Oxidative post-translational modifications of plant antioxidant systems under environmental stress
Abstract
Plants are often subject to environmental challenges posed by abiotic and biotic stresses, which are increasing under the current climate change conditions, provoking a loss in crop yield worldwide. Plants must cope with adverse situations such as increasing temperatures, air pollution or loss of agricultural land due to salinity, drought, contamination, and pathogen attacks, among others. Plants under stress conditions increase the production of reactive oxygen-, nitrogen-, and sulphur species (ROS/RNS/RSS), whose concentrations must be tightly regulated. The enzymatic antioxidant system and metabolites are in charge of their control to avoid their deleterious effects on cellular components, allowing their participation in signalling events. As signalling molecules, reactive species are involved in plant responses to the environment through post-translational modifications (PTMs) of proteins, which, in turn, may regulate the structure, function, and location of the antioxidant proteins by oxidative/nitrosative/persulfure modifications of different amino acid residues. In this review, we examine the different effects of these post-translational modifications, which are emerging as a fine-tuned point of control of the antioxidant systems involved in plant responses to climate change, a growing threat to crop production.
1 INTRODUCTION
In general terms, adverse environmental conditions generate an increased production of reactive oxygen, nitrogen and sulphur species (ROS/RNS/RSS), which trigger the antioxidant defence in plants through several mechanisms, including transcriptional and post-transcriptional control, post-translational modifications (PTMs), retrograde signalling, and protein–protein interactions. With regard to the oxidative modifications (oxiPTMs), they are very diverse and include, among others, specific reactions with protein thiols such as S-sulfenylation, S-glutathionylation, S-persulfidation, S-nitrosation, S-cyanylation, and S-acylation, as well as reactions with other amino acid residues in proteins that induce carbonylation, sulfoxidation or nitration. According to the UniProtKB/Swiss-Prot database, more than 450 different PTMs have been identified, the majority of them reversible and mediated by specific enzymes or by non-enzymatic compounds, such as the gasotransmitters nitric oxide (NO) and hydrogen sulfide (H2S), as well as ROS/RNS, which may exert synergistic or antagonistic actions (Mukherjee and Corpas, 2023). Maintenance of the cellular redox balance is essential for cell survival, and an increase in ROS/RNS/RSS can lead to oxidative stress conditions, potentially damaging lipids, proteins, and DNA. Certain protein amino acids are very sensitive to oxidative modifications, particularly methionine and cysteine residues, and the reversibility of some of these modifications may support a role for them in the regulation of protein activity, mainly under stress situations where concentrations of these species increase, and the functionality of the protein may be compromised. Thus, in addition to being key sites for redox-based PTMs, cysteine residues are also involved in ROS/RNS sensing and transduction of redox signalling (Foyer & Kunert, 2024).
The main plant antioxidant system comprises superoxide dismutases (SODs), which eliminate the highly reactive superoxide (O2−) to produce H2O2, a more stable and diffusible reactive specie that can be scavenged by a plethora of enzymes, including catalase (CAT); several peroxidases, including peroxiredoxin (PRX) and glutathione peroxidase (GPX); and components of the so-called ascorbate-glutathione (ASC-GSH) cycle: enzymes ascorbate peroxidase (APX), monodehydroascorbate/dehydroascorbate reductases (MDHAR/DHAR), and glutathione reductase (GR), together with the non-enzymatic antioxidants ascorbate (ASC) and glutathione (GSH). Furthermore, components of the redox system thioredoxin (TRX), nucleoredoxin (NRX), glutaredoxin (GRX), and sulfiredoxin (SRX) are also considered ROS scavengers, being able to revert the oxidised/nitrosated forms of the proteins, which usually recover their active reduced (red) state in this way (Dietz, 2011; Foyer et al., 2020; Martí et al., 2020). In this review, we summarize some of the identified plant oxiPTMs in targets, such as the antioxidant and redox proteins mentioned above, the situations in which they occur, and the effect (if known) on their catalytic activities, structure or localization.
2 ROS-DERIVED PTMS
Oxidative modifications of proteins are emerging as a fine mechanism of control of the protein function, as they are able to affect protein stability, structure, activity, location, and interaction with other proteins. Among oxiPTMs, thiol-based oxiPTMs occur on cysteine (Cys) residues (summarised in Figure 1). One particularity of the Cys residue is that it is one of the least abundant amino acids in proteins, and, when present, it is often very highly conserved and important from a functional point of view, so its modification may have significant consequences. Another interesting aspect is that Cys residues contain a polarizable sulphur atom and, therefore, their oxidation state can range from the fully reduced thiol/thiolate anion (-SH/-S−) to the fully oxidized sulfonic acid (-SO3H). Among ROS, H2O2 is the main responsible for oxidation of the thiolate to the sulfenic form (-SOH), which can generate an intra- or intermolecular disulfide bond (S-S) through the reaction with a neighbouring thiolate or can be reduced and reverted to -SH. A higher oxidative situation induces the generation of sulfinic (-SO2H) and -SO3H forms that are irreversible, except for some proteins, such as specific peroxiredoxins (PRXs), whose -SO2H can be regenerated by SRXs that, in turn, are regenerated by TRX (Iglesias-Baena et al., 2011; Sevilla et al., 2015).
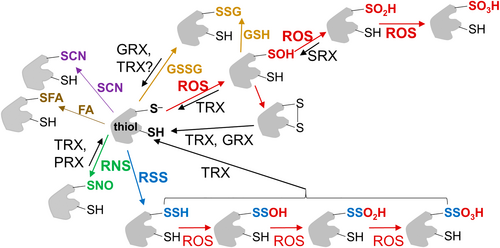
The fact that most thiol-based oxiPTMs are reversible is key for the plant responses under adverse situations. Thus, some components of the enzymatic antioxidant system, which are in charge of the regulation of ROS levels via their oxidation, may, in turn, be regulated and regenerated by means of the redox system. The reversibility of the redox reactions of the redox systems is considered a useful mechanism of control of the proteins' structure and function and a sensing mechanism in proteins involved in oxidative signalling events under stress. Rapid response of the antioxidant enzymes to abiotic and biotic stress conditions is primarily controlled by the redox state of thiol groups in their amino acid sequences, regulated by the redox system (Meyer et al., 2012; Lázaro et al., 2013; Jiménez et al., 2024). TRX and GRX contain two redox-active Cys arranged in a Cys-X2-Cys motif in charge of a thiol-disulfide exchange reaction attacking a disulfide bond of specific target proteins, whereas NRX contains three tandemly arranged TRX-like modules. TRX is considered to be the main reductant of sulfenic acids, while GRX is the main responsible for the reduction of glutathionylated proteins (Rouhier et al., 2008; Martí et al., 2009, 2020) (Figure 2). As a typical example, once oxidised, PRX can be converted back to the reduced thiol group by the action of TRXs and/or GRXs (Dietz, 2011; Sevilla et al., 2015). Regeneration of the reduced forms of TRX is then carried out by thioredoxin/ferredoxin reductases (at the expense of NADPH or ferredoxin in chloroplasts, respectively), and regeneration of glutathionylated GRX is carried out by GSH, which is converted to GSSG and regenerated to GSH by GR (Meyer et al., 2012). Furthermore, NRX1 has been reported to target several antioxidant enzymes, such as CAT1, CAT2, CAT3, and APX1 (Kneeshaw et al., 2017). As an example, CAT was maintained in a reduced state by interaction with NRX1, a process that is necessary for its H2O2-scavenging activity. Similarly, some TRXo targets were described in mitochondria, such as Mn-SOD (MSD1), PRXIIF, and GPX6 (Barranco-Medina et al., 2009; Martí et al., 2009; Yoshida et al., 2013). In root plastids, some identified antioxidant targets of TRXy1 were Cu, Zn-SOD (CSD2), CAT3, MDHAR6, and peroxiredoxin TPX1 (Marchand et al., 2010), whereas AtMDHAR6 was also identified as a target of AtTRXy2 (Vanacker et al., 2018). APX1, DHAR3, and type II-PRX were described as cytosolic TRXh3 targets identified by proteomic approaches (Marchand et al., 2004; Yamazaki et al., 2004), while 1-Cys PRX, 2-Cys PRX, APX1, CAT1, GPX, Cu, Zn-SOD, and DHAR2 were revealed as TRXh1 targets in wheat seeds (Wong et al., 2004).
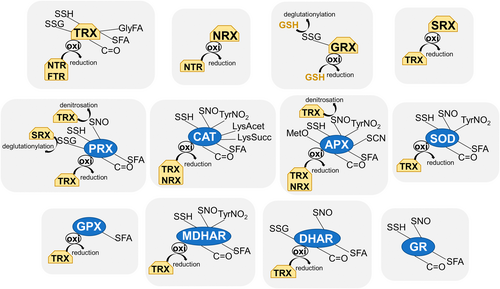
Oxidative stress is known to induce a loss of enzymatic activity, and H2O2 is recognized to inhibit Cu, Zn-SOD and Fe-SOD but not Mn-SOD isozymes (reviewed by del Río et al., 2018). Also, H2O2 induces changes in the oligomerization of certain proteins prone to oxidation, such as cytAtAPX1 (Kaur et al., 2021). Redox regulation influences both the structure and function of these proteins, which transition from peroxidases in the dimeric state to chaperones when forming high molecular weight complexes. Interestingly, heat stress favoured the formation of oligomeric complexes, whereas salt stress instigated the dissociation of the complexes, implying that specific abiotic stresses modulated the structural status of cytAtAPX1 in vivo. Usually, oxidation elicits an inactivation of the enzymatic activity, as is also reported in Arabidopsis DHAR2, in which H2O2 oxidised its Cys20 residue to sulfenic and sulfonic acids, decreasing its enzymatic activity (Waszczak et al., 2014). With regard to Arabidopsis MDHAR, the activity of root extracts was increased when TRXy1 (mainly found in non-photosynthetic tissues) was added. In leaf extracts, neither the addition of TRXy1 nor DTT or TRXf1 increased MDHAR activity, pointing to the specificity of the regulation (Marchand et al., 2010; Vanacker et al., 2018). This was corroborated using recombinant chloroplastic MDHAR6; its activity is induced by TRXy2 and not by other TRXs such as f, m or x (Vanacker et al., 2018). Concerning GPX, a TRX1-mediated redox regulation of longan fruit GPX has been described to be involved in senescence or quality deterioration of harvested fruits (Wu et al., 2021). CAT is another enzyme that undergoes oxidative modification, and during pepper fruit ripening, this oxidation leads to a decrease in its activity (Palma et al., 2020).
Similar to Cys, the sulphur-containing methionine (Met) residues are susceptible to H2O2, provoking sulfoxidation (MetO). This PTM inactivated banana cytAPX and can be partially reverted by Met sulfoxide reductase B2 (Xiao et al., 2021).
3 GLUTATHIONE-DERIVED PTM: GLUTATHIONYLATION
S-glutathionylation or thiolation is a PTM forming mixed disulfides (protein-SSG) between protein reactive thiols and GSH, being involved in the regulation of redox-based signalling events in the cell and serving as a protective mechanism against oxidative injury. This PTM alters protein function, interactions, and localization across physiological processes (Dixon et al., 2005).
Among the described targets for glutathionylation are AtMDHAR, AtDHAR1, AtDHAR2, At2-Cys PRX, AtGRX S12, TRXf (in Arabidopsis and Spinacia oleracea L.), and poplar TRXh2 and PRXIIB (Dixon et al., 2002, 2005; Gelhaye et al., 2004; Michelet et al., 2005; Noguera-Mazón et al., 2006) (Figure 2). With regard to DHAR, when incubated with GSSG, the putative active site cysteine of cytosolic Arabidopsis DHAR1 and DHAR2 formed a mixed glutathione disulfide (protein-SSG), whereas the plastidic/mitochondrial DHAR3 formed an intramolecular disulfide bridge (S-S) (Dixon et al., 2002). These authors showed that GSSG treatment of AtDHAR3 caused partial S-glutathionylation of Cys11 and disulfide bond formation between Cys25 and Cys28. The glutathionylation of AtDHAR1 in vitro with GSSG has been reported to occur in Cys20, identifying a spontaneous thiolation in the monomer and oligomer forms of the protein in the absence of reducing agents (Dixon et al., 2005). The glutathionylation of AtDHAR2 in its Cys20 has been described to protect the residue from irreversible oxidations (Waszczak et al., 2014). GSSG pre-treated AtDHAR1 was found to retain 77% of its activity, whereas AtDHAR3 maintained 95% of its activity (Dixon et al., 2002), being a good example of the fine regulation exerted by a PTM on different isozymes of a specific protein. Regarding PRX, changes in structure have been described for pea chloroplastic 2-Cys PRX, in which glutathionylation induced a change from decamer to dimer, and also for poplar 1-Cys PRXIIB, which changed from dimers to monomers (Noguera-Mazón et al., 2006; Calderón et al., 2017). However, this is not a general mechanism for PRXs, and, for example, glutathionylation of mitochondrial PRXIIF did not cause any change in oligomerization, although glutathionylation of the peroxidatic and resolving cysteines decreased the activity of the protein (Calderón et al., 2017). Another interesting point described by Calderón et al. (2017) was the different sensitivity of 2-Cys PRX and PRXIIF to small changes in the GSH/GSSG ratio, with implications in H2O2-dependent signalling events during stress. Moreover, pea SRX deglutathionylated 2-Cys PRX, but not PRXIIF. All this highlights the specificity of the glutathionylation-deglutathionylation processes.
Among the glutathionylated redox proteins identified in plants are Arabidopsis TRXf and poplar TRXh2, which are thiolated in an additional conserved cysteine different from the two active-site cysteines (Gelhaye et al., 2004; Michelet et al., 2005). Interestingly, thiolation of TRXh2 increased the redox potential of the enzyme, whereas thiolation of this Cys in TRXf slowed the metabolism due to the decreased ability to be reduced by ferredoxin-thioredoxin reductase, resulting in impaired light activation of target enzymes involved in carbon fixation in response to oxidative stress (Michelet et al., 2005).
4 H2S-DERIVED PTM: PERSULFIDATION
Protein persulfidation is a PTM of Cys residues (SH) to persulfides (-SSH) elicited by H2S, a ubiquitous gaseous signalling molecule present in both animal and plant systems and involved in the regulation of critical processes allowing the adaptation to changing and stressful situations (Aroca et al., 2021). H2S reacts with sulfenic acid (and not with thiols), and the generated persulfides are highly reactive to ROS, being oxidized to perthiosulfenic (–SSOH), perthiosulfinic (–SSO2H), and perthiosulfonic (–SSO3H) acids. Oxidised persulfides can then be reduced by TRXs and GRXs (Filipovic et al., 2019). Persulfidation causes changes in location and protein function through the alteration of catalytic activities. As an example, the nuclear or cytosolic localization of glyceraldehyde 3-phosphate dehydrogenase GapC1 is regulated by persulfidation (Aroca et al., 2017a). Treatments with H2S have been reported to increase stress resistance through the modulation of components of the antioxidant system, among others, although sometimes with different effects depending on the species. As an example, NaHS decreased the oxidative damage induced by NaCl in alfalfa, activating SOD, CAT, and APX, whereas H2S reduced the decrease in CAT and GR activities as well as the content of ASC and GSH in cucumber caused by salinity (Wang et al., 2012; Jiang et al., 2019). Several targets of persulfidation have been reported in Arabidopsis, including CAT1, CAT2, CAT3, PRXIIF, 2-Cys-PRXA, 2-Cys-PRXB, CytAPX1, MDHAR, GR1, GR2, TRXo1, and TRXm1 (Aroca et al., 2015, 2017b) (Figure 2). Specifically, persulfidation by H2S inhibited CAT activity in Arabidopsis but activated APX1 in Arabidopsis and tomato, and in this last species, persulfidation increased the resistance to oxidative stress induced by copper oxide nanoparticles (Aroca et al., 2015, Li et al., 2020). Also, the concentration of NaHS used for the treatment is important for the effect on the enzymatic activities, as it has been recently described in common bean nodules, where APX activity was only inhibited by >50 μM NaHS treatment whereas CAT activity was not affected in a concentration range of 0–500 μM and needed up to 5 mM to be inhibited (Matamoros et al., 2024). In addition to Arabidopsis, CAT has also been found in a persulfidated state in immature and mature pepper fruits (Muñoz-Vargas et al., 2024). The in vitro persulfidation assay using peroxisomes of Arabidopsis has revealed the inhibition by persulfidation of the enzyme (reviewed by Palma et al., 2020). The activation of cytAPX1 by NaHS at a low concentration has revealed a possible physiological role for sulfide in plants through protein persulfidation like the one that is well-established in animals (Aroca et al., 2015). It would be interesting to know whether the activating effect is a general mechanism of control of the enzymatic antioxidant activities and whether the TRX/GRX system could play a regulatory and significant role in the reversibility of the modification, mainly during oxidative signalling under stress, as it has been described in animal systems.
5 RNS-DERIVED PTMS: NITROSATION AND NITRATION
S-nitrosation (also known as S-nitrosylation, SNO) is a reversible PTM produced by the binding of NO to sensitive Cys in proteins, which may alter enzymatic activities by inactivation or activation (Wolhuter & Eaton, 2017). Nitration is produced by the reaction of peroxynitrite (ONOO− generated by the reaction of NO and O2−) with the binding of a nitro group (-NO2) to specific amino acids, tyrosine being the most studied (Ferrer-Sueta et al., 2018).
The modification of the activity of SOD by RNS has been reported (Figure 2). The S-nitrosation of Mn-SOD in isolated mitochondria treated with the NO donor DETA-NONOate or the in vitro treatment with GSNO of the Arabidopsis recombinant proteins MnSOD (AtMSD1), FeSOD (FSD3) and three Cu, Zn-SOD (CSD1-3) isoforms, did not affect their enzymatic activities (Martí et al., 2013; Holzmeister et al., 2015). However, mitochondrial AtMSD1 nitration in Tyr63 and apoplastic AtMSD2 in Tyr68 lead to a decrease in the activity, possibly by impeding the accessibility of the substrate. Other isoforms whose activities were affected by Tyr-nitration, although to a lesser extent, were peroxisomal AtCSD3 and chloroplastic AtFSD3; (Holzmeister et al., 2015; Chen et al., 2022). As to CAT, its nitrosation in pea, Arabidopsis, and pepper fruits decreased its activity (Palma et al., 2020), and the pepper enzyme was also inhibited by Tyr nitration (Chaki et al., 2015). With regard to PRX, in Arabidopsis and citrus, this peroxidase has been reported to be nitrosated under both normal and saline conditions (Tanou et al., 2009; Lindermayr et al., 2015), and this PTM in Cys121 induced a decrease in the peroxidase and peroxynitrite reductase activities of chloroplast PRXIIE during plant hypersensitive response, having as a consequence the increase in ONOO− and the subsequent nitration of several proteins (Romero-Puertas et al., 2007, 2008). Also, in our laboratory, mitochondrial PsPRXIIF in pea was found to be susceptible to nitrosation in catalytic Cys59 and Cys89, affecting its oligomeric state and inhibiting the peroxidase activity in favour of a transnitrosylase activity, nitrosated under long salinity stress (Camejo et al., 2013). The reversibility of nitrosation by the redox system and, consequently, of the peroxidase activity might act as a control mechanism of the function of PRXIIF in mitochondria under oxidative and nitrosative signalling events under stress situations (Martí et al., 2020).
The modulation of the antioxidant enzymes of the ASC-GSH pathway by RNS-derived PTMs has also been reported. Proteomics studies have pointed to the peroxidase activity of cytosolic APX as one of the targets of S-nitrosation and tyrosine nitration (Martí et al., 2013; Begara-Morales et al., 2014). Tyrosine nitration leads to irreversible inhibition of tobacco APX and CAT (Clark et al., 2000), whereas pea mitochondrial PsAPX and AtAPX1 peroxidase activities were reversibly regulated by S-nitrosation and irreversibly regulated by tyrosine nitration (Martí et al., 2013, Begara-Morales et al., 2014). However, these PTMs did not affect the chaperone activity or even the structural status of the AtAPX1 (Kaur et al., 2021). Also interesting is the involvement of cytosolic APX S-nitrosation in programmed cell death induced by heat shock and H2O2 in tobacco BY-2 cells, provoking a loss in the peroxidase activity but also acting as a signal for the ubiquitin-dependent protein degradation (De Pinto et al., 2013). In other species like Antiaris toxicaria (Pars.) seeds, the treatment with NO led to the nitrosation of APX, MDHAR, DHAR, and GR, but with a concomitant increase in their catalytic activities (Bai et al., 2011), whereas in cucumber this PTM inhibited the GR activity (Niu et al., 2019). Pea peroxisomal recombinant MDHAR can be nitrated in three Tyr residues by ONOO-, although nitrated Tyr345 was responsible for the inactivation of the enzyme (Begara-Morales et al., 2015). Also, nitrosation was able to inhibit MDHAR activity in vitro, while none of the RNS-derived PTMs affected the cytosolic or chloroplastic GR activities, which suggests that a mechanism exists to preserve redox status via reduced GSH (Begara-Morales et al., 2015). In pea mitochondria, and in contrast to an NO treatment, the peroxynitrite producer SIN1, together with the strong APX inhibition, moderately inhibited GR and DHAR, but did not affect MDHAR and Mn-SOD activities, being the response of these mitochondrial enzymes an important antioxidant defence against potential nitrosative stress induced by NO and ONOO− in this organelle (Martí et al., 2013).
Specific proteins have been reported as nitrosated by transnitrosation reactions, mainly in animal systems, and GSH and GSNO, together with reductants, such as cytosolic and mitochondrial TRXs, can mediate protein denitrosation (Benhar et al., 2008). In plants, TRX and NTR have been involved in denitrosation in processes such as plant immunity, protein translation, and root development. For example, TRXh5 carried out a selective denitrosation of excessive protein-SNO, which reinstated signalling by the immune hormone salicylic acid (Kneeshaw et al., 2014) and TRX/NTR mediated denitrosation and partial inhibition of cytAPX1 during auxin-mediated root development (Correa-Aragunde et al., 2013). The crosstalk between S-nitrosation and Tyr-nitration has been reported for PRX: interestingly, nitrosation of PRXIIE inhibited its peroxidase and peroxynitrite reductase activities, increasing ONOO- and nitration of proteins during the progression of the hypersensitive response to pathogens in Arabidopsis (Romero-Puertas et al., 2007). PRXIIE also displayed a trans-denitrosating activity on the bZIP67 transcription factor involved in fatty acid metabolism during seed development, thus destabilizing the bZIP67 function (Sánchez-Vicente et al., 2024). Another interesting result is the reported ability of bovine Cu, Zn-SOD to decompose GSNO in the presence of H2O2 to generate GSSG and NO (Singh et al., 1999), involving SOD in NO signalling, although the decomposition of nitrosothiols needs to be confirmed for plant Cu, Zn-SOD.
6 HCN-DERIVED PTM: CYANYLATION
In spite of its toxicity, cyanide generated in all organisms regulates different biological processes, and its reactivity with oxidized cysteine residues in disulfide bridge leads to S-cyanylation (SCN). A proteomic study of Arabidopsis roots identified cyanylated proteins involved in several metabolic pathways (García et al., 2019) and, among antioxidants, cytAPX1 and two proteins of the peroxidase family appeared as modified with this PTM (Figure 2), even if its effect on activities was not evaluated. The inhibitory effect of HCN on the active metal in metalloproteins, such as Cu, Zn-SOD and CAT, has been known for a long time, although the involvement of S-cyanylation was not described (Paci et al., 1988). Despite the fact that advances are being made in the methodology to identify proteins affected by this PTM, its biological significance is yet to be explored and represents an important challenge for future research.
7 FATTY ACIDS-DERIVED PTM: ACYLATION
Fatty acids (FA) react with thiols, provoking S-acylation (SFA, also known as palmitoylation), although its effect on plant proteins has been scarcely investigated. Recently, several antioxidant enzymes have been identified as S-acetylated in different Arabidopsis tissues, even if the effect of this PTM on catalytic activities was not determined: DHAR (isoforms 1 and 2), MDHAR1, APX (isoforms 1, 3, and 5), GR, GPX1, TRX family proteins, TRXf, 2-Cys PrxB, Cu, Zn-SOD (isoforms 1, 2, and 3), and CAT (isoforms 1, 2, and 3) (Kumar et al., 2022) (Figure 2). An interesting mechanism for palmitoylation together with myristoylation of AtTRXh9 is the described effect of this PTM on the N-terminal amino acids Cys4 and Gly2 (GlyFA), respectively, which is important for the association of the protein with the plasma membrane, allowing TRXh9 movement from cell to cell during the intercellular communication (Meng et al., 2010).
Like other acylation modifications of antioxidant proteins, acetylation (a carbonyl group linked to a methyl group) and succinylation (a divalent carboacyl group) of lysine residues in CAT have been detected and proposed to have a regulatory function in rice under oxidative stress situations (Zhou et al., 2018).
8 C=O-DERIVED PTM: CARBONYLATION
ROS can also exert their biological function through the introduction of carbonyl groups (C=O) into the side chain of Lys, Arg, Pro, and Thr via the Fenton reaction or through the peroxidation of lipids generating unsaturated aldehydes, which then form carbonyl adducts on Cys, His, and Lys residues in a non-enzymatic and irreversible process. Emerging roles are appearing that involve protein carbonylation in protein quality control, cell homeostasis, fruit ripening, and hormone signalling, although the biological significance of this PTM in growth and development is still quite unknown in plant systems (Camejo et al., 2015; López-Vidal et al., 2016). Among carbonylated proteins found in the mitochondrial matrix of rice leaves of plants grown during 7 days in constant light and 1 day in darkness was Mn-SOD, whereas CAT, PRX, and APX were identified as carbonylated when mitochondria were exposed to a metal-catalysed oxidation agent (Kristensen et al., 2004) (Figure 2). Additionally, APX, MDHAR, DHAR, and GR were found to be inhibited by carbonylation during seed dehydration of Antiaris toxicaria (Bai et al., 2011). Recently, the study of carbonylated proteins in Arabidopsis plants has identified among antioxidants Trxh3 under control conditions and 2-Cys PRX B under an H2O2 treatment (Fangue-Yapseu et al., 2022).
9 CONCLUSIONS AND PERSPECTIVES
Usually, stress situations in all organisms lead to controlled and specific changes in cell metabolism, with an increase, among others, in reactive oxygen, nitrogen, and sulphur species, provoking post-translational modifications due to the ability to tag specific amino acids in determined target proteins. The role of the different PTMs has not been well established because of the complexity of their underlying mechanisms. A specific PTM for a given protein seems to have different effects depending on the modification site and even causes opposite effects on different proteins (like the activation or inhibition of enzymatic activity). Moreover, one protein is usually a target of different PTMs, and due to the differential redox homeostasis in the specific cellular compartments, PTMs may affect the same protein in a distinct way depending on its location. This complicated scenario points to the necessity of understanding the selectivity of a given PTM, the sensitivity of the amino acid residues to a determined PTM, the effect of individual or combined PTMs on a protein, and the organelle-specific PTMs, all of them influencing protein structure, function, and/or location with consequences on signalling and cell metabolism that ultimately allow for a positive response of the plant to cope with the situation. There is still a lot to do and understand, but the role of modulation of cell metabolism by PTMs of proteins is growing and attracting the interest of researchers, not only in plants facing climate change and stress, but also in animal systems fighting against illnesses and disorders, all of them situations in which PTMs seem to act as key players for adaptation.
AUTHOR CONTRIBUTIONS
A.J., MC.M., and F.S. collected information, planned and prepared the manuscript. A.J. prepared the figures. All the authors have contributed to the article and approved the submitted version.
ACKNOWLEDGEMENTS
The authors want to thank Mr. A. Paredes for his help with the review of the English language. The authors apologize to fellow authors and colleagues whose research could not be cited or discussed owing to space limitations.
FUNDING INFORMATION
This study was supported by the Spanish grants Ministerio de Ciencia e Innovación (MICINN-FEDER) (PID2021-127335NB-I00 and Network RED2018-102397-T) and Murcia Regional Program “Fomento de la Investigación Científica y Técnica, Plan 2022” through the Fundación Séneca (22051/PI/22).
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.




