Transcriptome and metabolome analysis revealed that phenylpropanoid and flavonoid biosynthesis respond to drought in tiger nut
Abstract
Tiger nuts (Cyperus esculentus) have emerged as a novel oil crop, being utilized as raw materials for obtaining industrial ink. Drought is a serious stress that significantly affects the entire plant and reduces its yield. The seedling stage is crucial as it determines the future growth and yield. Consequently, it is essential to enhance the ability of tiger nuts to mitigate drought at the seedling stage. A comprehensive analysis was conducted on roots and leaves, including their phenotypes, physiological indicators, transcriptomes, and metabolomes. The results revealed that leaves and roots were affected by drought stress, as evidenced by phenotypic data such as leaf area and physiological indicators, including changes in peroxidase and catalase activity, malondialdehyde content, electrolyte leakage, and superoxide anion levels. Drought imposed greater effects on leaves. Phenylpropanoid and flavonoid biosynthesis were identified as candidate pathways using transcriptome and metabolome analysis, Real-Time Quantitative PCR (RT-qPCR), and physiological verifications. However, the response modes of the root and leaf parts differed based on the enriched pathways analysis, indicating that the changes in the content of some metabolites were contrasting between the roots and leaves. The study revealed the molecular mechanisms under drought, particularly the synergistic responses in leaves and roots, providing insights and a theoretical basis for enhancing the drought tolerance of tiger nuts.
1 INTRODUCTION
The demand for industrial ink is increasing with the continuous development of the industry. Oil crops have become essential raw materials for ink extraction. About 30% of prints in the United States utilize inks. Tiger nut (Cyperus esculentus) is a plant species in the family Cyperaceae native to Africa and first grew in arid desert areas (Zhang and Sun, 2023). After prolonged natural breeding, the plants have developed strong survival abilities, demonstrating tolerance to floods, droughts, salinity, alkalinity, and poor soil fertility. Moreover, it can be planted on land with varying fertility levels. It has been recognized as one of the “superfoods” of the 21st century. Due to its high oil content and strong stress resistance, it holds potential as an emerging economic crop (Codina-Torrella et al., 2015). However, tiger nuts are sensitive to the environment, with abiotic or biotic stresses (such as drought, cold, heat, pests, and diseases) significantly affecting plant growth and yield (Yu et al., 2024). Drought stress is one of the important abiotic stresses affecting crop growth, as it can occur at any stage of crop growth, leading to reduced yield or crop failure (Song et al., 2024). Drought decreases the metabolic activity of plant cells, affecting photosynthesis and respiration and decreasing the net assimilation rate of CO2 in photosynthetic carbon metabolism. Therefore, drought treatment can limit crop growth by reducing the accumulation of nutrients (Yan et al., 2023). Moreover, drought stress leads to disturbances in intracellular ion balance, including the imbalance of K+ and Ca2+ (Abideen et al., 2022). It causes a rapid increase in oxidative stress levels within plant cells, resulting in excessive accumulation of reactive oxygen species (ROS). The redox potential is altered by excessive ROS, impairing the functions of organelles, eventually leading to cell death and limited plant growth and development (Qi et al., 2018).
Plants have developed various ways to respond to drought through continuous evolution. These mechanisms are a mixed system rather than being regulated by a single pathway (Cheng et al., 2019). Drought promotes stomatal closure, reducing the rate of gas exchange and decreasing leaf transpiration in crops (Agurla et al., 2018). The synthesis of protective substances against dehydration maintains cell stability and normal function under drought, providing balance in intracellular water potential and reducing oxidative damage through cell membrane integrity (Razi and Muneer, 2021). Peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) are antioxidant enzymes that convert ROS into non-toxic molecules, preventing excessive accumulation of ROS (Yuan et al., 2019a). Additionally, abscisic acid (ABA) has been reported to increase under drought, and its synthesis has been associated with mitigating drought. Plants with strong drought resistance synthesize higher levels of ABA (Hussain et al., 2021). Although some mechanisms for responding to drought stress have been reported, the response mechanisms can vary across different crops (Godwin and Farrona, 2020).
The phenylpropanoid biosynthesis pathway is a metabolic pathway that regulates secondary metabolite synthesis (Dong and Lin, 2021). This pathway begins with the production of phenylalanine via glycolysis and shikimic acid. The compounds in this pathway are catalyzed by phenylalanine ammonia-lyase (PAL), cinnamyl-alcohol dehydrogenase (CAD), and cinnamoyl-CoA reductase (CCR). These reactions produce coumaroyl-CoA, which becomes a substrate for the subsequent metabolic reactions and is converted into various phenylpropanoid compounds such as lignin (Lam et al., 2022). The flavonoid biosynthesis is part of the downstream branch of the phenylpropanoid biosynthesis pathway (Liu et al., 2021). Coumarayl-CoA-generated chalcone is produced after the catalysis of chalcone synthase (CHS). Chalcone continues to be catalyzed by various enzymes to produce dihydroflavonol, anthocyanin, procyanidin, and leucoanthocyanidin by naringenin 3-dioxygenase, anthocyanidin synthase (ANS), and anthocyanidin reductase (Shen et al., 2022). The phenylpropanoid and flavonoid biosynthesis pathways have been found to respond to drought stress. The expression of the phenylpropanoid biosynthesis-related genes ZmCAD and ZmCOMT (phenylpropanoid biosynthesis-related genes) of maize (Zea mays) (Hu et al., 2009) inbred lines increased significantly under drought stress. Overexpression of these genes enhanced drought resistance under stress. Overexpression of BplMYB46, another phenylpropanoid biosynthesis pathway gene, increased lignin deposition and enhanced the tolerance to osmotic stress (Guo et al., 2017). As an important secondary metabolite, flavonoids contribute to crop growth and development, particularly in response to abiotic stress (Azuma et al., 2012). PFG3 is an Arabidopsis thaliana gene whose overexpression improved drought tolerance by regulating flavonoid accumulation, particularly the content of flavonols (Baozhu et al., 2022). Moreover, OsCHI2 promoted the concentration of flavonoids and enhanced the ability to resist drought (Jayaraman et al., 2021). Although some results have indicated associations between phenylpropanoid and flavonoid biosynthesis pathways with drought stress, there has been limited research on tiger nuts under drought. The mechanism of tiger nut response to drought stress at the seedling stage was investigated and analyzed through phenotypic, physiological, transcriptomic, and metabolomics studies, with leaves and roots at the seeding stage being the target tissues. Our results contribute to understanding the molecular mechanisms under drought stress in tiger nuts, particularly the responses between leaves and roots, providing insights for enhancing the drought tolerance of tiger nuts.
2 MATERIALS AND METHODS
2.1 Plant material and conditions
The local tiger nut variety “Jisha 2” grown in northeast China was used as the plant material. Seeds were disinfected by NaClO for 5 min and then planted in 0.5-gallon pots filled with uniform floriculture soil for growth in a greenhouse. The drought (D) and blank control (BC) groups were intercropped after emergence, with each group consisting of five biological replicates. Each pot was watered with 50 mL of purified water before the drought treatment. The D group was subjected to drought treatment using the persistence drought method, while the BC group continued to be watered. Sampling was conducted after six days (Jud et al., 2016), with roots and leaves under control and drought set as target sampling tissues (Sun et al., 2021).
2.2 Phenotypic and physiological analysis of drought treated and control plants
The aboveground phenotypes of each treated plant were examined using the Planteye F500 (Phenospex), while the root phenotypes of each treatment sample were scanned and estimated by WINRHIZOPRO-2004a (Delta-T). The activities of ROS-mitigating enzymes, including POD, SOD, CAT, and APX, were determined using different physiological indicators with the ELISA Kit (Mengxi). Moreover, the contents of malondialdehyde (MDA), hydrogen peroxide (H2O2), and superoxide anions (O2−) were determined using different physiological indicators using the ELISA Kit (Mengxi). Each group of physiological indicators was measured five times, with samples from different plants as five biological replicates.
2.3 Transcriptome analysis of the drought-treated and control group
The samples, including root and leaf tissues in BC and D groups, were denoted as BCroots, Droots, BCleaves, and Dleaves for transcriptome analysis, with each group consisting of three biological replicates. RNA from each group sample was isolated using the RNA Isolation Kit (RC411). Subsequently, RNA samples were sequenced using the Illumina HiSeq 2500 after confirming that the RNA quality in the sample was acceptable via the NanoDrop™. The reference genome used for sequencing was Cyperus esculentus, obtained from the CNGB database (accession: CNA0051961). Fragments per kilobase of exon model per million mapped reads were estimated using DESeq2. Furthermore, Log2(Fold-change) was utilized to estimate the differentially expressed genes (DEGs), while gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) databases provided comprehensive information for the enrichment analysis of DEGs.
2.4 Metabolome analysis of drought-treated and control plants
The samples, including root and leaf tissues in BC and D groups, were denoted as BCroots, Droots, BCleaves, and Dleaves for metabolome analysis, with each group consisting of six biological replicates. The 100 mg solid sample was placed in a 2 mL centrifuge tube, and a 6 mm diameter grinding bead and 800 μL of extraction liquid were added to extract metabolites. Furthermore, the sample solution was ground in a frozen tissue grinder for 6 min and then extracted by ultrasound at low temperature for 30 min. The sample was placed at −20°C for 30 min, then centrifuged at low temperature for 15 min. The supernatant was then transferred to the injection vial with internal intubation for machine analysis. The liquid chromatography-mass spectrometry (LC–MS) analysis was performed using Thermo Field's ultra-high performance liquid chromatography-tandem Fourier transform mass spectrometry UHPLC-Q exactive system (Thermo Fisher). The raw LC–MS data were imported into the metabolomics processing software Progenesis QI (Waters Corporation) for baseline filtering, peak identification, integration, retention time correction, and peak alignment. Finally, a data matrix of retention time, mass-charge ratio, and peak intensity was obtained. Simultaneously, the information of MS and MS/MS mass spectrometry were matched with MJDBPM, a proprietary database of plant metabolites built by Metabase, to obtain metabolite information. After searching the database, the metabolite data were uploaded to cloud.majorbio.com and analyzed following a standardized process. Significantly different metabolites were determined based on variable weight values and T-test P-values, with metabolites displaying more than a 1-fold difference between control and experimental groups and a P-value <0.05 (Ren et al., 2022). Differential metabolites were annotated using KEGG to determine the associated pathways.
2.5 q-RTPCR and enriched enzyme activity analysis
Six genes (CeHCT, CePOD, CeCHS, CeCOMT, CePAL, and CeCAD) enriched in phenylpropanoid and flavonoid biosynthesis pathways were validated for their expression levels. The RTq-PCR primers were designed by Primer, and CeUCE2 was used as a reference (Mu et al., 2022). The RTq-PCR program was performed using a 2× HotStart SYBR qPCR Mix (Genstar, China) and a LightCycler® thermocycler according to the product specification and procedural requirements. The expression was calculated using the 2–ΔΔCt method (Livak and Schmittgen, 2001). The activities of COMT, CHS, and CCR were tested with different ELISA kits (Mengxi China), respectively, following the methods provided in the respective kit manuals.
2.6 Statistical analysis
The data produced in this study, including phenotype, physiology, and gene expression, were analyzed using the Statistical Package for Social Sciences software. The results are presented as mean ± standard deviation and evaluated using analysis of variance through Duncan's multiple range tests while the P-value was set as 0.05.
3 RESULTS
3.1 Phenotypic analysis of drought-treated and control seedlings
In the phenotype analysis of tiger nut seedlings in BC and D groups, drought limited the growth of tiger nut plant leaves (Figure 1A), leading to a decrease in digital biomass. However, the leaf area decreased significantly compared to the BC treatment (Figures 1B-C). Root results indicated that the fresh and dry weight data in BC and D groups exhibited statistically non-significant changes under drought stress, while significant changes were observed in leaves (Figures 1D-E). These data suggest that leaves appear to be more stressed than roots.
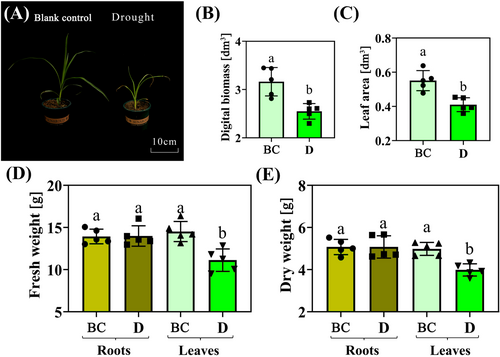
3.2 Physiological analysis of BC and D group samples in response to drought stress
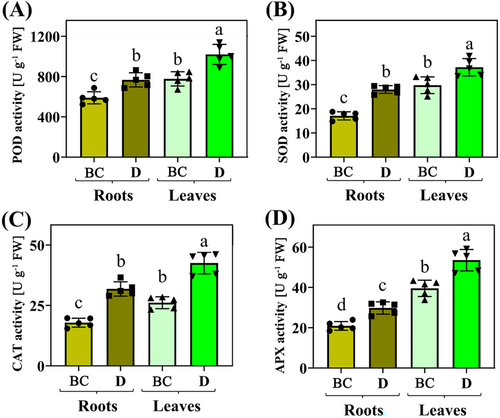
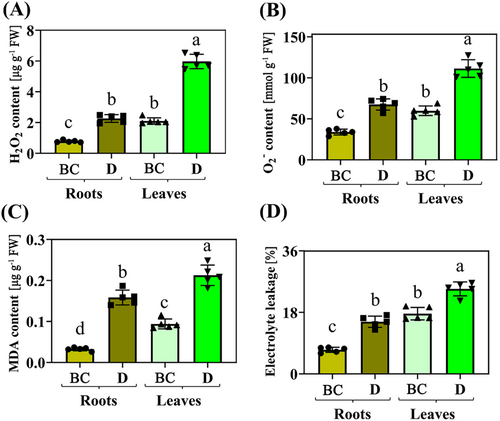
The activities of ROS-mitigating enzymes (SOD, POD, CAT, and APX) changed under drought in roots and leaves compared to CK treatment. The activities of enzymes (SOD, POD, CAT, and APX) were stimulated under drought in roots and leaves, although these activities were greater in leaves than those in roots (Figures 2A–D). Physiological indicators (such as MDA, H2O2, O2−, and El) under drought stress exhibited similar changes observed in the activities of the ROS system in roots and leaves compared to CK treatment. These indicators were stimulated under drought in roots and leaves, with physiological indicator data (such as activities of SOD, POD, CAT, APX activities and content of MDA, H2O2, O2−, and El) being greater in leaves than the roots (Figures 3A–D).
3.3 Transcriptome analysis of BC and D group samples
Roots and leaves of tiger nuts at the seedling stage, treated with drought and control conditions, were used for RNA-Seq analysis, resulting in 542 737 098 clean reads. The clean data exceeded 6.09 GB, while the Q30 base was above 94.39%. These results revealed that the quality control information of the RNA-Seq data was good and could be used for further analysis. The sequences of these samples were uploaded to NCBI with the accession number PRJNA1070209.
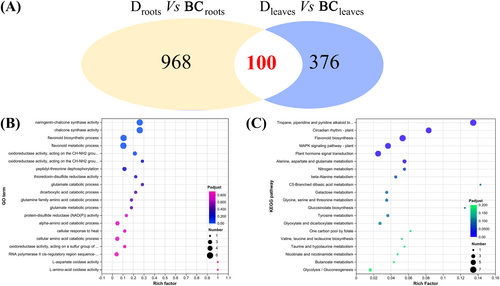
Based on RNA-Seq data, an inter-group differential gene expression analysis was performed to identify DEGs. The filtering threshold was set at |log2FoldChange| ≥ 1 and P-adjust <0.05. Compared to the control, 1068 DEGs were present in roots under drought stress, with 715 DEGs up-regulated and 353 DEGs down-regulated. A total of 476 DEGs in leaves were identified under drought stress (305 up-regulated and 171 down-regulated), which is fewer than in the roots (Table 1 and Figure 4). All these DEGs were considered candidate genes for enrichment analysis.
| DEGs comparison between different treatments | Total DEGs | Up-regulated DEGs | Down-regulated DEGs |
|---|---|---|---|
| Droots versus BCroots | 1068 | 715 | 353 |
| Dleaves versus BCleaves | 476 | 305 | 171 |
| Dleaves versus Droots | 9352 | 3743 | 5609 |
| BCleaves versus BCroots | 8840 | 3479 | 5361 |
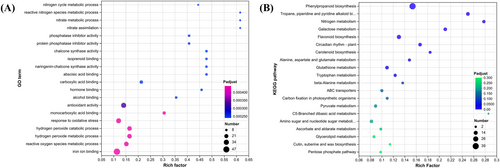
We analyzed the drought response mechanism in tiger nuts through RNA-Seq, targeting the leaf and root tissues. In roots, the DEGs in Droots versus CKroots were enriched in 79 GO terms such as nitrogen cycle, reactive nitrogen species metabolism, nitrate metabolism, nitrate assimilation, CHS activity, naringenin-CHS activity, and others. These GO terms were divided into two categories: Nitrogen metabolism and CHS, suggesting that nitrogen metabolism and CHS might be the candidate metabolic pathways under drought stress (Figure 4A). In the KEGG analysis of the DEGs in roots, 10 pathways were enriched significantly, including phenylpropanoid biosynthesis, nitrogen metabolism, flavonoid biosynthesis, carotenoid biosynthesis, and glutathione metabolism (Figure 4B). Nitrogen metabolism was identified in both enrichment analyses, while CHS was also involved in phenylpropanoid and flavonoid biosynthesis, indicating that these pathways might be candidates for responding to drought in roots.
In leaves, the DEGs in Dleaves versus CKleaves were significantly enriched in 21 GO terms. The terms with the lowest P-adjusted value were flavonoid biosynthesis and flavonoid metabolic processes along with CHS activity (Figure 5A). The enriched pathways included phenylpropanoid and flavonoid biosynthesis (Figure 5B), suggesting that these pathways might also be candidates for responding to drought in leaves.
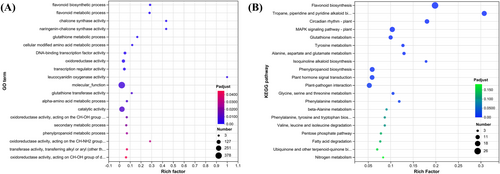
Similarly, some genes were identified as DEGs in roots and leaves under drought compared to control conditions, resulting in 100 common differentially expressed genes (co-DEGs; Figure 6A). Subsequently, these co-DEGs were subjected to enrichment analysis, as illustrated in Figures 6B-C. GO enrichment results revealed that chalcone and flavonoid biosynthesis might be candidates for responding to drought stress due to their low values (p < 0.05). However, flavonoid biosynthesis was an enriched pathway in the KEGG enrichment analysis, suggesting that the flavonoid synthesis pathway is one of the crucial pathways in response to drought. Additionally, chalcone plays an essential role in the enriched flavonoid biosynthesis pathway in GO and KEGG analyses. These results suggest that phenylpropanoid and flavonoid biosynthesis pathways might be candidates for responding to drought in leaves and roots through the RNA-Seq analysis.
3.4 Metabolome analysis of drought-treated and control plants
The samples, including root and leaf tissues in BC and D groups, were denoted as BCroots, Droots, BCleaves, and Dleaves for metabolome analysis. A total of four group samples were analyzed, with each group consisting of six biological replicates. Metabolites can be detected and analyzed in two modes: Positive ions and negative ions. The number of identifications under positive ions was 923, while the number under negative ions was 520 after preprocessing. The principal component analysis result indicated that the quality control data for the metabolomic analysis met the requirements, confirming that the metabolite data could be further analyzed (Figure 7A).
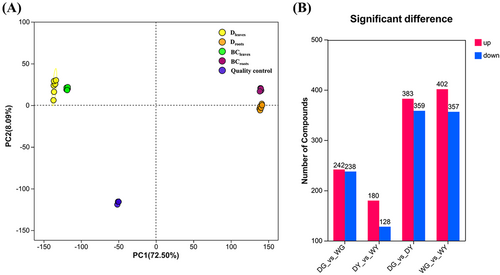
Moreover, fold change and P-value were used to screen the different altered metabolites (DAMs). Compared to CK, 480 DAMs were identified in roots under drought stress. Among these, 242 DAMs were up-regulated, while 238 DAMs were down-regulated. About 308 DAMs were identified in leaves under drought stress (180 up-regulated and 128 down-regulated). This number was less than the DAMS identified in the roots (Figure 7B). All these DAMs were set as candidate genes for enrichment analysis.
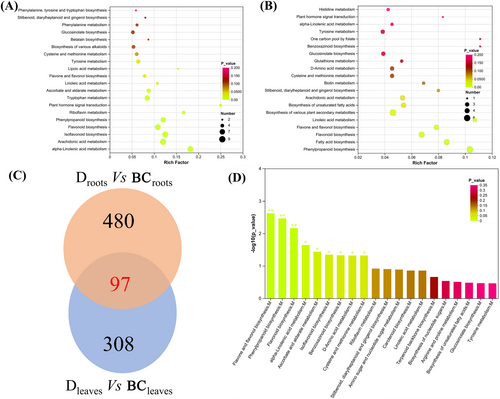
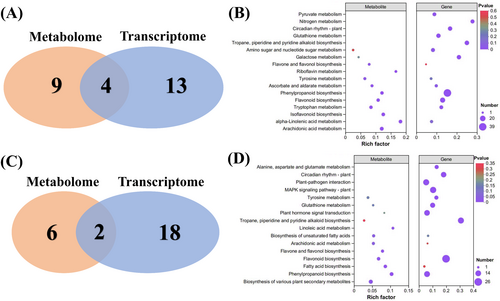
The mechanism of drought response in tiger nuts was investigated using metabolome analysis of these DAMs. The leaves and roots were the target tissues. In roots, the DAMs in Droots versus CKroots were enriched in 13 KEGG pathways (Figure 8A), while the DAMs in Dleaves versus CKleaves were enriched in eight KEGG pathways (Figure 8B). Five pathways were identified in roots and leaves under drought, including the phenylpropanoid and flavonoid biosynthesis pathways. Similarly, mutual metabolites, all identified as DAMs in roots and leaves during drought compared to CK, resulted in 97 candidate metabolites (Co-DAMs) (Figure 8C). These co-DAMs were subjected to KEGG enrichment analysis, suggesting the presence of phenylpropanoid and flavonoid biosynthesis pathways.
3.5 Transcriptome-metabolome analysis in response to drought
Four pathways enriched in roots were identified by combining transcription and metabolic analysis, including phenylpropanoid biosynthesis and flavonoid biosynthesis (Figures 9A-B). In leaves, phenylpropanoid and flavonoid biosynthesis were enriched by combining transcription and metabolic analysis (Figures 9C-D). These results suggest that phenylpropanoid and flavonoid biosynthesis respond to drought stress.
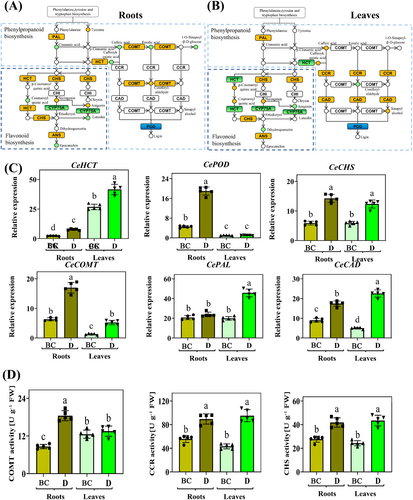
3.6 Enriched pathway analysis
Phenylpropanoid and flavonoid biosynthesis were identified as candidate pathways, enriched through transcriptomic and metabolomic analysis in leaves and roots. The data presented indicates that the expression of enriched genes and metabolite concentrations suggest distinct mechanisms in roots and leaves in response to drought stress (Figures 10A-B). In phenylpropanoid biosynthesis, the genes enriched in enzymes such as PAL and POD exhibited similar trends of change. The expression of genes enriched in HCT and COMT was up-regulated in roots. However, the expression of genes enriched in HCT was down-regulated and up-regulated in CCR and CAD. The contents of naringenin, CHS, and ANS in leaves and roots were up-regulated in flavonoid biosynthesis. However, the contents of other metabolites, including aoigenin and coumaroyl quinic acid, exhibited different trends of change. The expression levels of enriched genes such as CeHCT, CePOD, CeCHS, CeCOMT, CePAL, and CeCAD demonstrated similar changes in roots and leaves according to RTq-PCR analysis, and these trends were consistent with RNA-Seq results (Figure 10C). Furthermore, the activities of enriched enzymes (COMT, CAD, and CHS) revealed that phenylpropanoid and flavonoid biosynthesis were enriched in roots and leaves under drought. Conversely, the mechanisms of roots and leaves remain different (Figure 10D).
4 DISCUSSION
4.1 Phenotypic and physiological changes in leaves and roots
Drought, a type of water deficiency stress, causes various phenotypic changes (Kuromori et al., 2022). Roots directly sense drought stress, leading to retarded growth (Karlova et al., 2021), while leaves act as accepting tissues. The lack of available water during growth causes stomatal closure, resulting in retarded plant tissue growth and a decrease in biomass (Agurla et al., 2018). The results of phenotypes (digital biomass and leaf area) revealed an interesting phenomenon: The limitation ability of drought stress on the aboveground part (leaves) is greater than that on the underground part (roots). This phenomenon might be attributed to the ability of tiger nuts to suppress the growth of aboveground parts while maintaining the relative growth efficiency of roots. This may benefit tiger nuts by increasing water absorption and reducing water loss under water stress. Similarly, some crops, including maize (Zhang et al., 2022), soybean (Du et al., 2020), and rice (Xu et al., 2015), have been reported to exhibit this phenomenon under drought stress, suggesting that the response is a universal mechanism in plants.
Plants respond to drought stress with physiological responses as part of their evolution (Godwin and Farrona, 2020). The antioxidant enzyme system exhibited a significant effect in responding to drought stress, as the activities of some enzymes (such as SOD, POD, CAT, and APX) changed under drought stress. SOD catalyzes the conversion of O2− to H2O2. Subsequently, CAT, POD, and APX convert H2O2 to H2O, being a part of the enzymatic antioxidant defense system (Asada, 2006). However, some genes, such as ZMWRKY106 and ONAC066, may enhance drought stress through an antioxidant enzyme system (Wang et al., 2018; Yuan et al., 2019b). The resistance to drought is associated with the ability of the antioxidant enzyme system to remove ROS, as the activities of SOD and POD are up-regulated under drought (Zhang et al., 2022). In this study, the activities of SOD, POD, CAT, and APX were up-regulated in leaves and roots under drought (Figures 2A–D). The results were similar to soybeans (Zhang et al., 2022) and maize (Du et al., 2020) responding to drought. Furthermore, O2− and H2O2, as intermediate products of various biochemical reactions, exhibited strong oxidizing properties and the ability to damage membrane systems. However, the accumulation of contents under adverse conditions was an essential factor of damage (Yan et al., 2021a). To assess the degree of damage under stress, MDA is used as a byproduct of plant membrane lipid peroxidation (Xu et al., 2023). The contents of MDA, O2−, and H2O2 were tested, with drought stress stimulating the content of these physiological indicators. The results for tiger nuts were similar to those observed in other crops (Zou et al., 2021).
Breeding and cultivation are two important ways to enhance crop drought tolerance. Mining genotypes by comparing varieties with significant phenotypic differences is also an important approach to drought-tolerant breeding (Oladosu et al., 2019). Although the genome of tiger nuts has been identified, establishing it as a new economic crop and exploring different genotypes for molecular breeding remains a long and challenging process. Exploring novel pathways and enhancing drought tolerance through response mechanisms is an important way to address the current problem. Similarly, investigating drought-responsive pathways in other crops is also a meaningful approach (Zou et al., 2021).
4.2 Phenylpropanoid and flavonoid biosynthesis
The phenylpropanoid biosynthesis pathway is a crucial secondary metabolic pathway that is closely associated with drought response (Cesarino, 2019). It has been reported that the components enriched in this pathway might respond to drought stress. PAL catalyzes L-phenylalanine to produce trans-cinnamic acid, which converts phenylalanine into cinnamic acid (Xie et al., 2020). The expression of PAL is elevated at different rates in response to drought (Kisa et al., 2021). Increasing PAL activity may promote the accumulation of phenolic and flavonoid content, enhancing drought resistance (Hossain et al., 2020), thereby indicating its relation with drought stress. COMT catalyzes multi-step methylation reactions, while CAD catalyzes the conversion of cinnamaldehydes into corresponding alcohols, enhancing stress tolerance by regulating lignin synthesis (Khasin et al., 2021). The activities of COMT and CAD increased, suggesting that they might regulate metabolites and lignin synthesis in response to drought. COMT and CAD have been linked to drought (Zhou et al., 2020). The increased activity of COMT may enhance drought tolerance by regulating the synthesis of lignin and melatonin (Huang et al., 2024), while increasing CAD activity may enhance drought tolerance through H2O2 and lignin accumulation (Yan et al., 2021b).
Flavonoid biosynthesis pathway is a downstream branch of phenylpropanoid biosynthesis (Zhang et al., 2023). It reduces oxidative damage and acts as an antioxidant under stress (Collin et al., 2020). Treatment with flavonoid metabolites enhances stress tolerance (Liang et al., 2022). Drought altered the flavonoid synthesis by regulating the expression of CHS (Zhang et al., 2020). CHS is an enzyme that catalyzes the formation of naringen chalcone. The drought tolerance in crops might be associated with changes in enzyme activity and the expression of CHS (Li et al., 2015). Therefore, the activity and expression of CHS changed (Figure 10), indicating its significance in responding to drought. Moreover, an enriched pathway was identified in which CHS may be induced in response to drought (Zhang et al., 2023). These results revealed that phenylpropanoid and flavonoid biosynthesis pathways were crucial in the response of tiger nuts to drought, with COMT, CAD, and CHS being the enriched components.
4.3 Response mechanism in leaves and roots
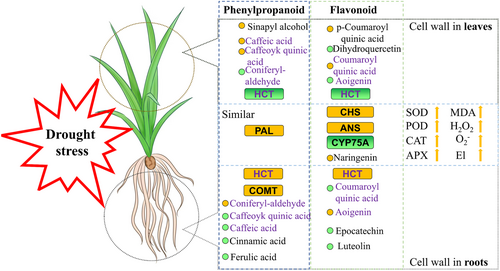
Plants exhibit tissue-specific expression, and the mechanisms by which different tissues respond to stress may vary (Cao et al., 2022). Although drought stress affects the entire plant system, some differences exist in the response and coordination in different tissues (Ackah et al., 2022). In this study, phenylpropanoid and flavonoid biosynthesis were co-enriched pathways through transcriptome and metabolome under drought stress. However, the mechanisms in roots and leaves were different. These findings were similar to the soybean (Du et al., 2020) and maize (Zhang et al., 2022) results. Although some metabolites and enriched enzymes (PAL, CHS, and ANS) exhibited similar changes under drought in roots and leaves, other metabolites and enzymes exhibited different responses. Some metabolites (caffeic acid, caffeoyl quinic acid, coniferyl-aldehyde in phenylpropanoid biosynthesis, and coumaroyl quinic acid and aoigenin in flavonoid biosynthesis) displayed opposite changes under drought stress, suggesting that the mechanism by which roots and leaves respond to drought stress differs in tiger nut (Figure 11). The impact of drought on the leaves of plants without significant changes in the roots, suggests the involvement of a different mechanism. Some crops exhibited a similar phenomenon; however, the mechanisms for drought stress in roots and leaves differed between rice and soybean (Xu et al., 2015), although the physiological changes of different organs were similar. Notably, one main planting variety has a distinct degree of individuality for excavator cultivation and may not be applicable to the entire species. However, Jisha 2, the main planting variety in northeast China (Mu et al., 2022), has a coverage rate that exceeds that of other varieties. This study offers valuable and referenceable information for improving local research, providing insights into the mechanisms underlying drought responses in leaves and roots.
5 CONCLUSION
The response of tiger nuts to drought stress was analyzed based on phenotypes. The results revealed that the restrictions on the leaves were greater than those caused by drought. The physiological indicators demonstrated that although all data varied in leaves and roots, the changes were more pronounced in the leaves. Phenylpropanoid and flavonoid biosynthesis were identified as candidate pathways using transcriptome and metabolome analyses, while the response modes in the roots and leaves differed according to enriched pathways analysis. The results provided insights into the molecular mechanism underlying drought in tiger nuts, particularly the synergistic response between leaves and roots. This understanding contributes to improving the drought tolerance of tiger nuts.
AUTHOR CONTRIBUTIONS
Z.Q. and Y.C. were responsible for the data curation and the writing of the original draft. Y.G., R.L. and H.L. did the data curation. J.Y, J.G. and M.L. participated in the conceptualization, methodology and software. C.L., Y.L., Q.X. and H.W. did the formal data analysis and preparation of the materials. J.L., X.S. took part in the methodology. Z.M. and J.D. did the conceptualization, data curation and funding acquisition. All authors reviewed manuscript.
ACKNOWLEDGMENTS
Thanks to Jilin Academy of Agricultural Sciences and Bayi Agricultural University for their support in terms of experimental materials and funds.
FUNDINGS
This study was financially supported by Heilongjiang Bayi Agriculture University (XYB202201) and Jilin Province agricultural science and technology innovation project (CXGC2023RCB005).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




