Comparison of different methods to evaluate tissue damage in response to leaf dehydration in Quercus ilex L. and Q. faginea Lam
Abstract
Determination of the point of critical damage in plant organs is crucial to elucidate the causes of plant mortality, but the different methodologies to quantify such damage have not been previously compared under the same experimental conditions. Here, we tested different indicators to evaluate damage in leaves of Quercus faginea and Q. ilex; in the latter case, 1- and 2-year-old leaves were included. The damage indicators were relative electrolyte leakage (REL), rehydration capacity (evaluated as the percentage loss of rehydration capacity; PLRC), chlorophyll fluorescence (maximum quantum yield of PSII; Fv/Fm), and the viability marker triphenyltetrazolium chloride (TTC). These damage indicators were evaluated in different sets of detached leaves for each species and leaf age dehydrated on the lab bench. Electrolyte leakage and PLRC showed a gradual response to decreasing relative water content, whereas Fv/Fm and TTC showed a threshold-like response, especially in the case of Q. faginea. Electrolyte leakage and TTC did not show differences between species and/or leaf ages. Measurement of Fv/Fm in dehydrating leaves proved to be the most straightforward, rapid and precise method for damage quantification, allowing for the differentiation in dehydration tolerance between Q. ilex and Q. faginea.
1 INTRODUCTION
In terrestrial plants, water stress due to limited water availability can ultimately lead to critical dehydration of the different organs and tissues (leaves, stems, and roots) beyond the point of recovery, eventually resulting in the death of the individual (Guadagno et al., 2017; Trifilò et al., 2023; Alon et al., 2024). In recent years, there has been an increasing number of studies concerning the causes of plant death and organ damage, with a special focus on the link between hydraulic failure and the death of the surrounding tissue, especially in leaves and stems (Brodribb et al., 2021; Mantova et al., 2021; Johnson et al., 2022; Mantova et al., 2023). Given the importance of drought stress in plant productivity and mortality events under present and future conditions (Xu et al., 2019; McDowell et al., 2022), furthering our knowledge regarding the response and timing of tissue dehydration under severe drought conditions is of great relevance for coping with climate change.
One of the key aspects to consider in the study of drought-induced mortality is the quantification of critical (i.e., irreversible) damage in plant tissues, especially the water status (in terms of water potential or water content) at which a significant level of damage occurs. There is increasing recognition of embolism formation being a key trigger of irreversible damage leading to plant mortality (Brodribb et al., 2021; Mantova et al., 2022, 2023). Xylem embolism thresholds—such as P50, the water potential at which 50% conductivity is lost—and their interaction with minimum water potential (i.e., the hydraulic safety margin) are emphasized as primary indicators of plant vulnerability to severe drought (Delzon & Cochard, 2014; Sanchez-Martinez et al., 2023; Ziegler et al., 2023). However, despite the development of new techniques such as the ‘optical method’ for evaluating embolism formation across different organs (Brodribb et al., 2016, 2017), most methodologies for the quantification of embolism formation are still relatively expensive and time-consuming, especially when considering high-resolution X-ray microtomography (Cochard et al., 2015), or constrained by the morphology of the organs to evaluate (e.g., thick or non-flat leaves are difficult to measure using the optical method). In addition, the measurement of embolism events represents the cause of damage, but it is not necessarily the measurement of damage per se. To this end, several methods have been utilized to characterise tissue damage (hence after, ‘damage indicators’), namely electrolyte leakage, rehydration capacity, viability markers and, in photosynthetic organs, chlorophyll fluorescence. The present work represents an effort to evaluate and compare these different indicators to quantify the damage caused by dehydration in leaves.
Electrolyte leakage has been a widely utilized technique to quantify damage in different plant organs in response to a wide array of environmental factors, such as water or osmotic stress (Bajji et al., 2002), heat and cold (Agarie et al., 1998; Campos et al., 2003), radiation (Fan & Sokorai, 2005), and biotic factors such as pathogens (Pike et al., 1998) and senescence (Rolny et al., 2011; Demidchik et al., 2014). The main principle is that the leakage of solutes from the cells affected by a given stress reflects the loss of integrity of cellular membranes (Leopold et al., 1981), resulting in an increase in organic and inorganic ions in the medium (Bajji et al., 2002), especially K+ (Demidchik et al., 2014). Although electrolyte leakage induced by water stress was primarily described with the application of polyethylene glycol (PEG; e.g., Bajji et al., 2002), it is also commonly used to evaluate the effects of pot drought and/or direct dehydration by excision of leaves or other parts of the plant (Blum & Ebercon, 1981; Trifilò et al., 2023). A potential drawback of this technique is that electrolyte leakage can also reflect increases in ion concentration without necessarily reflecting membrane damage, as reported for ammonium accumulation during leaf senescence (Rolny et al., 2011).
The leaf rehydration capacity is a widely used indicator of drought tolerance across different species and environments (John et al., 2018 and references therein). It evaluates the capacity of excised leaves to rehydrate after being subjected to different degrees of dehydration, originally considering as ‘drought-tolerant’ those species able to rehydrate (around 90% of initial weight) after experiencing substantial water loss (Oppenheimer & Leshem, 1966). More recently, John et al. (2018) reformulated this index as ‘percentage loss of rehydration capacity’ (PLRC), subsequently defining different PLRC thresholds (10, 25 and 50% loss of rehydration capacity) to evaluate drought tolerance in relation to other indices (e.g., water potential at turgor loss point and modulus of elasticity; Bartlett et al., 2012). A great advantage of the rehydration capacity method is the minimal equipment requirements, allowing for the screening of a high number of species in the field (Fortunel et al., 2023).
Several viability markers have been used to evaluate tissue damage, such as Evans blue (Baker & Mock, 1994), triphenyltetrazolium chloride (TTC) and neutral red (Oppenheimer & Leshem 1966). TTC is ubiquitously used to test the viability of seeds (International Seed Testing Association, 2014; Lopez Del Egido et al., 2017) but can also be applied to roots and leaves (Lin et al., 2001; Ruf & Brunner, 2003). The methodology is based on the reduction of the colourless TTC to red formazan by dehydrogenases present in the respiratory chain of mitochondria (Rich et al., 2001); the resulting red colouring of the samples is directly proportional to the respiratory activity of the tissue. Hence, the viability of a sample can be evaluated either with spectrophotometry or image analysis (Alejo-Jacuinde et al., 2022; Jaconis et al., 2021), with a diminished red colouration indicating greater tissue damage. This methodology has been utilized to evaluate different stresses in plant tissues, such as damage by drought (Nayyar et al., 2005), heat (Jaconis et al., 2021) and cold (Lin et al., 2001). Additionally, it has recently been described as a good evaluator of desiccation tolerance (i.e., recovery after extreme water loss in vegetative tissues) in leaves of Selaginella and fern species (Alejo-Jacuinde et al., 2022).
In the case of leaves or photosynthetic twigs (e.g., Johnson et al., 2022), tissue damage can also be evaluated using chlorophyll fluorescence. Among the different fluorescence parameters (see Murchie & Lawson, 2013), the maximum quantum yield of PSII (Fv/Fm) is the most commonly used to evaluate damage in leaves (e.g., Sancho-Knapik et al., 2018; Alonso-Forn et al., 2021). Fv/Fm decreases below 0.83 can result in the inactivation damage of PSII (photoinhibition) (Murchie & Lawson 2013). Several studies have used the decrease of Fv/Fm during dehydration as an indicator of cellular damage (Woo et al., 2008; Liu et al., 2014; Trueba et al., 2019; Mielke et al., 2024), attributed to the impairment of photosystem activity due to membrane damage (Guadagno et al., 2017). The use of this non-invasive technique allows to pair measurements of chlorophyll fluorescence with the monitoring of embolism formation, hence exploring the correlation between tissue damage and hydraulic failure in photosynthetic organs (Brodribb et al., 2021; Johnson et al., 2022). The recovery of Fv/Fm after rehydration is an additional measure of the viability of leaves subjected to dehydration; this procedure (together with TTC and electrolyte leakage) is regularly used to confirm the presence of desiccation tolerance mechanisms in the so-called ‘resurrection plants’ (Vander Willigen et al., 2001; Farrant et al., 2009; López-Pozo et al., 2019).
Most articles aiming at the quantification of tissue damage by dehydration include one or two of these methodologies. Some studies have combined two methods to evaluate if a given damage indicator (e.g., chlorophyll fluorescence, rehydration capacity) could be compared to a more established reference (usually considered to be electrolyte leakage and viability markers) (Oppenheimer & Leshem, 1966; Lin et al., 2001; Alejo-Jacuinde et al., 2022). However, to the best of our knowledge, no study has focused on the comparison among the four methods described above, which represent the most commonly utilized indicators for the quantification of critical damage in plant tissues. The objectives of the present study are twofold: (1) to compare the different damage indicators during leaf dehydration in order to establish if they present a similar response to water loss, and (2) to test which damage indicators enable the distinction of species' responses to dehydration in leaves, that is, to evaluate if the studied species present differences in dehydration tolerance. To this end, we evaluated the different methods in two Mediterranean oaks that co-occur in the inland areas of the Iberian Peninsula: the evergreen sclerophyllous Quercus ilex L. and the winter-deciduous malacophyllous Quercus faginea Lam. Although both species show a high degree of drought tolerance (Forner et al., 2018), they constitute two examples of different functional strategies to cope with drought in terms of leaf habit, photosynthetic, photochemical and hydraulic traits (Peguero-Pina et al., 2016; Gil-Pelegrín et al., 2017; Alonso-Forn et al., 2021).
2 MATERIALS AND METHODS
2.1 PLANT MATERIAL
The experiment was performed at the end of the 2023 summer (September and October) using adult trees (c. 5 m height) of Quercus faginea and Q. ilex growing outdoors in an experimental field at CITA de Aragón (41° 39’ N, 0° 52’ W, Zaragoza, Spain; mean annual temperature of 15.4°C, total annual precipitation of 298 mm). Plants are kept under well-watered conditions by regular drip irrigation. Measurements were conducted in fully-developed leaves from branches collected in the morning, recut under water to avoid embolism formation (Torres-Ruiz et al., 2015), and rehydrated for approx. 24 h in the lab under dark conditions. In the case of Q. ilex, 1-year-old (hence after ‘young’ leaves) and 2-year-old leaves (‘old’ leaves) from the same branches were considered for all measurements.
2.2 Chlorophyll fluorescence and rehydration capacity
A set of c. 40 leaves per species and leaf age was used to evaluate the dynamics of chlorophyll fluorescence and rehydration capacity during dehydration and subsequent rehydration. The maximum quantum yield of photosystem II (Fv/Fm) was measured using an FMS II modulated fluorometer (Hansatech Instruments, Norfolk, UK). Only leaves that showed Fv/Fm higher than 0.77 at this initial stage were used in the assay. Leaves were excised, embedded in moist paper and rehydrated through the petiole in 50 mL Falcon tubes for approx. 12 h to obtain the hydrated weight (HW). Then, leaves were left to dehydrate in the lab bench for a variable time period to obtain the full range of relative water content (RWC), from high hydration to complete water loss. The dehydration time ranged from a few minutes to 96 h, a similar time frame as described in Trifilò et al. (2023) for leaves of Q. ilex (c. 100 h). At this second stage, fresh weight (FW) and chlorophyll fluorescence were measured to obtain Fv/Fm during dehydration (CFD). Then, leaves were wrapped with moist paper and rehydrated through the petiole for 24 h in Falcon tubes to obtain the rehydrated weight (RW) and Fv/Fm after rehydration (CFR). We used hydration and rehydration periods of 12 and 24 h, respectively, following John et al. (2018), which showed no oversaturation effects when rehydrating for 8 to 24 h across different species. Two measurements (technical replicates) of Fv/Fm were performed at each stage in each leaf in different positions within the leaf after waiting several minutes. Leaves were kept under low light conditions throughout the assay to minimize non-dehydration effects that may cause additional photoinhibition (Trueba et al., 2019). Leaves were dark-adapted for at least 12 h in all cases before fluorescence measurements. Finally, leaves were dried in the oven for at least 72 h to obtain the dry weight (DW). Leaf relative water content was calculated as RWC = (FW − DW)/(HW − DW) × 100, and the percentage loss of rehydration capacity as PLRC = 100 − RW/HW × 100.
2.3 Relative electrolyte leakage
A second set of c. 65 leaves per species and leaf age was used for measurements of relative electrolyte leakage (REL) during dehydration. Leaves were excised and rehydrated as described above to obtain the hydrated weight (HW), and dehydrated in the lab bench for a variable time period (from a few min to c. 100 h) to obtain the complete range of relative water content. Fresh weight (FW) was measured, and immediately afterwards, two discs (1.1 cm in diameter) were cut from each leaf and placed in separate Flacon tubes with 15 mL distilled water and stirred periodically. After 1 h, initial electrical conductivity (ECi) was measured using a conductivity meter (Orion 5-Star, 013005MD conductivity cell; Thermo Fisher Scientific) twice in each sample to account for additional incubation time during conductivity measurements. Then, the tubes were boiled for 15–20 min, and the final electrical conductivity (ECf) of the solution was measured. Tubes were weighed before and after boiling to ensure minimal water loss during measurements that could affect conductivity. Finally, relative electrolyte leakage (REL) was calculated as REL = (ECi/ECf) x 100. REL for a given leaf is the average of the two discs (considered as technical replicates). RWC for a given leaf was calculated using dry weight (DW) estimated from leaf HW and mean leaf dry matter content (LDMC, see below) per species and leaf age.
2.4 Triphenyltetrazolium chloride staining
Where Rdisc,i and Rwhite,i correspond to the mean grey value (red in RGB) of the leaf discs and white reference at the i stage (after 24 or 48 h of TTC incubation), and Rdisc,o and Rwhite,o correspond to the mean grey value of the same samples and white reference prior to TTC incubation. RWC for a given leaf was calculated using dry weight (DW) estimated from leaf HW and mean LDMC (see below) per species and leaf age.
2.5 Leaf bulk structure
In the first set of leaves used for the chlorophyll fluorescence and rehydration capacity assay, leaf area (LA) and leaf thickness (LT) were measured in the hydrated stage. Photographs were taken on a flat surface and analysed using ImageJ; leaf thickness was assessed with a digital micrometre (Series 293 Digimatic micrometer, 0.001 mm resolution) as the average of six measurements along the leaf lamina. Leaf mass per area (LMA) was calculated as LMA = DW/LA, leaf density (LD) as LD = LMA/LT and leaf dry matter content (LDMC) as LDMC = DW/HW.
2.6 Statistical analysis
All statistical analyses were conducted using the R software (R Core Team, 2023). A unique categorical factor was considered in all analyses (‘Species, age’) consisting of three levels (Q. faginea, young Q. ilex and old Q. ilex leaves). Differences in leaf bulk structure were assessed by one-way ANOVA and Tukey's honest significant differences (HSD) test. To analyse the response of a given damage indicator (CFD, CFR, PLRC, REL and RI) to decreasing RWC, an extensive set of functions were tested for best model fitting (Table S1). Lineal, sigmoidal and exponential fittings were included following Trueba et al. (2019) and fitted using the functions lm and nls (‘stats’ package) for linear and nonlinear least squares, respectively. Weibull, log-logistic and other fittings (Table S1) were fitted as dose–response curves using the drm function (‘drc’ package; Ritz et al., 2015). Two models were evaluated for each fitting: a simpler model where no distinction between species and leaf age was made (‘pooled’ data), and a model considering the three levels separately as a fixed effect (‘separated’ data). The best model for a given damage indicator, including both different fittings and pooled vs. separated data, was selected based on the Akaike information criterion (AIC). Whenever appropriate, the differences among species and leaf age for the fitted parameters were assessed using the compParm function in the ‘drc’ package, which allows pairwise evaluation of significant differences (p < 0.05) in models containing two or more levels. The relative water content at which a given indicator changed by 10 or 50% (relative to its maximum value) was calculated using the ED50 function in the ‘drc’ package (i.e., an effective dose of 50%, equivalent to a change of 50%). The percentage changes are represented by subscript indices for each damage indicator. For example, CFD10 refers to the RWC at which Fv/Fm decreases by 10% during dehydration, while PLRC50 refers to the RWC at which the percentage loss of rehydration capacity increases by 50%. Confidence intervals (95%) were obtained using ED50 and plotted using the predict function from the ‘stats’ package (Ritz et al., 2015).
3 RESULTS
Both young (<1-year-old) and old (>1 year) leaves of Q. ilex presented higher leaf mass per area (LMA of 204 and 210 g m−2 for young and old leaves, respectively) than leaves of Q. faginea (LMA of 149 g m−2) (Table 1). Higher LMA in Q. ilex leaves resulted from the combination of thicker and denser leaves; young Q. ilex leaves presented the highest thickness (LT of 0.389 mm), while old Q. ilex leaves showed the highest density (LD of 0.596 g cm−3). Q. faginea presented lower LT and LD (0.298 mm and 0.498 g cm−3, respectively) than Q. ilex. Leaf dry matter content showed the same pattern as LD among the two species and leaf ages (Table 1).
| Species | LMA (g m−2) | LT (mm) | LD (g cm−3) | LDMC (g g−1) |
|---|---|---|---|---|
| Q. faginea | 148.5 ± 7.7 b | 0.298 ± 0.013 c | 0.498 ± 0.019 c | 0.460 ± 0.009 c |
| Q. ilex, 1 < year | 204.0 ± 19.6 a | 0.389 ± 0.042 a | 0.522 ± 0.020 b | 0.505 ± 0.017 b |
| Q. ilex, 1 > year | 210.0 ± 17.2 a | 0.352 ± 0.025 b | 0.596 ± 0.031 a | 0.542 ± 0.020 a |
| ANOVA | ||||
| F value | 192.6 | 100.6 | 188.7 | 279.8 |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
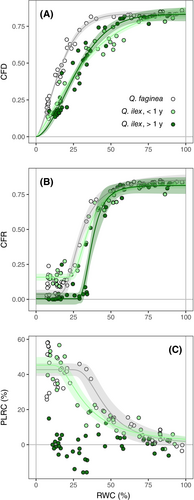
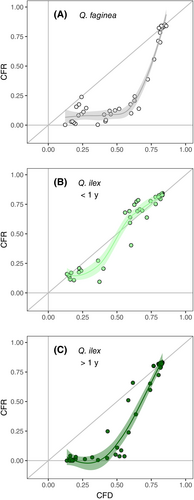
All indicators of leaf damage showed a significant change in response to decreasing water content. Different model fittings for each indicator are displayed in Table S2 for both ‘pooled’ and ‘separated’ data (i.e., if species and leaf age were considered jointly in the model or as a fixed effect). In the case of Fv/Fm (both CFD and CFR) and PLRC, significant differences were detected between species and leaf age (Figure 1). These three indicators were best described by a Weibull curve (type I for CFR and type II for both CFD and PLRC; Table S2). In the case of CFD, there was a clear threshold-like response: Fv/Fm only showed a minimal decrease from 100 to approx. 60% RWC in the case of Q. ilex (old and young leaves), and from 100 to 40% RWC in Q. faginea (Figure 1a). Then, Fv/Fm steeply decreased, reaching values around 0.1 at 15% RWC in both species and leaf ages. The decline of CFD was different among the two species (Table 2): Q. faginea showed an overall lower CFD50 (14.62%), whereas there were no differences between young and old Q. ilex leaves (26.45 and 25.72%, respectively). The steep decline of Fv/Fm below a certain RWC threshold occurred at a similar rate among the two species, as indicated by the lack of differences in the slopes of the respective fittings (Table S3). CFR was consistently lower than CFD measured in the same leaves (Figure 2), indicating a total lack of recovery of Fv/Fm after rehydration. Hence, although Fv/Fm showed a similar response during dehydration and after rehydration, CFR50 was slightly higher than CFD50 for the two species and leaf ages (Figure 1b, Table 2). Old Q. ilex leaves showed significantly higher CFR50 than young leaves and, as in the case of CFD50, Fv/Fm decrease in Q. faginea occurred at lower RWC than in Q. ilex (Table 2).
| Damage indicator | Q. faginea | Q. ilex, 1 < year | Q. ilex, > 1 year | Fitting | |
|---|---|---|---|---|---|
| Fv/Fm | CFD10 | 32.94 (29.79, 36.08) |
68.50 (52.92, 84.07) |
51.59 (43.60, 59.58) |
W2.3 |
| CFD50 | 14.62 a (13.89, 15.35) |
26.45 b (23.10, 29.80) |
25.72 b (23.61, 27.84) |
W2.3 | |
| CFR10 | 51.36 (39.74, 62.99) |
52.20 (45.37, 59.04) |
48.77 (39.45, 58.08) |
W1.4 | |
| CFR50 | 29.62 a (27.63, 31.62) |
34.74 b (33.18, 36.30) |
37.49 c (35.34, 39.64) |
W1.4 | |
| Rehydration capacity | PLRC = 10% | 60.75 (48.76, 72.73) |
54.76 (46.42, 63.10) |
W2.3 | |
| PLRC = 25% | 43.73 (37.19, 50.27) |
31.41 (26.20, 36.62) |
W2.3 | ||
| PLRC50 | 46.70 a (39.79, 53.61) |
33.69 b (28.47, 38.91) |
W2.3 | ||
| Electrolyte leakage | REL50 | 44.52 a (30.88, 58.15) |
37.82 a (22.39, 53.24) |
38.63 a (−5.38, 82.65) |
W2.3 |
| Red intensity (TTC) | RI10 | 22.87 (11.43, 34.31) |
20.26 (9.15, 31.36) |
26.95 (20.75, 33.15) |
W2.4 |
| RI50 | 13.15 a (1.88, 24.43) |
14.92 a (7.75, 22.09) |
23.30 a (18.43, 28.17) |
W2.4 | |
Although PLRC response was also best described by a Weibull curve, it did not display a clear threshold when compared to Fv/Fm (Figure 1c). In fact, AIC values for exponential fittings were relatively close (albeit slightly higher) to those of the Weibull fittings (Table S2), indicating a more gradual increase of PLRC with declining RWC. The data for old Q. ilex leaves was noisy, and PLRC did not show a significant response to declining RWC. PLRC in Q. faginea and young Q. ilex leaves showed a clear increase with decreasing RWC, and allowed differentiation between the two species. PLRC50 (when PLRC reached approximately 25%; Table 2) occurred at higher RWC in Q. faginea compared to young Q. ilex leaves (46.70 and 33.69%, respectively; Table 2).
No differences between species and leaf ages were detected for electrolyte leakage nor the TTC assay (Figure 3). REL showed a similar response to that of PLRC (Figure 3a); although it was best described by a Weibull curve, AIC values for exponential fittings were also close to those obtained for log-logistic and Weibull models (Table S2), thus indicating a gradual increase in REL with declining water content. However, in contrast with PLRC, no differences were detected among the fitted curves for either species or leaf ages (Table 2). REL50 (when REL reached approximately 17%) was 40.29% on average for both species and leaf ages. Despite lower AIC value for the ‘separated’ compared to the ‘pooled’ data model (Table S2), no clear differences among fitted parameters were detected for REL (Tables 1 and S3).
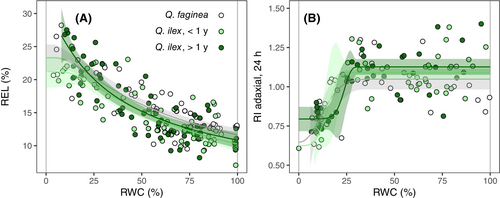
Regarding the TTC assay, only changes in red intensity in the adaxial surface of leaf discs after 24 h of TTC incubation are presented as indicators of tissue damage (Figure 3b). RI in adaxial and abaxial surfaces after 24 h showed a weak correlation (R2 = 0.19 for all pooled data; Figure S2a), and RI from the abaxial surface did not change with declining water content (Figure S2b). RI after extended incubation time (48 h) was slightly higher but positively correlated to the intensity obtained after 24 h of incubation (R2 = 0.55 for all pooled data; Figure S2c). RI in the adaxial surface did decrease with declining RWC, showing a threshold-like response best described by a type II Weibull curve fitting (Figure 3b, Table S2). No changes in RI occurred until leaves reached approx. 30% RWC, and then intensity declined sharply below 25% RWC. RI50 was 17.24% on average for both species and leaf ages. No differences were detected among either species or leaf ages (Table 2).
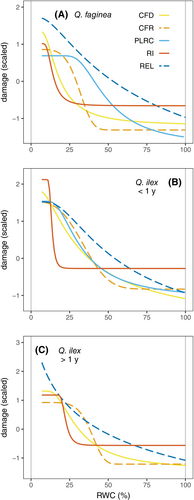
The different indicators of leaf damage are displayed for each species and leaf age in Figure 4. In all three species and leaf ages, red intensity (adaxial surface, 24 h of TTC incubation) presented the clearest threshold-like response, with significant changes only occurring at very low relative water content (approx. RWC below 25%). On the other hand, electrolyte leakage showed a gradual response to declining RWC. Indeed, RI50 and REL50 were the lowest and highest, respectively, across species and leaf ages (Table 2). The declines in Fv/Fm and rehydration capacity occurred between the responses of REL and red intensity, although some differences were detected among the two species. In the case of Q. faginea, the response of CFD resembled the response of RI (CFD50 of 14.62 and RI50 of 13.15%), whereas PLRC followed a gradual increase, more similar to that of REL (PLRC50 of 46.70% and REL50 of 44.52%; Figure 4a). In contrast, for both young and old Q. ilex leaves, the change in CFD showed an intermediate response, with an overall shape closer to that of REL (Figure 3b and c). The response of PLRC in the case of young Q. ilex leaves was similar to that of CFD and CFR (Figure 4b). Again, this is reflected in the sequence of indicator thresholds shown in Table 2, especially in the case of young Q. ilex leaves, where REL50, PLRC50, CFD50 and RI50 were 37.82, 33.69, 26.45 and 14.92%, respectively.
4 DISCUSSION
The present study compares four different methodologies as indicators of damage in leaves: electrolyte leakage, rehydration capacity, viability using TTC, and chlorophyll fluorescence (during dehydration and rehydration). Other studies have compared several damage indicators (Trueba et al., 2019; Abate et al., 2021; Alejo-Jacuinde et al., 2022; Azzarà et al., 2022; Trifilò et al., 2023; Sapes et al., 2024), but to the best of our knowledge this is the first study analysing four different indicators under the same experimental conditions. All damage indicators responded as expected to a decrease in relative water content in all cases (with the notable exception of PLRC in old Q. ilex leaves, discussed below), although the type of response (fitted curve) varied among them. Electrolyte leakage and PLRC showed a gradual response to dehydration, whereas Fv/Fm and the TTC assay displayed a ‘threshold-like’ response, with a similar, low CFD50 and RI50 (13–26% RWC) compared to REL50 and PLRC50 (33–47% RWC; Table 2). However, not all of the damage indicators showed differences when comparing the response of Q. faginea and Q. ilex: CFD50, CFR50 and PLRC50 were distinct among the two species, whereas no differences were detected in terms of RI50 and REL50 (Table 2).
Progressive leaf dehydration first results in the loss of volume (shrinking) of mesophyll cells, with only minor damage in the first stages (Momayyezi et al., 2022; Mantova et al., 2023), corresponding to the turgor loss point of Q. faginea and Q. ilex (82–87% RWC; Forner et al., 2018). This first stage is somewhat reflected by gradual increases in electrolyte leakage and PLRC (Figure 4). Both indicators are reported to increase during the first stages of dehydration and drought (RWC of 60–80%) across leaves, roots and stems (Azzarà et al., 2022; Trifilò et al., 2023). Only in the case of herbaceous Salvia species do PLRC and REL show a more threshold-like response (Abate et al., 2021). In any case, the two damage indicators are closely correlated (Azzarà et al., 2022; Trifilò et al., 2023), indicating that PLRC also likely reflects cell membrane damage, as established in the case of electrolyte leakage in water and osmotic stress experiments (Bajji et al., 2002; Guadagno et al., 2017). The gradual increase of PLRC with decreasing RWC would indicate progressive damage to a proportional number of cells, thus hindering the complete rehydration of the leaf (John et al., 2018). This gradual response and potentially heterogeneous damage of leaves contrasts with the threshold response of chlorophyll fluorescence and the TTC assay (Figure 4).
Testing the loss of rehydration capacity is the simplest and the least equipment-demanding of all the evaluated methods, in addition to being associated with other indices of drought tolerance, such as the turgor loss point (John et al., 2018). However, in the present study, we obtained some contradictory results regarding PLRC. First, it did not yield clear results in the case of old Q. ilex leaves (Figure 1c). In the previously detailed PLRC responses (John et al., 2018; Trueba et al., 2019), all species show a significant trend towards the loss of rehydration capacity with dehydration, although some of them presented a significant degree of noise in the PLRC-RWC relationship (r2 of 0.4–0.5). None of the studied species in the aforementioned works showed a lack of response of PLRC, as observed here for old Q. ilex leaves. Oversaturation could be a source of error in the measurement of PLRC. Although John et al. (2018) reported no oversaturation even after 24 h of rehydration across different species, other studies indicate that it may occur in the 4–20 h range (Arndt et al., 2015). In Trifilò et al. (2023), among 6 samples (leaves) of Q. ilex, only 1 did show signs of oversaturation in the 12–24 h range, with no clear differences among fully hydrated and dehydrated samples. The fact that we obtained a very different PLRC response between young and old leaves in the same species hinders the identification of any morphological or biochemical feature that could compromise this methodology in evaluating leaf damage. This result questions, to some extent, the applicability of this methodology across different species and/or phenological stages. In the case of Q. faginea, although PLRC showed a clear increase with progressive dehydration, there was significant noise in the 0–25% RWC range (Figure 1c), which could affect the accuracy of the fitted PLRC thresholds. As mentioned above, the rehydration assay can yield relatively noisy results, especially when samples reach extreme dehydration (John et al., 2018; Trueba et al., 2019). An additional shortcoming of PLRC is the contradictory results in terms of dehydration tolerance when compared to chlorophyll fluorescence. In the case of Fv/Fm during both dehydration and rehydration, Q. faginea showed a lower CFD50 and CFR50 compared to Q. ilex, whereas they showed the opposite trend in terms of PLRC (Table 2). These differences could arise from these two indicators reflecting different types of damage in leaves (see discussion below on the significance of Fv/Fm), with PLRC probably being more associated with cell membrane rupture (cytorrhysis; Mantova et al., 2023), as reflected by its closer relationship with electrolyte leakage (Figure 4; Abate et al., 2021; Azzarà et al., 2022; Trifilò et al. 2023). The complementary use of Fv/Fm and PLRC could give further insight into the mechanisms of leaf death, although more studies -and perhaps a refinement of the PLRC methodology- are required to ensure the universal applicability of the rehydration capacity assay. We advise to test the reliability of PLRC for a given species or set of leaves (to avoid potential oversaturation effects; Arndt et al., 2015; Trifilò et al., 2023) and to use it only when no other methodology is available or possible.
Despite being well-established methods to evaluate damage in all types of plant tissues, electrolyte leakage and the TTC assay did not allow for differentiation in the dehydration tolerance between the two species and leaf ages (Table 2). These two methods presented a higher degree of noise and scattering of data points (Figure 3), hindering the detection of the differences between Q. faginea and Q. ilex, in contrast with PLRC, CFD and CFR. To the best of our knowledge, the use of viability markers in plants is restricted to either seed viability (Lopez Del Egido et al., 2017) or to a qualitative test for establishing if a given leaf is dead or alive after desiccation (Alejo-Jacuinde et al., 2022). The TTC assay presented here aimed at a detailed quantification of the changes in red intensity, and although it clearly detected a critical RWC that indicates reduced mitochondrial activity (RWC range of 13–27%; Table 2), it did so only for the adaxial surface and with significant scattering in the datapoints (Figure 3b). The lack of response in the abaxial surface (Figure S2) could indicate a reduced penetration of TTC in the tissue, despite the relatively long time of incubation (24–48 h). The use of TTC may be more adequate in ‘softer’ tissues, such as the poikilohydric Selaginella species evaluated by Alejo-Jacuinde et al. (2022), with LMAs for Selaginella spp. described in the 4–55 g m−2 range (Campany et al., 2019; Carriquí et al., 2019), rather than the more robust and sclerophyllous leaves of Q. faginea and, especially, Q. ilex (LMA of 149–210 g m−2; Table 1).
The causes of cell death under dehydration are attributed mostly to loss of membrane function due to mechanical destabilization, caused by membrane electrolytes (Na+, K+), accumulation of reactive oxygen species (ROS), and/or the mechanical rupture of the membrane (Hincha et al., 1987; Farrant & Moore, 2011; Sancho-Knapik et al., 2011; Guadagno et al., 2017). Although it is assumed that changes in chlorophyll fluorescence and electrolyte leakage both reflect disruption of membranes (Guadagno et al., 2017), it is possible that their different timing of response reflects slightly different types of damage – or damage at different levels within the leaf. The exact causes of the decline of Fv/Fm are difficult to determine since PSII is affected by multiple environmental factors (Murchie & Lawson, 2013). Trueba et al. (2019) tested if the degradation of chlorophyll could be the cause of decreases in Fv/Fm during dehydration, but found no changes in chlorophyll content in excised leaves under low light conditions, the same procedure utilized here. High Fv/Fm values require intact photosystems (Franck et al., 2002) but not necessarily the maintenance of the complete electron transport chain, which would be reflected by other fluorescence parameters, such as the quantum yield of PSII (ΦPSII; Murchie & Lawson, 2013) or the fluorescence decrease ratio (Rfd; Perera-Castro et al., 2018). Decrease in Fv/Fm can thus be interpreted in terms of PSII downregulation or impaired functioning of some of its components (Kim & Lee, 2005; Foyer et al., 2017), most likely due to changes in the molecular configuration of chlorophyll a under lethal drought (Guadagno et al., 2021). Oxidative damage at the thylakoid level during drought stress can also directly affect PSII, leading to reduced Fv/Fm (Tambussi et al., 2000). Notably, the decrease in Fv/Fm was irreversible, indicated by the consistently lower Fv/Fm after rehydration (Figure 2; also reported in Trueba et al. (2019)), a further indication of substantial damage at the photosystem level. The same impairment as the one described here between electrolyte leakage and Fv/Fm has been described in other oak species (Sapes et al., 2024) and in the fern Mohria caffrorum (Farrant et al., 2009), with a clear threshold or ‘two-step’ response for CFD (Woo et al., 2008; Trueba et al., 2019). Notably, both Fv/Fm and the TTC assay are related to the functioning of chloroplasts and mitochondria since they reflect photosynthesis and respiration, respectively, and they show a distinct response to dehydration, with a sharp decline in CFD and red intensity at low RWC (Figure 4). Further studies are required to elucidate the distinct sensitivity of the different cell membranes and/or organelles, and the timing of their response upon water loss. Nonetheless, it is worth mentioning that such differentiation between gradual and threshold responses for the different damage indicators is evident only in Q. faginea, whereas it is not as clear in either young or old Q. ilex leaves (Figure 4). The differentiation between ‘gradual’ and ‘threshold’ changes in damage indicators could result from distinct leaf traits and/or growth forms (John et al., 2018; Abate et al., 2021).
The use of Fv/Fm as a damage indicator (both CFD and CFR) allows for clearly differentiating the two evaluated species in terms of dehydration tolerance: Q. faginea showed lower CFD50 and CFR50 (Figure 1; Table 2), indicating that its leaves can sustain greater water losses without significant photosystem damage compared to Q. ilex. In the latter species, young leaves present slightly lower CFR50 compared to old leaves, allowing for further differentiation of dehydration tolerance even at the intra-specific species. This result contrasts with electrolyte leakage and the TTC assay, where no differences were detected (Table 2). These two methods are usually taken as solid reflections of membrane damage and tissue viability; however, their use could be limited when evaluating the dehydration tolerance of closely related species. As discussed earlier, although differences were also detected in terms of PLRC, this indicator seems less reliable given the lack of response in some cases (old Q. ilex leaves) and the potential problems related to oversaturation.
A limitation of the present study is the use of excised leaves to evaluate dehydration instead of intact leaves in plants subjected to soil drought. The response to drought of these two species was evaluated in potted plants as in Alonso-Forn et al. (2021), describing a higher sensitivity of Fv/Fm in Q. ilex compared to Q. faginea to decreases in water potential, a similar response to the results presented here (Figure 1a). However, this was, in fact, related to an earlier decline in hydraulic conductance in Q. faginea, thus complicating the interplay between hydraulic failure and tissue dehydration, which in the case of oaks may involve hydraulic segmentation (Peguero-Pina et al., 2015). We thus acknowledge that further testing is required to ensure the applicability of the evaluated methods as indicators of drought tolerance. Nevertheless, our primary goal was to compare the different methods to test if they presented similar results, which we think will not be greatly compromised when evaluating the same methods with other experimental conditions (i.e., soil drought). In this regard, Trifilò et al. (2023) compared the results of very similar assays of electrolyte leakage and rehydration capacity among different species (including Q. ilex), and obtained the same results in terms of critical RWC (for a given REL and PLRC increase) in excised leaves and in potted plants experiencing soil drought (even considering the different timescales of water stress imposition). Hence, we are quite confident that the methods evaluated for excised leaves can indeed be representative of dehydration tolerance.
In conclusion, among all the tested methods, chlorophyll fluorescence offers the most precise, rapid and non-invasive method for quantifying lethal cellular damage. As shown in the present study, it agrees with other damage indicators such as viability markers (TTC), with the additional advantage of being faster and without involving time-consuming procedures required in the case of electrolyte leakage and, especially, the TTC assay (or other viability markers; e.g., Evans blue). It allows for differentiating the dehydration tolerance of closely related species, and, in the case of CFR, it even permits the differentiation between phenological stages (Table 2). On the other hand, CFD can be used in vivo without requiring any destructive sampling, with the additional advantage of enabling sequential measurements on the same tissue at different intervals. This allows for the simultaneous use of Fv/Fm and other physiological measurements, such as leaf shrinkage and embolism (Johnson et al., 2018, 2022; Brodribb et al., 2021). Its only major limitations are that it can only be applied to photosynthetic tissues and that it may be less useful to detect damage during the early stages of dehydration compared to PLRC and electrolyte leakage. This can be minimized by including the measurement of Fv/Fm after rehydration. Spatial heterogeneity in leaf damage can also introduce bias in chlorophyll fluorescence, especially in larger leaves. Increasing the number of measurements per leaf or using fluorescence imaging devices (Brodribb et al., 2021) are good solutions to reduce the impact of leaf heterogeneity. It is worth reminding that caution must be taken in order to upscale the results at the leaf level to the whole plant; nonetheless, we think that the present study provides a solid methodological basis for the use of different damage indicators in studies regarding leaf death caused by dehydration. Future studies are needed to pinpoint if the decrease of Fv/Fm is related to membrane damage or to other factors arising from extreme water loss.
AUTHOR CONTRIBUTIONS
Conceptualization and experimental design by MN, DSK, JJPP, and EGP. Measurements and data analysis performed by MN. First manuscript draft by MN. Subsequent manuscript writing, review, and editing by all authors. Funding acquisition by DSK, JJPP and EGP.
ACKNOWLEDGEMENTS
This research was supported by Grant PID2022-136478OB-C32 funded by MICIU/AEI/10.13039/501100011033 (Spain) and by “ERDF A way of making Europe”, by grant CNS2022-136156 funded by MCIN/AEI/10.13039/501100011033 (Spain) and European Union (‘Next Generation EU’/PRTR) and by Gobierno de Aragón S74_23R research group. MN was supported by the postdoctoral fellowship Juan de la Cierva-Formación (FJC2020-043902-I), funded by MCIN/AEI/10.13039/501100011033 and the European Union (‘Next Generation EU’/PRTR).
FUNDING INFORMATION
Grant PID2022-136478OB-C32 (MICIU/AEI/10.13039/501100011033, “ERDF A way of making Europe”). Grant CNS2022-136156 (MCIN/AEI/10.13039/501100011033, European Union Next Generation EU/PRTR). Gobierno de Aragón S74_23R research group. Grant FJC2020-043902-I (MCIN/AEI/10.13039/501100011033, ‘NextGenerationEU/PRTR’).
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as all new created data is already contained within this article.




