The Rootstock's Cotyledon-Regulated Fructokinase ClFRK1 Contributes to Graft Union Formation in Watermelon
Abstract
Grafting is a traditional horticultural practice that enhances plant resilience against biotic and abiotic stresses. However, the influence of specific tissues, such as rootstock cotyledons, on graft union formation is not well understood. This study investigates the impact of rootstock cotyledon removal on graft healing in watermelon and its underlying mechanisms. Our results indicate that grafting with rootstock cotyledons (+C) consistently resulted in higher survival rates and better growth outcomes compared to grafting without rootstock cotyledons (-C). This effect was more pronounced in cultivated watermelon rootstocks, which have lower hypocotyl sugar content than wild watermelon rootstocks. Transcriptomic analysis revealed that cotyledon removal disrupted sugar metabolism and affected gene expression related to cell division and tissue development. A fructokinase, ClFRK1, was identified among the candidate genes positively correlated with graft survival rate and healing degree. Silencing ClFRK1 reduced callus proliferation, delayed graft healing and reduced survival rate. Conversely, fructose treatment increased ClFRK1 expression levels at the graft junction, which promoted callus proliferation and vascular reconnection. We propose a novel regulatory model for how ClFRK1 regulates graft union formation. These findings underscore new insights into the interactions and synergistic processes between the graft interface and non-grafted organs during graft union formation and also enrich our understanding of fructokinase.
1 INTRODUCTION
Grafting, a longstanding horticultural technique, is widely employed to improve plant resilience to both biotic and abiotic stresses (Xiong et al., 2021; Xu et al., 2022; Pu et al., 2024). Although grafting is a traditional method, recent advancements have broadened our understanding of the biological processes behind it. For example, RNA-seq studies have shed light on transcriptomic reprogramming occurring at the graft junction (Menkyl et al., 2018). More recently, the role of cell wall modifications and cell adhesions, and the process of vascular development during post-graft wound repair have been described (Kurotani et al., 2020; Notaguchi et al., 2020; Thomas et al., 2022; Zhang et al., 2022; Huang et al., 2023). Moreover, the hormone auxin has been shown to play a crucial role, particularly in WOX13-dependent callus formation (Zhai et al., 2021; Serivichyaswat et al., 2022; Tanaka et al., 2023). Furthermore, the PAT gene family is crucial for the regulation of wound healing during gymnosperm grafting in Norway spruce and Arabidopsis (Feng et al., 2024). Despite these advances, the influence of specific tissues, such as rootstock cotyledons, on the graft healing process remains less understood.
As eudicots, cucurbit crops, including watermelon, possess two large cotyledons, which are typically retained on the rootstock during grafting. When grafting techniques preserve the rootstock cotyledon, it leads to higher cultivation costs associated with managing rootstock regrowth. In contrast, grafting performed after removing both cotyledons from the rootstock may have the potential to enhance grafting efficacy by preventing rootstock regrowth and lowering the production costs for transplantation—which is particularly relevant for watermelon (Devi et al., 2020a; Liu et al., 2021). However, removing rootstock cotyledons can impair seedling development (Hanley et al., 2004; Hanley & May, 2006; Hanley & Fegan, 2007; Yi & Wang, 2016) and negatively impact post-grafting survival and fruit quality (Memmott & Hassell, 2010; Guan & Zhao, 2015; Pu et al., 2024). Although numerous studies have established that cotyledons are crucial for regulating graft union formation, the precise mechanisms by which they influence the graft healing process remain incomplete.
Sugar is the main provider of energy and carbon skeleton, influencing the grafting healing process. Grafting causes asymmetric distribution and response of sugars above and below the grafting interface due to the interruption of plant vascular channels. For example, the sugar-induced gene ADP-glucose pyrophosphorylase ApL3 was rapidly upregulated at the scion graft interface with high sugar levels, while the sugar-repressed genes DARK INDUCIBLE 6 (DIN6), GLUTAMATE DEHYDROGENASE 1 (GDH1), and SUGAR TRANSPORTER PROTEIN 1 (STP1) were rapidly upregulated at the rootstock graft interface with low sugar levels (Melnyk et al., 2018). This asymmetric sugar response may play an important role in the formation of the grafting event (Yin et al., 2012; Melnyk et al., 2018). Exogenous glucose and sucrose treatment can promote graft union establishment and increase graft survival (Miao et al., 2021; Devi et al., 2021; Kurotani et al., 2022). Silencing the Cucurbita moschata MALTOSE EXCESS PROTEIN 1a (CmoMEX1a) gene in pumpkin rootstock cotyledons inhibits maltose export, leading to reduced root growth in grafted seedlings (Pu et al., 2024). In addition, heterografts influence the sugar distribution between scion and rootstock after successful graft establishment, with the rootstock cotyledons acting as a buffer in this process (Pu et al., 2024).
Plant fructokinase (FRK) is a highly efficient fructose-phosphorylating enzyme that regulates the concentration of fructose in cells, as well as the distribution and flow of organic carbon between cells. It plays a crucial role in regulating plant growth and development, metabolism and responses to environmental stress (Granot et al., 2014; Stein & Granot, 2018; Ye & Zhou, 2021). Suppression of fructokinase encoded by LeFRK2 in tomato stem inhibits growth and causes wilting of young leaves (German et al., 2003). LeFRK4 and SlFRK4 play a role in the development of stamens and pollen anthers (German et al., 2002; David-Schwartz et al., 2013). FRKs play a role in the accumulation of carbohydrates: cellulose (Roach et al., 2012; Su et al., 2021), starch (Zhang et al., 2023), and soluble sugar (Zhao et al., 2024). Research has also found that fructokinase-like genes in Arabidopsis are important for seed oil accumulation (Stein et al., 2017). The research on the function of fructokinase in plant vascular development is another hot topic. LeFRK2 is required for phloem and xylem differentiation (Damari-Weissler et al., 2009). Suppression of SlFRK3 leads to xylem development defects and causes grafting failure (Stein et al., 2016). SlFRK1 and SlFRK2 play a role in the normal development of phloem fibers (Stein et al., 2018). Fructokinase and Sucrose synthase are essential for meristem and vascular development (Lugassi et al., 2022). Research found that fructokinase might be regulated by the level of fructose and ATP. Gonzali et al. (2001) and Petreikov et al. (2001) found that high concentrations of fructose inhibited the activity of fructokinase. Fructose had an inhibitory effect on OsFRK1, but the activity of OsFRK2 was not sensitive to high concentrations of fructose (Guglielminetti et al., 2006). High levels of ATP inhibited the activity of fructokinase (Gonzali et al., 2001; Riggs & Callis, 2017).
Here, we investigated the impact of rootstock cotyledon removal on graft healing across various watermelon rootstock resources. Our findings revealed that grafting with rootstock cotyledons (+C) consistently resulted in higher survival rates and better growth outcomes than grafting without rootstock cotyledons (-C). The detrimental effects of cotyledon removal were more pronounced in cultivated watermelon rootstocks than in wild ones, likely due to the higher hypocotyl sugar content in wild rootstocks, which correlates positively with graft survival rates. Transcriptome analysis indicated that cotyledon removal disrupted sugar metabolism, reduced energy availability, and decreased the expression of genes related to cell division and tissue development. Among these genes, the expression of a fructose metabolism-related gene, ClFRK1, was found to be strongly correlated with graft healing. Our study leveraged transcriptome data and sugar treatments to explore the role of rootstock cotyledons in regulating graft healing through their impact on sugar metabolism at the healing interface. Additionally, the positive effects of exogenous sugar treatments on graft healing emphasize the critical role of carbohydrates in supporting successful graft integration. Overall, these findings highlight the importance of rootstock cotyledons in the graft healing process and suggest that enhancing sugar availability could improve grafting outcomes, particularly in cultivated watermelon varieties with naturally lower sugar levels.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
In this study, the watermelon (Citrullus lanatus) cultivar 97103 (W) was used as the scion in all grafting combinations. Information on the eight watermelon rootstocks used in this study can be found in Table S1. Watermelon cultivars 97103 (W) and wild watermelon Yongshi (Y) were the primary rootstocks used for further grafting experiments involving scion growth measurements, paraffin section observation, sugar content determination, and RNA-seq analysis after grafting. All plants were grown in a controlled environment with a day/night (14/10 h) cycle at 300 μmol m−2·s−1, 24°C, and 60% relative humidity.
2.2 Plant grafting and the culturing of grafted seedlings
Grafting was performed 8–12 days after sowing, after the emergence of the first true leaf. Two grafting methods were used: grafting with one rootstock cotyledon (+C), following the method described by Xu et al. (2022), and grafting without rootstock cotyledons (-C), in which all cotyledons and growth points of the rootstock were completely removed (Figure 1A).
The grafted seedlings were immediately placed in a healing box with a transparent plastic cover to maintain high humidity (95%–100%) and kept in a growth room with a 14-hour light/10-hour dark cycle at 24°C. The lighting schedules for the seedlings were as follows: on 0 Days After Grafting (DAG), they were kept in darkness. On 1 DAG, they received supplemental lighting at 100 μmol m−2·s−1 for 30 minutes in both the morning and afternoon. On 2 DAG, the lighting duration was extended to 1 hour in the morning and afternoon. On 3 DAG, the seedlings were exposed to supplemental lighting at 100 μmol m−2·s−1 for 14 hours. On 4 DAG, the light intensity was raised to 300 μmol m−2·s−1 for 30 minutes in both the morning and afternoon. On 5 DAG, the lighting at 300 μmol m−2·s−1 was extended to 1 hour in both the morning and afternoon, with the cover removed for 30 minutes during each period. From 6 to 8 DAG, the seedlings received 14 hours of supplemental lighting at 300 μmol m−2·s−1, with the cover removed for 1 hour in each period. On 9 to 11 DAG, they continued to receive 14 hours of lighting at 300 μmol m−2·s−1, with the cover removed for 14 hours. On 12 DAG, the cover was permanently removed, and seedlings were transferred to normal growth conditions.
Three weeks after grafting, the surviving grafts were transferred to a greenhouse located at the Wuhan Academy of Agricultural Sciences (Wuhan, China). Between 15–20 plants were established, and manual pollination was performed, resulting in one to two fruits retained per plant. This cultivation phase extended from August to November 2021.
2.3 Survival rate, scion growth, adhesive force testing, and fruit yield measurement
The survival rate was calculated 14 days after grafting using the formula: Survival rate = (number of survived grafted seedlings / total number of grafted seedlings) × 100%. Grafted seedlings were considered successful if they showed clear growth of the scion and maintained an upright posture. Each graft combination was repeated three times, with 24 grafted seedlings per replicate. For scion fresh weight, 15–24 grafted seedlings were weighed 14 days after grafting, and the average weight was calculated. The true leaf area was determined by laying each leaf of every grafted seedling flat on a ruler, photographing it, and calculating the leaf area using ImageJ (https://imagej.nih.gov/ij/) (Figure S7).
When the watermelons matured, the weight and fruit Brix (%) of each watermelon fruit were measured. The ATAGO(PAL-1) Portable Digital Fruit Brix Meter (ATAGO U.S.A. Inc.) was used to measure the Brix (Brix, %) values of each watermelon fruit.
2.4 Sample preparation
At 0, 6, 24, 72, 120, and 168 hours after grafting (HAG), the scions and rootstocks were separated, and the graft union regions of rootstocks were sampled as depicted in Figure S2, frozen in liquid nitrogen and stored in a − 80°C freezer for RNA-seq, biochemical analyses and qRT-PCR. For each graft combination, six grafted seedlings were randomly selected, and the entire sampling procedure was repeated four times.
2.5 Microscopy
Graft union sites from the rootstock and scion were collected at 24, 72, and 168 hours after grafting (HAG). The graft unions were initially fixed in a 70% FAA solution (ethanol: formaldehyde: acetic acid = 18:1:1, v/v/v) for 24 hours and then transferred to 70% ethanol for long-term preservation at 4°C. Paraffin embedding and sectioning were performed according to Xiong et al. (2021). Sections were cut vertically at 10 μm thickness using a rotary microtome (Leica RM2255), then dewaxed, rehydrated, and stained with 1% safranin for 2 minutes. After dehydration, the sections were counterstained with 1% fast green in 90% alcohol for 15 seconds, cleared, and mounted with neutral balata. Imaging was performed using a fluorescence microscope (Leica DM6B).
To monitor the proliferation of callus tissue at the incision sites under various treatments, watermelon seedlings were incised as depicted in Figure S8. The development of callus tissue at these sites was imaged daily using a stereo microscope Leica M205FA (Leica Microsystems GmbH). The management of both cut and grafted watermelon seedlings was maintained consistently throughout the observation period.
2.6 Metabolite quantification
The plant tissues were sampled and prepared as described above. The following quantification kits were used in this experiment: Plant Sucrose Content Assay Kit, AKPL006M; Plant Fructose Content Assay Kit, AKPL007M; D-Glucose Content Assay Kit, AKSU001M; Fructokinase (FRK) Activity Assay Kit, AKSU084M (Beijing Boxbio Science & Technology Co., Ltd). Each sample was tested thrice with three technical replicates. Absorbance values for sucrose (wavelength: 480 nm), fructose (wavelength: 480 nm), glucose (wavelength: 505 nm), and fructokinase (wavelength: 340 nm) using a TECAN Spark® Multimode Microplate Reader (Tecan Group Ltd.) were recorded for calculating the concentration or enzyme activity.
2.7 Transcriptome analysis
RNA-seq was performed by Biomarker Technologies Corporation, Beijing (http://www.biomarker.com.cn/). Nucleic acids were extracted using the following kits: Tiangen DP411 (Tiangen Biotech Co.), CLB + Adlai RN40 (Aidlab Biotechnologies Co.), CTAB+Adlai RN40 (Aidlab Biotechnologies Co.), and Tiangen DP762-T1C/TRIzol (Tiangen Biotech Co.). The concentration of the extracted nucleic acids was determined using a Nanodrop2000 (Thermo Fisher Scientific Co.), and their integrity was assessed with an Agilent2100 LabChip GX (Agilent Technologies Co.). Library construction was carried out using the Hieff NGS Ultima Dual-mode mRNA Library Prep Kit for Illumina (Yeasen Biotechnology Co.), and product purification was performed using HieffNGS DNA Selection Beads (Yeasen Biotechnology Co.).
Significant differences in gene expression were identified using a false discovery rate (FDR) of ≤0.01 and an absolute log2(fold change) (|log2(FC)|) greater than 1 (where FC is the fold change calculated by FPKM values). FPKM stands for fragments per kilobase of exon per million reads mapped. Gene functions were annotated using the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases. Functional enrichment analyses of DEGs were performed to identify significant enrichments in specific GO terms or KEGG pathways. GO terms and KEGG pathways with a corrected P-value ≤0.05 indicate significant enrichment. Protein sequences were extracted from the provided link and submitted to STRING database to generate a protein–protein interaction network for HUB gene identification, focusing on the model plant Arabidopsis thaliana, with an interaction score threshold of 0.40.
2.8 Identification of the Watermelon FRK gene family
Watermelon Citrullus lanatus subsp. vulgaris cv. 97103 v2 genome was obtain from CuGenDBv2 (http://cucurbitgenomics.org/v2/), while the Arabidopsis TAIR10_genome was sourced from the Arabidopsis Information Resource (https://www.arabidopsis.org/download/list?dir=Genes/TAIR10_genome_release). Using the Arabidopsis FRK gene family data from Riggs et al. (2017), we obtained gene and protein information for AtFRKs in Arabidopsis thaliana. An initial selection of watermelon ClFRKs was performed using Tbtools-blast with the AtFRKs protein sequences. Conserved domains within AtFRKs were identified using the NCBI's CD-Search Tool. Watermelon ClFRKs were screened based on conserved domains (pfkB: PF00294, bac_FRK: cd01167) and compared with AtFRK1-7. A phylogenetic tree was constructed using MEGA11 with the protein sequences, employing the Maximum Likelihood (ML) method.
2.9 Exogenous sugar treatments
Sucrose, fructose, and glucose solutions (Hefei Bomei Biotechnology Co.) were prepared by dissolving the sugars in deionized water to achieve the desired concentrations. Approximately 2 mL of the solution was sprayed evenly onto the watermelon rootstocks 12–16 hours prior to grafting, ensuring the liquid was nearly dripping off. This spraying process was repeated one hour after grafting.
2.10 RNA extraction, cDNA synthesis and qRT-PCR
RNA was extracted using the TranZol protocol (Code: ET101-01, Beijing TransGen Biotech Co.). cDNA synthesis followed the procedures outlined in the TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (Code: AT311-02, Beijing TransGen Biotech Co.). Primers were designed using Primer3Plus (https://www.primer3plus.com/index.html). Real-Time Quantitative Reverse Transcription PCR (qRT-PCR) was conducted using the 2 × TransStartTM TOP Green qPCR SuperMix Kit (Code: AQ131-01, Beijing TransGen Biotech Co.) on a QuantStudio 7 Flex Real-Time PCR System (Applied Biosystems). ClADP (Watermelon ADP/ATP transport protein) served as the reference gene, and relative gene expression levels were calculated using the 2-ΔΔCt method (Livak & Schmittgen, 2001). Primer sequences are provided in Table S2.
2.11 Molecular cloning and transformation
The Cucumber Green Mottle Mosaic Virus (CGMMV) vector was used for VIGS experiments to silence the ClFRK1 (Cla97C01G005540) gene (Liu et al., 2020). The 431–686 bp region of the ClFRK1 gene was selected as the silencing fragment. This fragment was amplified by PCR and cloned into the CGMMV vector. Primer sequences are listed in Table S2. The vector was assembled through homologous recombination following digestion with BamHI. Agrobacterium strain GV3101 harboring the CGMMV vectors was cultured at 28°C in LB medium supplemented with appropriate antibiotics. After 24 hours, the Agrobacterium cells were harvested and resuspended in infiltration buffer (10 mmol/L MgCl2, 10 mmol/L MES, 100 μmol/L acetosyringone), adjusting the optical density to OD600 = 0.4. The seed infection method was employed as follows: watermelon seeds were first sterilized in a 55°C water bath for 30 minutes. The seed coats were then removed, and the seeds were incubated at 30°C for 48 hours to promote germination. Subsequently, the germinated seeds were soaked in 40 mL of Agrobacterium inoculum, ensuring they were partially immersed, and incubated in the dark at 20–23°C for 24 hours. After incubation, the inoculated seeds were placed on sterile filter paper to absorb any excess inoculum before sowing.
3 RESULTS
3.1 Removal of rootstock cotyledon decreases the graft survival rate and delays the healing process
To explore the role of rootstock cotyledons in the graft recovery process, we utilized two grafting methods. In one method, we retained one rootstock cotyledon (+C), and in the other, we removed both cotyledons (-C) (Figure 1A). For these experiments, the watermelon cultivar 97103 (Citrullus lanatus) was grafted onto eight different watermelon rootstocks (Figure 1B, Table S1), and the graft survival rate was assessed 14 days after grafting (DAG). We observed that the survival rate significantly decreased following the removal of the rootstock cotyledons (Figure 1B, C). Notably, grafts without rootstock cotyledons exhibited higher survival rates in the four wild watermelon rootstocks compared to the four cultivated watermelon rootstocks, where the survival rates were lower (Figure 1C).
Next, we selected one wild rootstock, called “Yongshi” (Y), and one cultivar rootstock, known as “97103” (W) to further investigate cotyledon-related survival rates. We observed a slight decrease in survival rate for W/Y -C (from 100% to 66.4%) and a substantial decrease for W/W -C (from 97.1% to 27.8%) (Figure 1C). During the healing process, we monitored the total area of true leaves of the scion and found that the growth rate was fastest in W/Y + C, followed by W/Y -C, and slowest in W/W -C (Figure 1D). The fresh weight of the scion at 14 days after grafting (DAG) corroborated these observations, showing a significant decrease in scion fresh weight due to cotyledon removal, with a larger reduction in W/W -C (19.09%) compared to W/Y (51.29%) (Figure 1E).
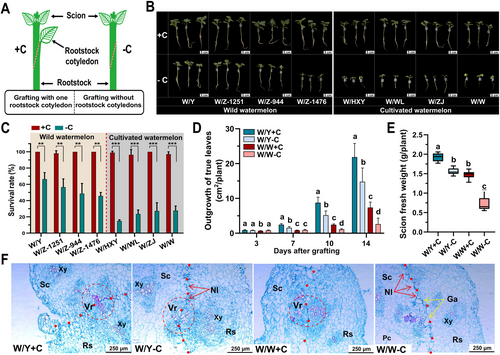
To better understand the observed differences in graft survival rates and growth outcomes, we examined paraffin sections of the graft surface at 3, 5, and 7 DAG. At 3 DAG, no significant differences were observed among W/Y + C, W/Y -C, and W/W + C; however, W/W -C showed gaps at the graft surface that were not filled with cellular tissue (Figure S1). By 5 DAG, W/Y + C and W/W + C exhibited minimal necrotic tissue and showed signs of vascular reconnection, with W/Y + C demonstrating more pronounced reconnection. By contrast, W/Y -C and W/W -C still exhibited a distinct necrotic layer (Figure S1). At 7 DAG, differences between graft combinations became more evident. W/Y + C and W/W + C showed no necrotic layer, whereas W/Y -C and W/W -C had persistent necrotic layers (Figure 1F). Completed vascular reconnection was observed in either W/Y + C or W/Y -C, but in W/W, it only occurred in W/W + C but not in W/W -C (Figure 1F). These results suggest that the absence of rootstock cotyledons impaired graft healing, particularly in cultivated watermelon rootstocks, leading to increased graft failure.
3.2 Impact of cotyledon removal on gene expression at the graft junction
To better understand the effects of cotyledon removal, we conducted RNA-seq analyses on the rootstock portion of the graft junction at 0, 6, 72, 120, and 168 hours after grafting (HAG) (Figures 2A-E and S2). The Principal Component Analysis (PCA) clustering revealed distinct differences in transcriptome profiles not only between grafts with and without rootstock cotyledons but also between the W/Y and W/W graft combinations, with the greatest variation occurring at 6 HAG (Figure 2A). Next, differentially expressed genes (DEGs) were analyzed by DESeq2 (Fold Change≥2, FDR <0.01). Overall, 1947 genes were upregulated and 3110 genes were downregulated in W/W -C, whereas 2122 and 2494 were up- and downregulated in W/Y -C, respectively (Figure 2B). Interestingly, the highest number of DEGs (3200 genes) was observed at 168 HAG in W/W, while the highest number of DEGs (2944 genes) was observed at 6 HAG in W/Y (Figure 2B).
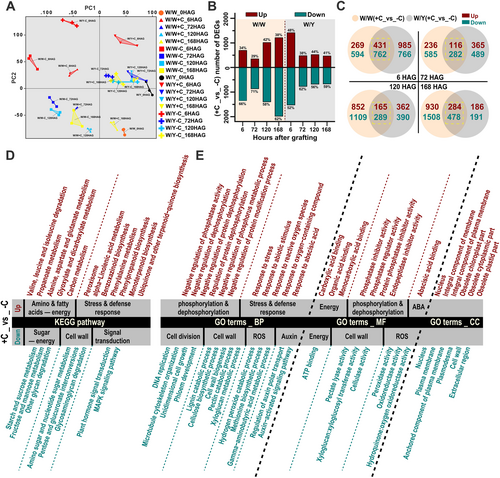
To further compare DEGs in W/W and W/Y following cotyledon removal, we generated Venn diagrams to visualize the proportion of shared and unique DEGs at each time point (Figure 2C). Next, KEGG and GO enrichment analyses were performed on DEGs shared between W/W and W/Y to obtain a broad picture of biological processes among different post-grafting stages (Figures 2D-E and S3). Among all time points, the downregulated DEGs were significantly enriched in KEGG categories, including ‘starch and sucrose metabolism’ (ko00500), ‘fructose and mannose metabolism’ (ko00051) and ‘other glycan degradation’ (ko00511). Meanwhile, the upregulated genes were significantly enriched in the metabolic pathways of various amino acids such as ‘valine, leucine and isoleucine degradation’ (ko00280) and ‘alanine, aspartate and glutamate metabolism’ (ko00250). Additionally, genes related to ‘propanoate metabolism’ (ko00640) and ‘glyoxylate and dicarboxylate metabolism’ (ko00630) were significantly upregulated (Figure 2D). Consistent with our hypothesis, these results suggest that cotyledon removal impacts key metabolic processes across sugars, amino acids, and fatty acids.
GO term enrichment analysis of the DEGs at different time points revealed that downregulated genes were significantly enriched in biological processes related to cell division and tissue development, such as ‘DNA replication’ (GO:0006260), ‘unidimensional cell growth’ (GO:0009826), and ‘microtubule cytoskeleton organization’ (GO:0000226). Processes related to cell wall component biogenesis and degradation, including ‘cell wall biogenesis’ (GO:0042546), ‘cellulose biosynthetic process’ (GO:0030244), ‘lignin catabolic process’ (GO:0046274), and ‘pectin catabolic process’ (GO:0045490) were also significantly enriched (Figure 2E, Figure S3). These results indicate that the removal of cotyledons led to the attenuation of these processes, consistent with the observed persistence of necrotic layers and gap formation at the graft junction in grafts without rootstock cotyledons at 168 HAG (7 DAG), compared to grafts with cotyledons where these issues were less apparent (Figure 1F). Moreover, downregulated genes were also enriched in processes related to reactive oxygen species (ROS) degradation, such as ‘hydrogen peroxide catabolic process’ (GO:0042744) and ‘peroxidase activity’ (GO:0004601). Conversely, upregulated genes were significantly enriched in stress- and defense-related pathways, including the ‘response to reactive oxygen species’ (GO:0000302), ‘abscisic acid-activated signaling pathway’ (GO:0009738), and ‘response to abiotic stimulus’ (GO:0009628) (Figure 2E). These findings suggest that the removal of cotyledons led to reduced ROS degradation at the graft junction, triggering a stronger stress and defense response. In terms of molecular function, downregulated genes were significantly enriched in ‘ATP binding’ (GO:0005524), reflecting the reduced energy supply due to impaired sugar metabolism. These findings align with the KEGG pathway enrichment results, reinforcing the conclusion that the removal of cotyledons weakens sugar metabolism, leading to an energy deficit that impairs the graft healing process.
3.3 Protein–protein interaction network analysis of graft phenotype-related genes
Grafts without rootstock cotyledons (-C) had lower survival rates as well as weaker growth and healing compared to grafts with rootstock cotyledons (+C). Further, we found that the W/W grafting without cotyledons (W/W-C) had lower survival rates, weaker growth and healing compared to the W/Y grafting without cotyledons (W/Y-C) (Figure 1). Expanding on this result, by comparing W/W and W/Y, we found that the W/W-C combination exhibited reduced healing (delayed about 3 days) and survival rates (58.18% lower) relative to W/Y-C (Figures 3A, 1C, and S1). To investigate the underlying genetic factors, we established four differential comparison groups: (1) graft-induced genes “0 HAG_vs_+C up” (encompassing all DEGs upregulated in the four graft combinations at 6, 72, 120, and 168 HAG compared to 0 HAG); (2) genes that responded to rootstock cotyledon removal, “W/W + C_vs_W/W-C down” (including all DEGs downregulated in W/W-C compared to W/W + C), and (3) “W/Y + C_vs_W/Y-C down” (including all DEGs downregulated in W/Y-C compared to W/Y + C); and (4) DEGs between different watermelon species grafted without cotyledons, “W/W-C_vs_W/Y-C up” (including all DEGs upregulated in W/Y-C compared to W/W-C) (Figure 3B). Venn analysis of these groups identified 290 hub genes associated with graft phenotype (Figure 3B).
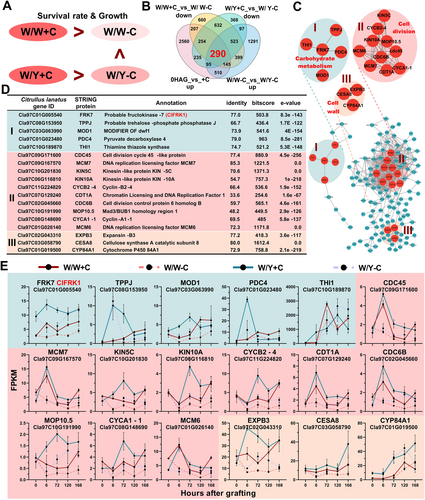
Subsequent protein–protein interaction (PPI) network analysis of these 290 hub genes identified three primary interaction regions: Region I (carbohydrate metabolism), Region II (cell wall processes), and Region III (cell division) (Figure 3C). In Region I, the central enzyme fructokinase FRK7, which is involved in fructose metabolism, played a crucial role. Region II showed strong interactions among proteins involved in DNA replication, cell cycle, and cell division, including Cell Division Cycle proteins (CDC45, CDC6B), Kinesins (KIN5C, KIN10A), and Cyclins (CYCA1-1, CYCB2-4). In Region III, key cell wall enzymes such as Expansin (EXPB3) and Cellulose Synthase (CESA8) were identified (Figure 3D, E). Notably, the expression patterns of FRK7 aligned with the observed graft phenotypes (Figure 1B, D, E), suggesting a potential role for FRK7 in response to cotyledon removal and graft healing. Further characterization of the watermelon FRK gene family revealed that FRK7 is equivalent to ClFRK1, located on chromosome 1 (Figure S4).
3.4 Exogenous sugar treatment promotes graft healing in watermelon
To further evaluate the potential changes in sugar content at the graft junction after cotyledon removal, we measured sucrose, glucose, and fructose levels of the rootstock cotyledon and the graft junction. Interestingly, each sugar decreased during the first 24 hours after cotyledon removal, then continuously increased thereafter (Figure 4A). In grafts with cotyledons (W/W + C), sucrose and fructose levels at the graft junction were high and similar to those in the rootstock cotyledons, whereas the contents of these sugars were lower in grafts without cotyledons (W/W-C) (Figure 4A). Glucose content was highest in the rootstock cotyledons, followed by the graft junction in W/W + C, and was lowest in the graft junction of W/W-C (Figure 4A).
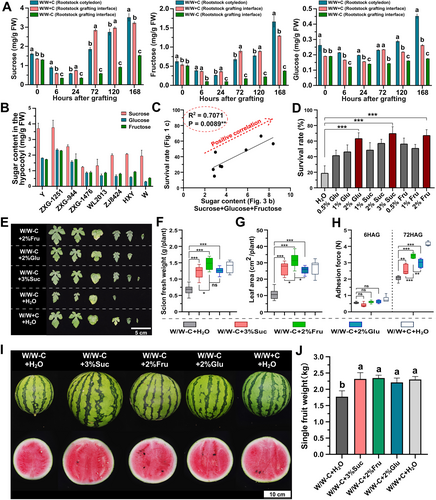
Moreover, the sugar content of the hypocotyls of the eight watermelon rootstocks was measured. The results showed that the sugar content (sucrose, glucose, and fructose) among the rootstocks showed a strong positive correlation (R2 = 0.7071, p = 0.0089) with graft survival rates when these rootstocks were used without cotyledons (Figures 1C and 4B, C). To further investigate the relationship between sugar content and graft survival rate, we applied exogenous sucrose (Suc), glucose (Glu), and fructose (Fru) treatments to grafts without rootstock cotyledons. Three sugar treatments significantly improved graft survival rates and their optimal concentrations were 3%, 2%, and 2%, respectively (Figure 4D). Optimal sugar treatments also increased scion fresh weight, leaf number, leaf area at 14 DAG, and adhesive force at 72 HAG (Figure 4E, H). Taken together, these results provide strong evidence for high sugar content as a crucial determinant for the grafting success of a rootstock, and fructose shared the best performance in each character.
Next, we evaluated the effects of different sugar treatments during the graft healing period on the development of watermelon self-grafts (W/W) (Figures 4I, J and S5). Grafted plants were treated with various sugars during the healing period and then transplanted into the greenhouse 3 weeks after grafting. At 7 weeks after grafting, all sugar treatments increased the single fruit weight by approximately 33% in W/W-C, matching the weight observed in W/W + C (Figure 4I, J). Additionally, these treatments mitigated the losses in scion length caused by the removal of rootstock cotyledons (Figure S5 A, B).Bby contrast, the central and edge Brix values of the fruit were unaffected (Figure S5 C). Given the common use of pumpkin as a rootstock for watermelon in agricultural settings, we extended our sugar treatment tests to watermelon/pumpkin (W/P) grafts. Compared to W/P-C, the grafting survival rate increased by 48.1% in glucose treatment, 50.0% in sucrose treatment and 54.2% in fructose treatment. (Figure S6). Moreover, sugar treatments enhanced the growth of both the scion and rootstock in W/P-C. (Figure S6 A, B); Similar increases in single fruit weight (3%Suc: 29.6%, 2%Fru: 35.2%, and 2%Glu: 32.6%) and scion length (3%Suc: 8.2%, 2%Fru: 21.8%, and 2%Glu: 34.6%) were also observed (Figure S6 C-E).
3.5 Fructose regulates watermelon grafting healing through CIFRK1
Sugars played a crucial role in the graft healing processes, and the absence of cotyledons during grafting, which led to reduced sugar availability at the grafting interface, is a primary cause of grafting failure (Figure 4C). Protein–protein interaction (PPI) network analysis identified ClFRK1 as a key gene in the fructose metabolism pathway (Figure 3A-E). Based on our results, we hypothesized that ClFRK1 was activated by fructose and regulated the graft healing process.
To test this hypothesis, we first used a virus-induced gene silencing (VIGS) approach to silence the expression of ClFRK1 during the graft healing period (Figure 5). In grafts with rootstock cotyledons (+C), silencing of ClFRK1 led to a significantly reduced survival rate in the ClFRK1-silenced rootstock combination (CIFRK1-VIGS) compared to non-inoculated (NI) and empty vector-inoculated control rootstocks (VC) (Figure 5). This outcome was comparable to grafting without rootstock cotyledons (-C). Exogenous application of fructose to the hypocotyls of intact watermelon seedlings significantly increased ClFRK1 expression and fructokinase activity (Figure 5). Additionally, the PPI results linked several cell division-related genes with ClFRK1, supporting its involvement in cell division processes. Callus tissue observations revealed significantly less callus formation in ClFRK1-silenced grafts compared to NI grafts (W/W + C) and were similar to those observed in W/W-C (Figure 5). Exogenous fructose treatment mitigated these adverse effects caused by the removal of rootstock cotyledons and silencing of ClFRK1 (Figure 5). Microscopic observations at 168 hours after grafting (HAG) revealed no necrotic layers and extensive xylem reconnection between the scion and rootstock in both W/W + C and W/W-C treated with fructose. By contrast, W/W-C and ClFRK1-silenced combinations exhibited considerable necrotic layers and gaps, with no evident xylem reconnection (Figure 5). Importantly, these results suggest that high ClFRK1 expression is positively associated with callus proliferation at the grafting junction, indicating a critical role in promoting graft healing.

4 DISCUSSION
Low grafting efficiency and high management costs are limiting factors for the widespread application of grafting techniques (Devi et al., 2020b). Methods that retain the rootstock cotyledon increase cultivation costs due to the need for manual removal of rootstock regrowth (Liu et al., 2021). Conversely, removing both cotyledons from the rootstock could significantly improve the efficacy of grafting by preventing rootstock regrowth and reduce the associated production costs (Devi et al., 2020a). Therefore, grafting without cotyledons could be a viable solution to these challenges. However, our findings indicate that grafting without cotyledons results in lower survival rates and reduced growth (Figure 1A-F), aligning with previous reports by Memmott et al. (2010), Guan et al. (2015), and Dabirian & Miles (2017). Interestingly, here we found that wild watermelons exhibited notably higher survival rates and better healing outcomes compared to cultivated watermelons when grafted without rootstock cotyledons (-C) (Figure 1A-F).
Further, we revealed that wild watermelons had higher stem sugar content than cultivated varieties (Figure 4B), which was positively correlated with graft survival (Figure 4C). We reasoned that the higher sugar content in the wild watermelon varieties may contribute to faster healing in grafts. Consistent with this, our observations showed a significant decrease in sugar content at the graft union following grafting without rootstock cotyledons (-C), supporting the role of cotyledons as essential carbohydrate sources (Daley & Hassell, 2014). Interestingly, we also discovered that exogenous application of sugars to rootstocks before grafting positively influenced grafting efficiency, with treatments of 3% sucrose, 2% fructose, and 2% glucose improving survival rates by 70.1%, 67.6%, and 63.6%, respectively (Figure 4D). Notably, this finding is also consistent with studies demonstrating the importance of sugar metabolism and sugar signaling in the wounding response (Melnyk et al., 2018; Miao et al., 2021).
Next, we investigated the role of the graft phenotype-related gene, ClFRK1, which is involved in the fructose metabolism pathway in watermelon, focusing on its response to grafting and exogenous sugar treatments (Figures 3A-E and 4A-J). ClFRK1 was upregulated in grafts with one rootstock cotyledon and downregulated in grafts without cotyledons. Wild watermelons, which have higher sugar content, exhibited higher ClFRK1 expression compared to cultivated varieties. Additionally, treatment with 2% exogenous fructose activated ClFRK1 expression and increased fructokinase activity. Conversely, inhibiting ClFRK1 expression markedly reduced grafting success and callus tissue at the wound surface, suggesting that fructose may regulate graft formation through ClFRK1 (Figure 5A-G). In summary, ClFRK1 is crucial for callus formation at the graft interface, likely by promoting cell division and differentiation through fructokinases (Figure 5F). Other genes associated with plant regeneration and callus formation at the graft junction, such as CELL DIVISION CYCLE 45 (CDC45) and CDC6B (Stevens et al., 2004; Bömer et al., 2021), DNA Replication Licensing Factors MCM6 and MCM7 (Dresselhaus et al., 2006; Long et al., 2019), KINESIN-LIKE PROTEINS KIN5C and KIN10A (B. Liu et al., 1996; Hotta et al., 2022), and Cyclins CYCA1-1 and CYCB2-4 (Polko et al., 2015) may play also significant roles in this process, although their interactions with ClFRK1 requires further investigation. According to the above results, ClFRK1 affected the proliferation of calli through energy, cell division regulatory genes, and the synthesis of cell wall components, and played an active role in the graft union formation process in Watermelon (Figure 6).
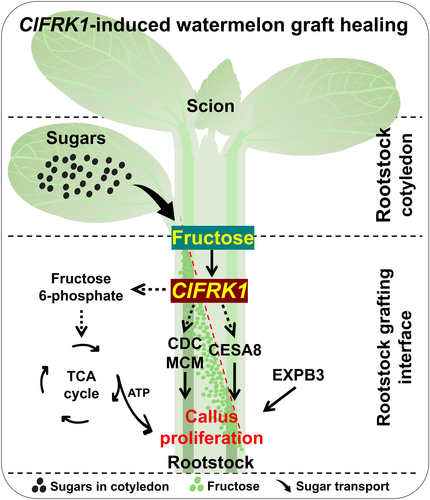
In conclusion, we assessed the effects of grafting without cotyledons on watermelon fruit yield and quality in watermelon/watermelon (W/W) and watermelon/pumpkin graft (W/P) combinations (Figures 4I, J and S6). Grafting without cotyledons resulted in lower scion length and fruit weight compared to grafting with one cotyledon; however, these negative impacts could be alleviated by exogenous sugar treatments during graft healing. While fruit Brix levels showed no significant differences, the weight of fruits from grafts without cotyledons was significantly lower than those from grafts with one cotyledon or those receiving sugar treatments. This suggests that grafting without cotyledons may delay the growth and development cycle, likely due to inadequate root system or vascular bundle development, thereby affecting nutrient uptake and fruit development. Nevertheless, preventing rootstock regeneration through grafting without cotyledons, combined with the positive impact of CIFRK1 expression and exogenous sugar treatments, has proven effective in enhancing the grafting process. These findings provide a strong foundation for developing successful grafting strategies in watermelon cultivation.
AUTHOR CONTRIBUTIONS
Y. H., M. N., Z. -L. B., A. K. and M. X. designed the experiments. A. K., M. X. and C. -L. H. conducted the experimental work and performed data analysis. B. -P. Z. and H. -L. Z. carried out the sugar content tests. X. -S. W. and Y. A. were involved in plant cultivation and grafting. Z. -H. Z. completed the transcriptome sampling. A. K. and M. X. wrote the manuscript. Y. H., M. N. and Z. -L. B. reviewed and revised the manuscript.
ACKNOWLEDGEMENTS
We thank Professor Lin Zongcheng and Professor Munenori Kitagawa for their critical reading of the manuscript. We also would like to thank Scientific Editing (www.alpublish.com) for their expertise in language editing during the preparation of this manuscript.
FUNDING INFORMATION
National Natural Science Foundation of China (31972434); Young Scientist Fostering Funds for the Notional Key Laboratory for Germplasm Innovation and Utilization of Horticultural Crops (11909920008); China Agriculture Research System of MOF and MORA (CARS-25); Hubei Provincial Key Research and Development Program (2023BBB033); Fundamental Research Funds for the Central Universities (2662024JC004); Huazhong Agricultural University-Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences Cooperation Fund (SZYJY2021005).
Open Research
DATA AVAILABILITY STATEMENT
The transcriptomics raw data generated in this study have been deposited in the National Center for Biotechnology Information (NCBI) SRA database under accession code PRJNA1149428 (https://dataview.ncbi.nlm.nih.gov/object/ PRJNA1149428?reviewer = 8fobh9m0k69nnsr0apa6cml5gb).




