Transcriptional Regulation Mechanisms in AsAFL1-mediated Drought Tolerance for Creeping Bentgrass (Agrostis stolonifera)
Abstract
Drought stress is a major environmental stress that impairs plant growth and development. The At14a-like1 (AFL1) gene encodes a stress-induced membrane protein involved in endocytosis, signal transduction, and proline accumulation. The objective of the present study was to investigate biological functions and underlying mechanisms of AFL1 regulation of drought tolerance in a perennial grass species, creeping bentgrass (Agrostis stolonifera). AsAFL1 was cloned from creeping bentgrass, and its expression was induced by drought stress. Motif analysis showed that AsAFL1 has five epidermal growth factor structural domains and one β1-integrin structural domain. Transient expression in tobacco epidermal cells indicated that AsAFL1 was localized at the plasma membrane. Overexpression of AsAFL1 in creeping bentgrass significantly enhanced drought tolerance, as manifested by significantly increased leaf relative water content, chlorophyll and proline contents but lower electrolyte leakage and malondialdehyde content. Comparative transcriptomic and weighted correlation network analysis (WGCNA) revealed that AsAFL1-mediated drought tolerance was related to transcriptional regulation of genes involved in phytohormone (abscisic acid, auxin, and strigolactone) biosynthesis and signaling, redox homeostasis, and biosynthesis of second metabolites (lignin, cutin, suberin and wax), as well as nutrient transport and mobilization.
1 INTRODUCTION
Drought stress is a major abiotic stress adversely affecting plant growth and development (Razi and Muneer 2021, Vadez et al. 2024). As sessile organisms, plants have evolved different adaptive mechanisms to tolerate drought stress, including signal perception and transduction, osmotic adjustment, synthesis of protective proteins, and activation of antioxidant systems (Haghpanah et al. 2024). Emerging evidence has suggested that, upon exposure to abiotic stresses, the communication among cell wall-plasma membrane-cytoskeleton in plant cells plays important roles in signal transduction and regulation of plant responses to environmental cues, including osmotic stress (Liu et al. 2015b, Liu et al. 2015a). Integrins are a large family of integral membrane proteins characterized as cell surface receptors and key signaling components in animal cells (Swatzell et al. 1999; Baluska et al. 2003; Soni and Bacete 2023). Till now, integrins have not yet been identified in plants but integrin-like proteins have been found in various plant species (Nick 2013). It has been proven that integrin-like proteins are the primary signal-perceiving molecules and are responsible for osmotic stress-induced abscisic acid (ABA) accumulation in plant cells (Lü et al., 2007). However, the function of integrin-like proteins in regulating plant drought tolerance remains largely unknown.
AT14a is one of the earliest identified integrin-like proteins in plants isolated by immuno-screening an Arabidopsis thaliana expression library with an anti-integrin antibody (Nagpal and Quatrano 1999). AT14a contains a small domain with high sequence similarities to integrins in animals and fungi. It localizes in the plasma membrane and functions in mediating cell wall-plasma membrane-cytoskeleton continuum in Arabidopsis cell (Lü et al. 2012). Further study found that Arabidopsis suspension cells overexpressing AT14a exhibited enhanced tolerance to PEG-induced osmotic stress damage through regulation of antioxidant enzyme activities (Wang et al. 2014). Recently, Xin et al. (2023) found that overexpression of Arabidopsis AT14A improved drought tolerance in tomato (Solanum lycopersicum), and the enhanced drought tolerance was attributed to enhanced proline content and antioxidant enzyme activities, which helped to maintain water retention capacity and free radical scavenging ability under drought stress.
A cluster of AT14A-related genes has been found in Arabidopsis, including At14a-Like1 (AFL1) (Kumar et al. 2015, Langhans et al. 2017). Protein structure analysis showed that Arabidopsis AFL1 contains a small domain with similarity to mammalian β1 integrin and is like At14a (Kumar et al. 2015, Langhans et al. 2017). The transcript level and protein abundance of AFL1 was strongly induced by low water potential stress and overexpression of AtAFL1 led to enhanced growth and increased proline accumulation in Arabidopsis under PEG-induced osmotic stress (Kumar et al. 2015). At the plasma membrane, AFL1 interacts with Adaptor protein2-2A (AP2-2A), colocalizes with clathrin light chain (CLC), and is associated with clathrin-coated vesicle formation during low water potential (Kumar et al. 2015). In addition, AFL1 is also involved in endocytosis and actin filament organization, which are crucial for plants to cope with turgor pressure changes under low water potential conditions (Kumar et al. 2019). Although physiological evidence supports the positive regulatory role of AFL1 in osmotic stress tolerance, the molecular mechanism underlying AFL1-mediated drought tolerance in plants is not yet well understood.
The objectives of this study were to characterize the function of AsAFL1 in regulating drought tolerance and to elucidate key molecular factors and related pathways underlying AsAFL1-enhanced drought tolerance in creeping bentgrass (Agrostis stolonifera), a cool-season perennial grass species widely used as turfgrass and forage grass. Understanding the roles and mechanisms of AsAFL1 regulating drought tolerance is particularly important for developing strategies to improve drought tolerance for drought-sensitive cool-season grass species, such as creeping bentgrass.
2 MATERIALS AND METHODS
2.1 Gene cloning, plasmid construction, and generation of transgenic plants
The coding sequence of AsAFL1 was amplified from the cDNA of creeping bentgrass (Agrostis stolonifera, cv. ‘Penn A4’), using the primer set AsAFL1 (Table S1) with restriction enzymes BamHI and NotI recognition sites and cloned into pMD19-T vector. AsAFL1 was excised from pMD19-T with BamHI and NotI digestion and ligated to pENTR™1A. Linearized pENTR™1A-AsAFL1 was recombined using Gateway™ LR Clonase™ II (Invitrogen) into destination vectors, such as pEarlygate103 for subcellular localization assay and pVT1629 (Xu et al. 2011) for genetic transformation of creeping bentgrass.
Agrobacterium-mediated genetic transformation was conducted following the protocol described by Luo et al. (2004). Embryogenic calluses induced from mature seeds of ‘Penn A4’ were infected with Agrobacterium tumefaciens EHA105 containing pVT1629-AsAFL1 plasmid. Plants generated from different calluses were considered independent transgenic lines, and hygromycin-resistant putative transgenic lines were confirmed by GUS staining, regular PCR amplification, and sequencing.
2.2 Subcellular localization and phylogenetic analysis
AsAFL1 was subcloned into pEarlyGate103 vector to generate AsAFL1-GFP fusion gene driven under the control of the cauliflower mosaic virus (CaMV) 35S promoter. The recombinant plasmid was transformed into Agrobacterium strain EHA105, which was injected into the lower epidermis of one-month-old tobacco (Nicotiana tabacum) plants, and Arabidopsis Calcineurin B-Like (AtCBL1) was used as the plasma membrane-located marker (Batistic et al. 2008). After 48 hours incubation, the GFP signal (excitation at 488 nm and scanning at 505–530 nm) was observed under a Zeiss LSM 780 laser scanning microscope.
Full-length amino acid sequences of AFL1 in Hordeum vulgare, Triticum aestivum, Aegilops tauschii, Zea mays, Brachypodium distachyon, Oryza sativa, Sorghum bicolor, Setaria viridis, Panicum miliaceum, and Leersia perrieri were obtained by BLAST search in UniProt database (https://www.uniprot.org/) and aligned using BioEdit sequence alignment editor (Hall 1999). The phylogenetic tree was constructed by using the Neighbor-joining method in MEGA11.0 (Kumar et al. 2018). The conserved structural domains of the gene were analyzed using the SMART database (http://smart.embl-heidelberg.de/).
2.3 Plant materials, growth conditions, and stress treatments
Seeds of creeping bentgrass (cv. ‘PennA4’) were germinated in petri dishes for 14 days and uniform seedlings were transplanted and cultivated hydroponically with 1/2 Hoagland's nutrient solution (Hoagland and Arnon 1950) in a controlled-environment growth chamber (MT8070iE, Xubang). The chamber conditions were maintained at 25/15°C (day/night), 70% relative humidity, and 14/10-hour photoperiod with photosynthetically active radiation of 650 μmol m−2 s−1.
To detect AsAFL1 expression profiles in different organs, fresh samples of leaf blades, leaf sheaths, stems, and roots of one-month-old creeping bentgrass plants were collected and frozen in liquid nitrogen immediately. For detection of AsAFL1 expression profiles under different abiotic stresses, one-month-old plants were exposed to PEG6000-induced drought stress (15% polyethylene glycol 6000 solution, w/v), salt stress (150 mM NaCl), heat stress (38°C), and cold stress (4°C) for 48 hours (Zhang et al., 2023). Leaf and root samples were collected at 0, 3, 12, 24, and 48 hours under each treatment and stored at −80°C for further analysis.
For comparison of drought tolerance between wild type (WT) and AsAFL1-overexpressing transgenic lines (OE3 and OE7), stolons of these plants were cut and planted in uncovered plastic turnover box (43 cm in length, 295 cm in width, and 13 cm in height) filled with a mixture of soil and peat (3:1; v/v). Plants were watered every 2 days and fertilized twice a week by using Miracle-Gro fertilizer (The Scotts Company LLC). After one month of establishment in the growth chamber, WT and AsAFL1-OE plants were exposed to either control (plants were irrigated every 2 days, soil relative water content was kept at approximately 90%) or drought stress (withholding irrigation for 20 days, soil relative water content decreased to 2%) (Figure S1). Fully expanded leaf samples were collected at 10 and 20 d of drought stress treatment and used for further physiological and transcriptomic analysis. All the treatments were conducted in a growth chamber under the same environmental conditions as described above.
2.4 RNA isolation and qRT-PCR analysis
Total RNA isolation, cDNA synthesis, and qRT-PCR were performed following the protocol described in Zhang et al. (2023). AsACTIN was selected as the reference gene for creeping bentgrass (Xu and Huang 2018). Each biological sample has two technical replicates, and all genes were calculated based on the equation 2-ΔΔCt (Livak and Schmittgen 2001). The full sequences of Agrostis stolonifera genes were obtained by aligning the amino acid sequences of Arabidopsis and rice (Oryza sativa spp. japonica) as a query to a local transcriptome database. Specific primers were designed using Primer Premier 6.0 software (Premier Biosoft International). All primer sequences used in the study are listed in Table S1.
2.5 Measurements of physiological parameters
Physiological parameters, including leaf relative water content (RWC), electrolyte leakage (EL), and chlorophyll content, were measured at 20 days of treatment. Leaf RWC was determined according to the method of Barrs and Weatherley (1962), and calculated based on fresh weight (FW), turgid weight (TW), and dry weights (DW) using the following formula: RWC = (FW–DW)/(TW–DW) × 100 (Katuwal et al. 2023). Leaf EL was measured following the method described by Jespersen and Huang (2015). About 0.1 g of fresh leaf sample was immersed in 35 mL deionized water and an initial conductance (Ci) was measured using a conductivity meter (YSI Incorporated) after incubation in a shaker for 24 hours. Leaf tissues were autoclaved at 121°C for 20 min and a final conductance (Cmax) was measured after shaking for an additional 24 hours. Leaf EL was calculated as: EL = Ci/Cmax × 100%. For leaf total chlorophyll detection, fresh leaves (0.1 g) were soaked in 10 mL dimethyl sulfoxide for 72 hours and the absorbance of the extract at 663 and 645 nm was determined with a spectrophotometer (Spectronic Instruments). Chlorophyll content was calculated following the formula described in Barnes et al. (1992).
2.6 Determination of malondialdehyde (MDA) and proline content
Fresh leaves (0.3 g) were ground to powder in liquid nitrogen and homogenized in 6 mL of phosphate buffer (50 mM, pH 7.8) containing 0.1% trichloroacetic acid (w/v). After centrifugation at 15000 g for 30 min at 4°C, 2 mL of the supernatant was used to measure MDA content as described by Heath and Packer (1968) and Dhindsa and Matowe (1981). Proline content was quantified using a modified acid-ninhydrin assay (Abrahám et al., 2010). Briefly, the reaction mixture, consisting of 2 mL supernatant, 2 mL acetic acid, and 2 mL acid-ninhydrin regent was heated in a water bath at 100°C for 30 min, and cooled on ice. Then, the reaction mixture was extracted with 4 mL toluene and the chromophore containing toluene was aspirated. The absorbance was measured at 520 nm with a spectrophotometer and proline content was calculated from a standard curve.
2.7 Determination of lignin content
Lignin contents were measured following an acid-insoluble lignin method by Dence (1992) with modifications. About 0.2 g of fresh leaf sample was ground into fine powder in liquid nitrogen and immersed into 1% acetic acid (v/v). After centrifugation, the precipitate was washed with 1% acetic acid, soaked in ether-ethanol (1:1, v/v) for 3 mins, and then immersed into 72% sulphuric acid (w/w) at room temperature for 16 hours to fully digest cellulose. After boiling and centrifugation, the insoluble residue (lignin) was collected and washed with distilled water. The acid was removed with a hot water bath and the residue was dried to a constant weight in an oven at 105°C. Half mL of 10% barium chloride (w/v) and 5 mL of distilled water were added to the extraction, mixed thoroughly, centrifuged and washed twice with distilled water. Ten mL of 10% sulfuric acid (w/w) and 10 mL of 0.1 M potassium dichromate solution were added to the precipitate and boiled for 15 minutes. The residue was rinsed with 15–20 mL distilled water and transferred to a beaker for titration. After adding 5 mL of 20% potassium iodide (m/v) and 1 mL of 0.5% starch (m/v), the solution was titrated with 0.2 M sodium thiosulfate, and the lignin content was calculated following the formula described in Dence (1992).
2.8 Measurement of leaf water loss rate
To determine leaf water loss rate, stolons of WT and AsAFL1-OE plants were cut and cultivated in hydroponic culture in plastic containers for 2 weeks; then, 6 leaves (including 3rd and 4th mature leaves from the top of WT and AsAFL1-OE plants) were detached and weighed immediately after detachment. The leaves were placed on plates under normal air conditions, and the weights of the leaves were subsequently recorded at 3, 4, 6, 7, and 8 hours, respectively. The leaf water loss rate was represented by calculating the change in mass relative to the initial mass (Meng et al. 2022).
2.9 Transcriptomic analysis
Leaves harvested from WT and AsAFL1-OE plants after 10 and 20 days of control and drought conditions were immediately frozen in liquid nitrogen and stored at −80°C. RNA-Seq analysis was carried out by Gene Denovo Co. (Guangzhou, China) using the paired-end technology of the Illumina HiSeq 2000 platform. Leaves from one pot of plants were regarded as one biological replicate, and three biological replicates of WT, AsAFL1-OE3, and AsAFL1-OE7 lines were performed for RNA-seq analysis in each treatment. Library construction, sequencing, and transcriptomic analysis were performed following the same procedures as described before (Yu et al. 2021). The original transcriptome data were deposited at NCBI (BioProject: PRJNA957937). The FPKM (Fragment Per Kilobase of transcript per Million mapped reads) values of genes values were used for identifying the differentially expressed genes (DEGs) based on a threshold of false discovery rate (FDR) ≤ 0.001 and an absolute value of log2 ratio ≥1. Weighted correlation network analysis (WGCNA) between gene expression level and physiological parameters was performed using the OmicShare tools (https://www.omicshare.com/tools). A regulation overview of DEGs involved in different pathways was visualized by MAPMAN (Thimm et al. 2004).
2.10 Statistical analysis
The analysis of variance was tested to determine treatment and transgenic effects using a statistical program (SPSS 13.0). The least significant difference (LSD) was used to examine the significance of differences between WT and AsAFL1-OE lines under well-watered conditions or drought stress (p < 0.05).
3 RESULTS
3.1 Phylogenetic analysis, subcellular localization, and expression profiling of AsAFL1
The full-length cDNA sequence of AsAFL1 was cloned from creeping bentgrass. Phylogenetic analysis reveals that AsAFL1 shares 93.5%, 93%, and 93% sequence identity with HvAFL1, TaAFL1, and AetAFL1 in Hordeum vulgare, Triticum aestivum, and Aegilops tauschii, respectively (Figure 1A). Comprising 383 amino acids, AsAFL1 is characterized by the presence of a conserved low-complexity structural domain (LCD) and transmembrane region (TM) (Figure 1B). Motif analysis showed that AsAFL1 has five epidermal growth factor (EGF_1) structural domains and one β1-integrin structural domain (Figure 1C). LCD can assist in the correct assembly of the fibrous cytoskeleton or participate in the formation or assembly of various functional organelles through its phase separation (Zhou et al. 2022), and EGF structural domains have Ca2+-binding ability (Davis 1990), suggesting AsAFL1 may act as a membrane receptor involved in cytoskeleton maintenance and vesicle formation.
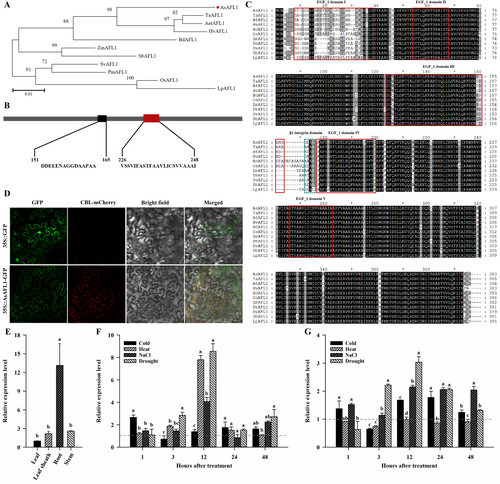
The subcellular localization of AsAFL1 was investigated through the transient expression of AsAFL1-GFP fusion protein in tobacco leaves. As shown in Figure 1D, the fluorescence signal of the AsAFL1-GFP fusion protein was co-localized with a plasma membrane marker, CBL1n-mCherry (Batistic et al. 2008), indicating that AsAFL1 was a plasma membrane protein.
The expression pattern of AsAFL1 was characterized using qRT-PCR analysis. The transcript level of AsAFL1 was highest in roots, which was about 13, 6 and 5 times higher than that in leaves, leaf sheath and stem (Figure 1E). To reveal stress-dependent-specific expression profiles of AsAFL1, seedlings were exposed to different abiotic stresses, including drought (15% PEG 6000), salt (150 mM NaCl), heat (38°C), and cold (4°C) stresses. The highest expression level of AsAFL1 was found after 12 hours of PEG-induced drought stress, which was about 8 times and 3 times higher in leaves and roots than that of the control, respectively (Figure 1F,G). Similarly, AsAFL1 showed a general up-regulation pattern when exposed to heat and salt stresses in leaves for 12 hours, and a down-regulation pattern when exposed to heat stress in roots for 12 hours. The results showed that cold, heat, salt and drought all induced the expression of AsAFL1 in leaves (Figure 1F,G).
3.2 Overexpression of AsAFL1 enhanced drought tolerance in creeping bentgrass
To investigate the function of AsAFL1 regulating plant drought tolerance, overexpression transgenic lines of AsAFL1 driven by the maize (Zea mays) ubiquitin promoter in creeping bentgrass were generated by Agrobacterium-mediated transformation (Figure S2). A total of 10 overexpression lines (OE) were obtained and confirmed by the presence of HPTII gene and positive GUS staining signals (Figure S3A,B). The AsAFL1-OE3 and OE7 lines had 2.94- and 2.84-times higher expression levels of AsAFL1 than the WT plants, respectively (Figure S3C).
To assess the drought stress tolerance between AsAFL1-OE and WT plants, one-month-old seedlings were exposed to drought by withholding irrigation for 20 days and re-watered for 25 days. Phenotypically, after 20 days of drought treatment, AsAFL1-OE lines (OE3 and OE7) showed vigorous growth and had fewer yellow leaves than WT (Figure 2A). Under drought stress, leaf RWC, chlorophyll content and proline content of AsAFL1-OE lines were higher than those of WT, while EL and MDA were lower than those of WT (Figure 2B). Under control conditions, there were no significant differences in leaf RWC, chlorophyll content, EL, MDA and proline content between AsAFL1-OE and WT plants (Figure 2B).
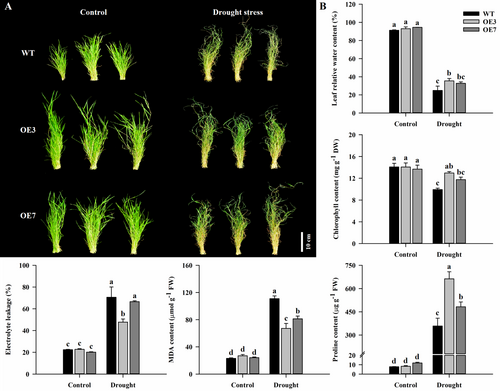
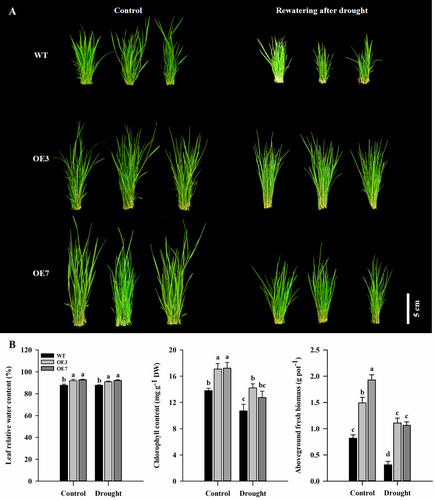
After re-watering for 25 days, the growth performance and biomass of WT were significantly lower than that of AsAFL1-OE lines (Figure 3A,B). Leaf RWC of OE3 and OE7 was 3.70% and 5.0% higher than that of WT, while leaf chlorophyll content of OE3 and OE7 was 32.6% and 18.89% higher than that of WT, respectively (Figure 3B). The aboveground biomass of OE3 and OE7 was 128.84% and 119.52% higher than that of WT, respectively (Figure 3B).
3.3 Transcriptomic comparison between AsAFL1-OE and WT plants
Transcriptomes of AsAFL1-OE (OE3 and OE7) and WT plants were compared at 10 and 20 days of drought stress using Illumina-based sequencing. Approximately 127 million bp of clean reads were assembled into 139326 unigenes with an N50 length of 1499 bp and a mean unigene length of 912 bp (Table S2). Principal-component analysis (PCA) revealed that the samples clustered into three separate groups (Figure 4A). Principal component 1 (PC1) separated 20-day-drought-stress samples (D20) from 10-day-drought-stress (D10), 10-day-control (C10), and 20-day-control (C20) samples, and accounted for 69.5% of the total variance, while principal component 2 (PC2) distinguished the WT from AsAFL1-OE (OE3 and OE7) samples and accounted for 12% of the total data variance (Figure 4A). Setting the cut-off value of fold change (FC) > 2 and false discovery rate (FDR) < 0.05, eight sets of differentially expressed genes (DEGs) were identified, including up-and down-regulated genes in WT and AsAFL1-OE (OE3 and OE7) at 10 and 20 days under control and drought stress conditions (Figure 4B).
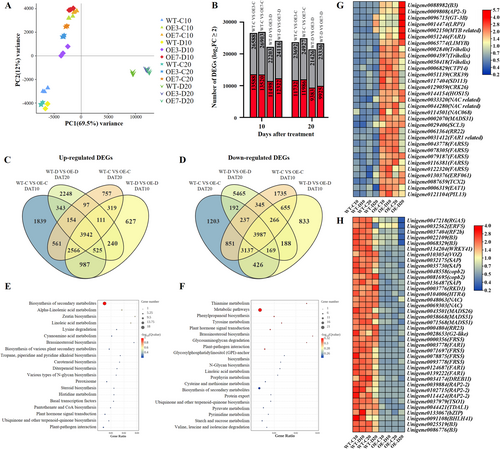
Venn diagrams showed that a total of 240 genes were up-regulated, and 188 genes were down-regulated in plants overexpressed AsAFL1 relative to WT (WT-D vs. OE-D) at 10 and 20 days of drought treatment, while 561 genes and 851 genes exhibited up-regulation and down-regulation, respectively in transgenic plants relative to WT (WT-C vs. OE-C) at 10 and 20 days of well-watered conditions (Figure 4C,D). KEGG pathway enrichment analysis on these up- or down-regulated DEGs due to AsAFL1 overexpression showed that the up-regulated DEGs were mainly enriched in biosynthesis of secondary metabolites, α-linolenic acid metabolism, and zeatin biosynthesis (Figure 4E). The down-regulated DEGs were mainly enriched in pathways including thiamine metabolism, metabolic pathways and phenylpropanoid biosynthesis (Figure 4F).
Further examination of the transcription factors (TFs) among the AsAFL1-responsive DEGs revealed that, compared with WT, there were 35 and 47 TFs up- and down-regulated in AsAFL1-OE plants under both well-watered and drought stress conditions, respectively (Table S3). TFs, including B3 (Unigene0088982), FAR-RED IMPAIRED RESPONSE 1 (FAR1, Unigene0051246), APETALA2/ETHYLENE-RESPONSIVE FACTOR (AP2-5, Unigene0009808), LATERAL ROOT PRIMORDIUM 1 (LRP1, Unigene0014474), GT-3B (Unigene0096715), and MYB-associated (Unigene0002150), exhibited significant increases in OE3-D20 and OE7-D20, compared to WT-D20 (Figure 4G). TFs such as NAC-related 2 (Unigene0069303), RAP2-2 (Unigene0039884), MADS33 (Unigene0006471), and ETHYLENE RESPONSE TRANSCRIPTION FACTOR 5 (ERF5, Unigene0032562) were significantly down-regulated in OE3-D20 and OE7-D20 compared to WT-D20 (Figure 4H).
3.4 Overexpression of AFL1 up-regulated multiple metabolic pathways
To further identify specific pathways regulated by AsAFL1, weighted correlation network analysis (WGCNA) was performed between transcriptomic data and three physiological parameters (leaf RWC, chlorophyll, and MDA) in WT and AsAFL1-OE plants exposed to 20 days of well-watered condition and drought stress. A total of eight modules were classified for the highly correlated genes (Figure 5A). Among the eight modules, brown, turquoise, pink and yellow modules were significantly correlated with physiological parameters (Figure 5B). After 20 days of drought treatment, genes in turquoise module were specifically up-regulated in WT, and genes in brown module genes were specifically up-regulated in the AsAFL1-OE lines (Figure 5C). Further KEGG analysis of these two modules revealed that overexpression of AsAFL1 affected genes involved in pathways of phytohormone signaling, carotenoid biosynthesis, biosynthesis of various plant secondary metabolites, flavonoid biosynthesis, and cutin, suberin and wax biosynthesis (Figure 5D).
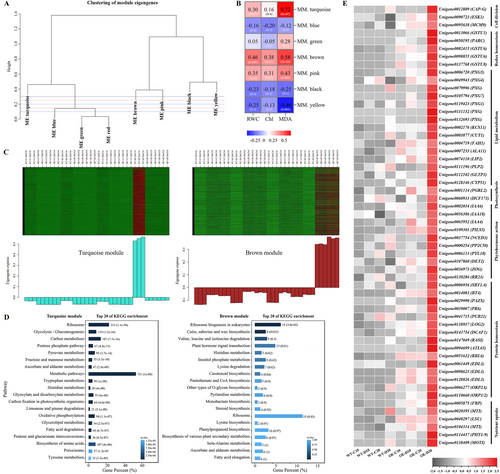
As shown in Figure 5E, genes involved in redox homeostasis (GLUTATHIONE S-TRANSFERASE, GSTU1, GSTU6, GSTU8), lipid metabolism (CALEOSIN/PEROXYGENASE, CLO/PXG; KCS; CUT1; GLYCOLIPID TRANSFER PROTEIN 3, GLTP3; LANOSTEROL 14ALPHA-DEMETHYLASE, CYP51), and nutrient transport (RESPONSE TO LOW SULFUR, LSU; PHOSPHATE TRANSPORTER 1, PHT1; NITRATE TRANSPORTER 1.1, NRT1.1; MOLYBDENUM TRANSPORTER TYPE 1, MOT1) were significantly up-regulated in AsAFL1-OE plants at 20 days of drought stress, compared with WT (Figure 5E).
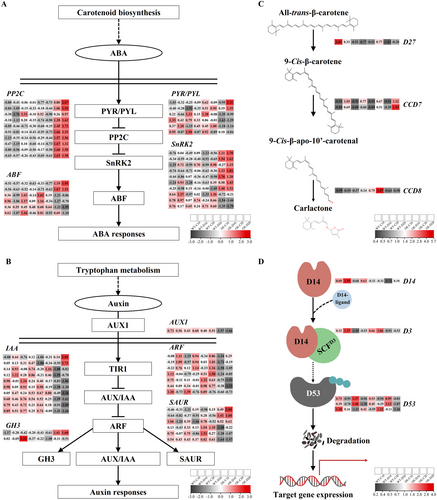
In the phytohormone signaling pathway, DEGs exhibited consistent differences in AsAFL1-OE lines compared with WT, which were mainly found in ABA, auxin, and SL signal transduction pathways (Figure 6). In the ABA signal transduction pathway, after 20 days of drought treatment, AsAFL1-OE plants showed significantly higher expression levels of PYR/PYL, PP2C, SNF1-RELATED PROTEIN KINASES 2 (SnRK2), and ABSCISIC ACID-RESPONSIVE ELEMENT BINDING FACTOR (ABF) compared to WT (Figure 6A). Similarly, GRETCHEN HAGEN3 (GH3), SMALL AUXIN-UP RNA (SAUR), and IAA involving in auxin signal transduction pathway, were significantly up-regulated in AsAFL1-OE plants compared with WT after 20 days of drought stress treatment (Figure 6B). Overexpression of AsAFL1 significantly enhanced the expression levels of three key genes in SL biosynthesis pathway, DWARF 27 (D27), CAROTENOID CLEAVAGE DIOXYGENASE 7 (CCD7) and CAROTENOID CLEAVAGE DIOXYGENASE 8 (CCD8), after 20 days of drought treatment (Figure 6C). In addition, the expression level of several key genes (DWARF14, D14; DWARF3, D3; and DWARF53, D53) involved in SL signal transduction pathway were significantly affected in AsAFL1-OE plants (Figure 6D).
3.5 Overexpression of AsAFL1 promoted wax formation, increased lignin content, and prevented water loss in creeping bentgrass under drought stress
According to KEGG enrichment (Figure 5D) and Mapman analysis (Figure S4), ‘Phenylpropanoid biosynthesis’ and ‘Cutin, suberin and wax biosynthesis’ pathways were significantly enriched in AsAFL1-OE plants after 20 days of drought stress. A total of 301 and 83 unigenes were enriched in the ‘Phenylpropanoid biosynthesis’ and ‘Cutin, suberin and wax biosynthesis’ pathways, respectively (Tables S4 and S5). As shown in Figure 7A, genes encoding key enzymes for the initiation of lignin biosynthesis, phenylalanine ammonialyase (PAL) and cinnamate-4-hydroxylase (C4H), were significantly up-regulated by 20 days of drought stress, and the expression level of 4CL was significantly higher in AsAFL1-OE plants than that in WT. After 20 days of drought stress, AsAFL1-OE plants showed significant up-regulated levels of CAD and PRDX6, which function in the final steps of monolignal metabolism. The lignin contents in WT and AsAFL1-OE lines after 20 days of drought treatment were further determined. The results showed that overexpression of AsAFL1 significantly increased the lignin content in creeping bentgrass (Figure 7B). Under control condition, the lignin contents of OE3 and OE7 were increased by 23.78% and 39.77%, respectively, compared to WT. After 20 days of drought stress, the lignin contents of OE3 and OE7 were 11.25% and 16.31% higher than that of WT, respectively (Figure 7B).
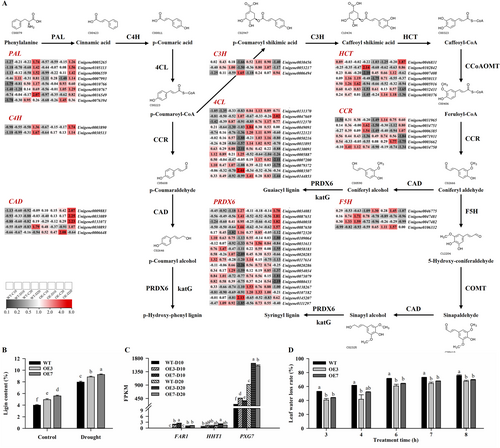
The expression level of three genes (omega-hydroxypalmitate O-feruloyl transferase, HHT1; alcohol-forming fatty acyl-CoA reductase, FAR; and PXG) involved in the ‘Cutin, suberin, and wax biosynthesis’ pathway, was significantly higher in AsAFL1-OE lines than in WT in the 20-day drought treatment (Figure 7C). The results of the water loss rate of creeping bentgrass leaves also revealed that the water loss rate of WT leaves was significantly higher than that of AsAFL1-OE lines, implying that the leaves of AsAFL1-OE lines may have a much thicker cuticle, thus reducing water evaporation (Figure 7D). These results demonstrated that overexpression of AsAFL1 significantly increased the lignin content as well as the formation of cutin, suberin and wax in transgenic creeping bentgrass under drought stress, which may contribute to enhanced drought tolerance in AsAFL1-OE plants.
To validate the RNA-Seq data, qRT-PCR analysis of 8 identified DEGs (CHI3, HSP70, P5CS1, ARF9, TSIT1, ERD7, SnRK2 and IAA6) were conducted (Figure S5). Results between qRT-PCR and RNA-Seq were consistent, confirming the reliability of the transcriptome analysis.
4 DISCUSSION
AsAFL1 gene, cloned from creeping bentgrass, was found to localize at the plasma membrane, and its expression was induced by drought stress. Overexpression of AFL1 significantly promoted growth and drought tolerance of creeping bentgrass, as exemplified by higher RWC, chlorophyll and proline contents, and lower EL and MDA content in AsAFL1-OE lines compared with WT plants under drought stress. The comparative transcriptome analysis showed that AsAFL1-mediated improvement in drought tolerance was mainly related to the up-regulation or down-regulation of genes in hormone biosynthesis and signaling (PYR/PYL, PP2C, SnRK2, ABF, GH3, SAUR, IAA, D27, CCD7, CCD8, D14, D3, and D53), redox homeostasis (GSTU1, GSTU6, and GSTU8), and secondary metabolism, including biosynthesis of lignin (PAL, C4H, 4CL, CAD, and PRDX6) and cutin, suberin and wax (PXG, KCS, CUT1, GLTP3, CYP51, HHT1, and FAR), as well as nutrient transport (LSU, PHT1, NRT1.1, FBP, MOT1, and MT).
Plant hormones are key signaling molecules in plant growth, development, and environmental stress responses (Waadt et al. 2022). In this study, the core genes in ABA signaling (PYR/PYL, PP2C, SnRK2, and ABF), auxin signaling (GH3, SAUR, and IAA), SL biosynthesis (D27, CCD7, and CCD8) and signaling (D14, D3, and D53) showed significantly altered expression levels in AsAFL1-OE lines and WT. GH3-5/WES1 functions in Arabidopsis temperature and drought resistance by participating in salicylic acid/ABA-mediated plant defense response pathways (Park et al. 2007). Loss of IAA6 reduced glucosinolate levels and further decreased drought tolerance in Arabidopsis (Salehin et al. 2019). Overexpressing AtSAUR32 improved drought tolerance by interaction with clade A-PP2C proteins and increased sensitivity to ABA responses (He et al. 2021). In SL signal transduction pathway, the degradation of D53 can stimulate the expression of genes related to the synthesis of flavonoids and SL, which enhances plant growth, development, nutrient uptake, and drought tolerance (Zhou et al. 2013). These results demonstrated that improved drought tolerance by AsAFL1-overexpression may involve the activation of coordinated ABA, auxin, and SL signaling pathways.
Drought stress typically enhances the production of reactive oxygen species (ROS) and causes nutrient depletion, hindering plant development and growth (Huang et al. 2014, Vadez et al. 2024). Numerous studies have proven that the GSH system is one of the most important endogenous antioxidant systems that maintains cellular redox balance while preventing oxidative damage and cell death (Tan et al., 2023). It has been demonstrated that TaBZR2, a BES/BZR-type TF, positively activates TaGST1 to scavenge drought-induced ROS accumulation in wheat (Cui et al. 2019). Overexpressing OsGSTU6 in rice contributes to cadmium stress tolerance by involving intracellular ROS homeostasis (Jing et al. 2021). Overexpression of CsGSTU8 in Arabidopsis resulted in improved drought tolerance, as evidenced by increased scavenging of ROS during drought conditions (Zhang et al. 2021). In this study, expression levels of GSTU1, GSTU6, and GSTU8 were significantly enhanced in AsAFL1-OE lines after 20 days of drought stress, demonstrating that AFL1 may play a positive role in the control of intracellular redox homeostasis via ROS scavenging.
Phenylpropane metabolism is an important pathway to produce secondary metabolites, such as lignin, flavonoids, and phenolic compounds, contributing to plant development and plant-environment interactions (Dong and Lin 2021, Jogawat et al. 2021). In this study, KEGG enrichment and Mapman analysis revealed that DEGs involved in phenylpropanoid, cutin, suberin and wax biosynthesis pathways were significantly enriched in AsAFL1-OE plants under drought stress. In addition, genes encoding key enzymes involved in lignin synthesis pathway, such as CAD and PRDX6, were significantly up-regulated in AsAFL1-OE plants. Furthermore, compared with WT, the lignin contents in AsAFL1-OE lines were significantly increased under both control and drought stress conditions, demonstrating that overexpression of AsAFL1 promoted lignin biosynthesis and increased lignin accumulation.
Lipid metabolism supplies the precursors for the biosynthesis of wax and cutin, which serve as barriers to protecting plants from biotic and abiotic stresses (Pollard et al. 2008; Lewandowska et al. 2020; Philippe et al. 2020). Caleosin/peroxygenases (CLO/PXGs) are lipid-associated proteins that stabilize lipid droplets and are involved in cutin synthesis (Lequeu et al. 2003, Hanano et al. 2023). Suppressing CUT1, which encodes a very-long-chain fatty acids condensing enzyme in cuticular wax production, resulted in waxless stems and siliques and conditional male sterility in Arabidopsis (Millar et al. 1999). Overexpression of 3-ketoacyl CoA synthase gene (KCS20), which is critical for cuticular wax and suberin biosynthesis, increased the wax content by 10% in Arabidopsis leaves (Lee et al. 2009). The CYP51G subfamily encodes obtusifoliol 14α-demethylase, a crucial enzyme in sterol and brassinosteroid production that affects plant development and stress response (Jiao et al. 2022). In this study, lipid metabolism-associated genes (CLO/PXGs, KCS, CUT1, GLTP3, and CYP51), cutin, suberin, and wax biosynthesis genes (HHT1 and FAR), were significantly increased in AsAFL1-OE lines, and the water loss rate of AsAFL1-OE plants was significantly lower than that of WT, implying that overexpression of AsAFL1 promoted cutin and wax formation, thus preventing water loss under drought stress.
Like organic metabolites, mineral nutrient availability also plays essential roles in plant adaptation to drought stress (Kumari et al., 2022). In this study, overexpression of AsAFL1 significantly up-regulated the expression level of genes associated with the transport of nutrients in plants exposed to 20 days of drought stress, such as transporter proteins for nitrate (NRT1.1), phosphate (PHT1), sulfur (LSU), ferric iron (FBP), molybdate (MOT1), and heavy metals (metallothionein, MT). The transporter proteins control nutrient transport and mobilization in plants that regulate plant tolerance to abiotic stress tolerance (Krouk et al. 2010, Bouguyon et al. 2015, Giehl and von Wirén 2015, Arisumi et al. 2023). These results suggested that overexpression of AsAFL1 could promote the absorption and transport of nutrients, mainly nitrate, phosphate, sulfur, ferric iron, and molybdate, to maintain sufficient nutrition or nutrient balance, which could contribute to improved drought tolerance.
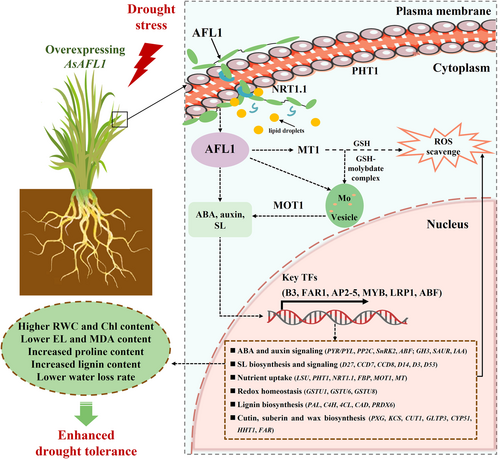
In conclusion, the current study demonstrates that overexpression of AsAFL1 significantly enhanced drought tolerance in creeping bentgrass, as evidenced by notable increases in leaf relative water content, chlorophyll levels, and membrane stability under drought stress. The overexpression of AsAFL1 also up-regulated the biosynthesis and signaling pathways of ABA, auxin, and SL, redox homeostasis, and synthesis of essential secondary metabolites, including lignin, cutin, suberin, and wax. Additionally, it increased the expression of genes controlling mineral nutrient transport. Regulation of all these factors collectively contributes to greater drought resilience in creeping bentgrass (see Figure 8). However, the mechanisms involved in the signaling transduction pathways influenced by AsAFL1 for regulating the molecular and metabolic pathways demonstrated in Figure 8 deserve further investigation to unravel the upstream molecular components of this functional candidate gene for genetic improvement of plant drought tolerance across different plant species.
AUTHOR CONTRIBUTIONS
Xiaxiang Zhang and Bingru Huang designed the research. Zhenzhen Tan, Yiting Wang, and Hengyue Jiang performed the research. Zhenzhen Tan, Yiting Wang and Yu Liu analyzed the transcriptomic data. Zhenzhen Tan and Xiaxiang Zhang prepared the original draft. Xiaxiang Zhang, Bingru Huang, Ya Li, Xiaoxian Zhong, Lili Zhuang, and Zhimin Yang revised the manuscript. All authors reviewed and approved the final version of the manuscript for submission.
ACKNOWLEDGEMENTS
This research was supported by Jiangsu Funding Program for Excellent Postdoctoral Talent to Zhenzhen Tan (2024ZB830), Fundamental Research Funds for the Central Universities (XUEKEN2022020), and Central Finance Forestry Science and Technology Promotion Demonstration Funding Project (Su [2023] TG1).
Open Research
DATA AVAILABILITY STATEMENT
The original transcriptome data were deposited at NCBI (BioProject: PRJNA957937). The data that supports the findings of this study are available in the supplementary material of this article.




