to chilling injury at low temperatures. HT treatment has been reported to alleviate chilling injury in postharvest fruits during low-temperature storage in loquat (Xu et al., 2019), citrus (Lafuente et al., 2017), papaya (Shadmani et al., 2015), etc. In loquat, previous studies have identified numerous lignin monomer synthesis genes that participated in fruit chilling injury and lignification, including PAL, 4CL, CCOAOMT, and CAD family members, for example, EjPAL1 (Li et al., 2017), Ej4CL1 (Li et al., 2017), EjCCOAOMT1 (Liu et al., 2015) and EjCAD3 (Xu et al., 2019), and the studies on their regulatory mechanisms have been conducted (Liu et al., 2015; Li et al., 2017; Xu et al., 2019; Zhang et al., 2020). EjAP2-1 and EjHAT1 were found to be involved in loquat fruit chilling-induced lignin synthesis as repressors (Xu et al., 2019; Zeng et al., 2015), and EjMYB8 and EjERF39 were found to act as activators to regulate loquat fruit chilling-induced lignin synthesis (Zhang et al., 2020).
Previous studies have focused primarily on upstream monomer synthesis genes in loquat, with fewer investigations on monomer polymerization genes. Zhang et al. (2022) reported that EjPRX12 is induced by low temperature and that overexpressing EjPRX12 in Arabidopsis could increase the lignin content. Here, we systematically identified LAC family members involved in monomer polymerization on the basis of the genome. Wild loquats, apples, and pears originated from the same ancestor and experienced two whole-genome duplication (WGD) events. The second WGD occurred approximately 17.6–43.2 MYA, followed by the evolutionary divergence of loquat, apple, and pear varieties and the later differentiation of cultivated and wild loquat varieties. In loquats, compared with the last common ancestor, 104 loquat gene families, including LAC, have undergone significant expansion to enhance environmental adaptability (Jing et al., 2023). During long-term domestication and selection, important genes have been further strengthened in cultivated loquat. The expression level of EjLAC12 in cultivated loquats is significantly higher than that in wild loquats (Figure 2A), suggesting that EjLAC12 may play a positive role in fruit quality, potentially by enhancing fruit resistance through lignification or improving fruit flavor via the regulation of flavonoid metabolism. The expression of EjLAC12 is significantly upregulated in the later stage of fruit development in both cultivated and wild loquats, which is consistent with the development pattern of lignin in loquat fruits (Chen et al., 2012).
The gene expression analysis in the transcriptome of postharvest 0°C and HT treatment revealed that repressors EjAP2-1 and EjHAT1 were up-regulated in their expression by HT treatment, and activators EjERF39 and EjMYB8 were down-regulated in their expression by HT treatment, thereby mitigating postharvest low-temperature-induced lignin synthesis in loquat fruits. The expression of genes EjPAL1, EjCCOAOMT1 and EjCAD3 in the lignin synthesis pathway was down-regulated by HT treatment to reduce lignin synthesis in the fruits. The expression patterns of the above known lignin synthesis-related genes were consistent with those reported in previous studies, indicating that the postharvest HT treatment of loquat fruits and its transcriptomic data are reliable. For laccases in loquat fruits, the differences of chilling-induced lignin accumulation occurred mainly in the first two days as reported by Liu et al. (2019), and the expression of EjLAC12 also generated the largest difference in the first two days. EjLAC12 had the highest expression in fruits, and other EjLACs were at lower expression levels. Overall, the expression of EjLAC12 was also positively correlated with changes in its lignin content during postharvest 0°C and HT treatments (Figure 2B). Additionally, EjLAC12 ranks among the top 10 genes, excluding heat shock proteins, with the highest importance score for DEGs in the transcriptome of loquat fruits under 0°C and HT treatment (Liu et al., 2019).
Phylogenetic tree analysis of the LACs revealed that EjLAC12 clustered together with Arabidopsis AtLAC5 and poplar TrLAC90 (Supplementary Figure S2). Ranocha et al. (1991) reported that TrLAC90 could catalyze the polymerization of lignin in vitro. Yonekura-Sakakibara et al. (2021) reported that AtLAC5 and AtDP1/AtDIR12 could catalyze the biosynthesis of guaiacyl-type lignin via the oxidation of coniferyl alcohol and lignan. After mutation of AtLAC5, the contents of the guaiacyl-type lignin polymer units and feruloylcholine (FC)-coupled lignans decreased. Zhuang et al. (2020) reported that other members of the third subfamily of Arabidopsis, AtLAC3, also participate in the lignification of the Casparian strips. Numerous studies have also shown that laccase catalyzes the polymerization of lignin monomers to produce large molecules of lignin mainly in secondary cell walls (Dixon et al., 2019; Yonekura-Sakakibara et al., 2021). Prediction of the structure of EjLAC12 using DeepTMHMM indicated that EjLAC12 was a secreted protein (Supplementary Figure S5). The results of subcellular localization using onions indicate that EjLAC12 localizes to the cell membrane, plasmolysis gap and cell wall (Figure 3).
Arabidopsis AtLAC4, 5, 10, 12 and 17 can catalyze the polymerization of G-type lignin monomers (Blaschek et al., 2023; Yonekura-Sakakibara et al., 2021; Zhao et al., 2013). ChLAC8 can oxidize the lignin polymerization of caffeyl alcohol (C-type) and sinapyl alcohol (S-type) but not G-type, and Gln289 is important for caffeyl alcohol binding in the active site of ChLAC8. EjLAC12 does not contain this Gln at the same site, implying that EjLAC12 cannot catalyze caffeyl alcohol. Our metabolomic results also showed that there was no difference in the content of caffeyl alcohol (POS_t100, in Supplement Table S4) in transgenic tomatoes overexpressing EjLAC12. Protein sequence and secondary structure analyses of EjLAC12 with the laccases that catalyze G-type or S-type lignin polymerization revealed that most of the protein domains of laccases with lignin-catalytic activity are very conserved. Since ChLAC8 cannot catalyze G-type lignin polymerization although it can catalyze S-type lignin polymerization, we compared it with the laccases preferred to catalyze G-type lignin polymerization and found some important site differences containing residues Arg98, Arg101, Trp136, Thr254, Tyr296, Thr311, Met545 and Leu562 (Supplementary Figure S4), which may be very important for catalyzing G-type lignin monomers.
Hydrochloric acid-phloroglucinol was available to visualize the lignin distribution of conifer aldehyde units (Blaschek et al., 2020). Quantitative analysis of lignin-stained sections by hydrochloric acid-phloroglucinol and lignin content assays in the fruits and stems of EjLAC12-OE transgenic tomato plants revealed that overexpressing EjLAC12 can accelerate conifer aldehyde units lignin polymerization (Figures 4, 5). The metabolomic analysis also revealed that the substrate (coniferyl alcohol) of the conifer aldehyde units lignin polymers decreased after the overexpression of EjLAC12 (Supplementary Table S4, POS_q538), suggesting that EjLAC12 played a positive role in lignin polymerization when coniferyl alcohol was used as a substrate. Collectively, these studies indicate that the role of EjLAC12 in lignin deposition is conserved with that of its orthologs in other species.
Metabolic regulation in plants is a complex network, and overexpression of EjLAC12 may affect the flow and direction of metabolic branches by influencing the nodes in the metabolic regulatory network. KEGG enrichment analysis revealed that the metabolites in the biotin biosynthesis and riboflavin metabolism pathways were down-regulated after overexpression of EjLAC12. KEGG enrichment analysis revealed that overexpression of EjLAC12 resulted in the down-regulation of metabolites in the biotin biosynthesis and riboflavin metabolism pathways, and up-regulation of metabolites in the pentose and glucuronate interconversions, amino sugar and nucleotide sugar metabolism, and flavonoid biosynthesis pathways (Figure 6). In addition to lignin polymerization, laccase, as a multicopper oxidase, can regulate a variety of physiological and biochemical metabolites, including lignin, flavonoids and salvianolic acid. Salvia miltiorrhiza SmLAC7 and SmLAC20 can regulate the biosynthesis of rosmarinic acid/salvianolic acid B and lignin biosynthesis in the phenolic acid metabolic pathway (Li et al., 2019; Xu et al., 2016). Hu et al. (2018) reported that the overexpression of GhLAC1 promoted the biosynthesis of lignin in cotton and that the inhibition of the expression of GhLAC1 promoted the synthesis of flavonoid secondary metabolites and jasmonic acid in cotton. Overexpression of EjLAC12 led to an increase in flavonoids suggesting that EjLAC12 may be involved in these biosynthetic pathways either directly or indirectly, and that flavonoids, such as taxifolin and pinocembrin, are commonly recognized to play a role in defense against biotic and abiotic stresses (Laoué et al., 2022; Zhang et al., 2024), suggesting that EjLAC12 may be able to mitigate chilling injury caused by postharvest low temperature in loquat fruits by regulating flavonoids following HT treatment (Laoué et al., 2024; Zhang et al., 2024). Interestingly, we also detected increased accumulation of salvianolic acid D (Supplementary Table S4, NEG_q1051) in EjLAC12-OE tomatoes, which implies the potential role of EjLAC12 in salvianolic acid oxidation, which needs future investigation.
5 CONCLUSIONS
Laccase, a multicopper oxidase, plays a pivotal role in regulating a multitude of physiological and biochemical processes. Loquat laccase family members were systematically identified on the basis of the loquat genome. Comparative analysis of different loquat species revealed that the expression of EjLAC12 in cultivated loquat was markedly higher than that in wild loquat, suggesting a significant role for EjLAC12 in long-term selection and domestication strategies aimed at improving fruit quality or resistance. Analysis of LAC expression during postharvest low-temperature storage and subcellular localization revealed that EjLAC12 may be involved in the regulation of fruit lignin synthesis. Further studies in the EjLAC12-OE tomato revealed that it not only catalyzes guaiacyl-type lignin polymerization but also affects the content of flavonoids. Consequently, as a multifunctional oxidase, loquat EjLAC12 is involved not only in the metabolic regulation of lignin synthesis during the developmental and postharvest stages but also in the metabolic regulation of fruit flavonoids.
AUTHOR CONTRIBUTIONS
X.M. designed the experiments and acquired the funding. C.S. and L.S. performed all experiments and wrote the original draft. D.S., L.Z. and C.X. analyzed the transcriptomic and metabolomic data.
ACKNOWLEDGEMENTS
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
FUNDING INFORMATION
This research was financially supported by the Natural Science Foundation of Shandong Province (ZR2021QC100), the National Natural Science Foundation of China (32302621), the Key Research and Development Program in Linyi (2023029), Linyi University Research Supporting Project (2021PTXM015).
Open Research
DATA AVAILABILITY STATEMENT
Data are contained within the article and Supplementary materials.




