Sphingolipid long chain bases as mediators of cell death in olive fruit abscission
Abstract
Plant sphingolipids are lipophilic membrane components essential for different cellular functions but they also act as signaling molecules in various aspects of plant development. However, the interaction between plant sphingolipids and abscission remains largely uncharacterized. Here, the possible role of sphingolipids in regulating fruit abscission was examined in the abscission zone (AZ) of olive fruit. To this end, sphingolipid levels were manipulated through the application of exogenous sphingolipid long-chain bases (LCBs) or biosynthesis inhibitors, and their effects on fruit abscission as well as sphingolipid LCB/gene expression, hormones, reactive oxygen species (ROS) and cell death levels were examined in the AZ of olive fruit. Our data indicated that exogenous sphinganine (d18:0) induced fruit abscission, whereas the application of sphingosine (d18:1) or phytosphingosine (t18:0) or their phosphorylated derivatives did not have an effect on fruit abscission. Moreover, inhibition of LCB kinase or ceramide synthase, which increases sphingolipid LCB levels in the AZ, reduced fruit break strength. This induction of fruit abscission is associated with elevated ROS levels and cell death in the AZ enriched in salicylic acid (SA) and jasmonic acid (JA). Along the same line, programmed cell death (PCD) was particularly evident on the distal side of the AZ. These data suggest that endogenous d18:0 plays a key cellular role as signaling molecule functioning upstream of the SA/JA signaling pathway in mediating PCD spatially regulated in the AZ during fruit abscission. Overall, the findings reported here provide insight into the complex connection between PCD and plant sphingolipid LCBs, uncovering their interaction in the abscission process.
1 INTRODUCTION
Plant organ abscission has been studied not only regarding the molecular and physiological aspects of the process but also in relation to its significant implications in agriculture (Tranbarger et al., 2017; Tranbarger and Tadeo, 2020; Li et al., 2021; Shi et al., 2023; Li and Su, 2024). Research has addressed the scarcely understood regulatory steps of abscission in crop species, including its activation and the signaling mechanisms developed in the fleshy fruit and that result in features that have an impact on crop production and yield (Estornell et al., 2013; Botton and Ruperti, 2019; Meir et al., 2019; Ma et al., 2021; Dutta et al., 2022; Shi et al., 2023). In the case of olive (Olea europaea L.), a major oil fruit tree crop worldwide, understanding fruit abscission would facilitate the mechanical harvest of fruit, either for oil or table olives (Barranco et al., 2004; Gomez-Jimenez et al., 2010a; Goldental-Cohen et al., 2017; Parra and Gomez-Jimenez, 2020).
Abscission of plant organs is regulated by complex signaling networks that mediate specific and dynamic plant responses upon activation by various types of exogenous and endogenous cues (Estornell et al., 2013; Patharkar and Walker, 2016, 2018, 2019; Meir et al., 2019). These responses include cell separation events underlying in the abscission zone (AZ) during organ detachment, such as protein degradation, cell wall remodeling, middle lamellar dissolution, cell membrane permeability loss, reactive oxygen species (ROS) accumulation, and programmed cell death (PCD) events (Cho et al., 2008; Bar-Dror et al., 2011; Gil-Amado and Gomez-Jimenez, 2012; Parra et al., 2020; Schuster and van der Hoorn, 2020; Yu et al., 2023).
A connection between plant organ abscission and sphingolipids has been identified in olive, indicating that sphingolipids, sterols, remorins, and signaling proteins, which contribute to the formation of membrane nanodomains, should be considered in AZ activation during the abscission process (Gil-Amado and Gomez-Jimenez, 2013). Additionally, an increase in polar lipids, sphingolipids, and endocytosis has been observed during olive fruit abscission (Parra-Lobato et al., 2017).
The structural role of complex sphingolipids as critical building components of the plasma membrane, nanodomains, and the endomembrane system is well recognized (Chen et al., 2009; Li et al., 2016; Luttgeharm et al., 2016; Michaelson et al., 2016; Cassim et al., 2020; Huby et al., 2020; Carmona-Salazar et al., 2021; Haslam and Feussner, 2022; Bahammou et al., 2024). Beyond this structural function, sphingolipid precursors, such as long-chain bases (LCBs) and ceramides (Figure 1), have signaling roles in multiple cellular and regulatory processes, including vesicle trafficking, PCD, plant development and stress responses (Shi et al., 2007; Alden et al., 2011; Saucedo-García et al., 2015, 2023; Qin et al., 2017; Ali et al., 2018; Huby et al., 2020; Zhang et al., 2020; Liu et al., 2021, 2022a; Zeng and Yao, 2022; Devendrakumar et al., 2024; Fougère et al., 2024).
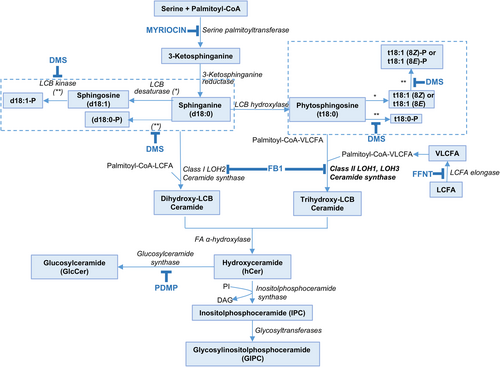
The presence of a rheostat between LCBs/ceramides and their phosphorylated counterparts—previously described in animal cells and linked to PCD—is also believed to exist in plants, where it likely regulates cell fate. According to this model, LCBs and ceramides can trigger PCD, whereas ceramide phosphates and LCB-Ps promote cell survival (Shi et al., 2007; Alden et al., 2011). Notably, the widest documented participation of LCBs and ceramides as transducers in signaling pathways leading to PCD is the physiological context of plant defense against pathogens (Zeng and Yao, 2022 for a recent review). The currently accepted model contends that pathogen signals trigger de novo synthesis of LCBs, which are transiently accumulated in the plant host, acting as second messengers (Peer et al., 2010; Saucedo-García et al., 2011). Other transducers in this PCD signaling wire are the protein kinases MPK6 (Saucedo-García et al., 2011) and a 14–3-3-regulated CPK3 (Lachaud et al., 2013), which are activated downstream of the LCB surge. ROS have been consistently associated with the LCB-mediated transducing route towards PCD. They increase at high levels upon LCB build-up, and their origin has been assigned to ROS-generating systems at the plasma membrane or the chloroplast (Peer et al., 2011; Zavafer et al., 2020; Saucedo-García et al., 2023).
The intervention of plant hormones in the PCD mediated by sphingolipids has more experimental support when the role of ceramide as a second messenger is considered. Jasmonic acid (JA), salicylic acid (SA) and ethylene are hormones associated with the signaling role of ceramides. This support is based mainly on the relationship between ceramide accumulation or depletion and hormone levels (Liang et al., 2003). More recently, it has been shown that JA modulates sphingolipid metabolism and accelerates cell death in the ceramide kinase mutant acd5 (Huang et al. 2021). Moreover, it has been demonstrated that sphingolipid-induced PCD is a salicylic acid and EDS1-dependent phenotype in Arabidopsis FATTY ACID HYDROXYLASE (Fah1, Fah2) and CERAMIDE SYNTHASE (Loh2) triple mutants (König et al., 2022). Likewise, disruption of Arabidopsis neutral CERAMIDASES 1 and 2 results in specific sphingolipid imbalances triggering different hormone-dependent plant cell death programmes (Zienkiewicz et al., 2020). A close association of LCBs with defense signaling hormones can be found in the early experiments performed by Stone et al. (2000) and Asai et al. (2000) using a Fumonisin B1 model that evokes an LCB accumulation. The interpretation of these experiments pointed out to the involvement of LCBs in the SA and JA pathways.
Given that AZ cells undergo changes that culminate in the final detachment of the fruit, it is feasible that a population of cells undergoing PCD is required. However, the association between LCBs and cell death during plant organ abscission remains unexplored. The purpose of the present study was to investigate whether plant sphingolipids might drive PCD in the abscission process. To this end, sphingolipid levels in the AZ were manipulated through the application of exogenous LCBs or sphingolipid biosynthesis inhibitors to olive trees (Figure 1). Afterwards, we examined the effects of altered sphingolipid levels on fruit abscission as well as sphingolipid LCB content, gene expression, hormone, ROS and cell death levels. The results described in this work provide significant evidence of the connection between sphingolipid LCBs and PCD spatially regulated in the AZ during the abscission process. We further show that the inhibition of ceramide synthase or LCB kinase activity, which is associated with the accumulation of LCBs in the AZ, led to the induction of this process. In this context, we propose that sphinganine (d18:0), the most elemental form of sphingolipid LCBs, acts as a positive regulator of the abscission process via an enhancement of ROS and cell death levels in the AZ along with the activation of SA and JA pathways.
2 MATERIALS AND METHODS
2.1 Plant material and chemical treatments
Twenty-year-old olive trees (Olea europaea L.) grown under drip irrigation and fertirrigation in an orchard near Badajoz (Spain) were studied. The table olive cultivar ‘Manzanilla Sevillana’ was chosen due to its high fruit detachment force (FDF) at the commercial harvest stage corresponding to 140 days post-anthesis (DPA) (Camarero et al., 2024). This olive cultivar exhibits massive natural fruit abscission after ripening, as do other olive cultivars (Gomez-Jimenez et al., 2010a). Fruit AZs were collected at two developmental stages, the commercial harvest stage (140 DPA) and the fully ripe stage (169 DPA), using 3 olive trees per developmental stage (Gil-Amado and Gomez-Jimenez, 2013; Parra et al., 2020; Parra and Gomez-Jimenez, 2020). At each stage, 150 fruit AZs per tree were collected for analysis. Fruit AZs from each tree formed a biological replicate. For the cytological study of the fruit AZ, freshly excised AZ samples were stained with 0.04% (w/v) toluidine blue as previously described (Parra and Gomez-Jimenez, 2020). In addition, for an examination of the proximal and distal fracture planes of the fruit AZ by scanning electron microscopy (SEM) during natural fruit abscission, following critical-point drying, samples were mounted onto steel stubs, coated with gold–palladium and viewed using an LEO 1430 VP SEM (Gomez-Jimenez et al., 2010a; Corbacho et al., 2013; Parra et al., 2013).
Fruit abscission experiments were performed in planta as previously described (Parra-Lobato and Gomez-Jimenez et al., 2011; Gil-Amado and Gomez-Jimenez, 2012). Fruit treatments were applied as an aqueous solution (250 mL per branch) on olive trees (3 per treatment) at 140 DPA (Corbacho et al., 2018; Inês et al., 2019). In an effort to avoid contamination during spraying, at least one guard tree was used to separate each of the test trees, and the trees were sprayed only with weak or no wind. For each treatment, 3 branches (1 branch per tree) were sprayed with exogenous LCBs or their phosphorylated derivatives. These compounds were originally dissolved in 0.1% ethanol or 0.1% methanol, respectively, at the following concentrations: 1 mM dihydrosphingosine (d18:0, sphinganine, Sigma-Aldrich), 1 mM sphingosine (d18:1, Sigma-Aldrich), 1 mM phytosphingosine (t18:0; Sigma-Aldrich), 1 mM d18:0-P, 1 mM d18:1-P or1 mM t18:0-P (Avanti Polar Lipids). These four species of dihydroxylated and trihydroxylated LCBs are precursors of complex sphingolipids. Similar treatments with inhibitors of sphingolipid biosynthesis were also included (Figure 1): 10 mM fumonisin B1 (FB1; Sigma-Aldrich), 10 mM N,N-dimethylsphingosine (DMS; Sigma-Aldrich), 10 mM myriocin (Sigma-Aldrich), 10 mM d-l-threo-1-phenyl-2-decanoyl amino-3-morpholino-1-propanol (PDMP; Enzo Life Sciences AG) or 10 mM flufenacet (FFNT; Sigma-Aldrich). For the purposes of this study, treated and untreated fruit AZ samples were collected from each tree after 3 and 6 days of treatment. Freshly excised AZ samples were immediately used for in vivo measurements of ROS and cell death levels or were frozen in liquid nitrogen and stored at −80°C for subsequent use.
2.2 Fruit break strength
Fruit break strength or fruit detachment force (FDF), defined as the kg force (kgf) necessary to separate the fruit from the parent plant at the AZ site, was measured as described elsewhere (Parra-Lobato and Gomez-Jimenez, 2011).
2.3 Sphingolipids analysis
Total sphingolipids were analyzed using their released LCB and adding C20-4-SPH (d20:1) as internal standard, as previously described (Markham et al., 2006; Corbacho et al., 2018; Inês et al., 2018). HPLC analyses were carried out using an Agilent Technologies HPLC Infitinity 1260 by reverse phase HPLC on a 2.1 mm × 150 mm Eclipse XBD-C18 Narrow-bore column (Agilent Technologies, Inc.). Elution was performed at (0.4 mL/min) with 20% solvent RA (5 mM potassium phosphate, pH 7), 80% solvent RB (100% methanol) for 7 min, increasing to 90% solvent RB by 15 min, followed by isocratic flow for 10 min before increasing to 100% solvent RB by 30 min with a 3-min 100% solvent RB wash before returning to 80% solvent RB and re-equilibrating for 2 min. Fluorescence was excited at 340 nm and detected at 455 nm. The results were analyzed and integrated by OpenLAB®.
2.4 Quantitative RT-PCR analysis
Isolation of total RNA, qRT-PCR and data calculation were performed as previously described (Gil-Amado and Gomez-Jimenez, 2013; Parra-Lobato et al., 2017; Briegas et al., 2020; Parra and Gomez-Jimenez, 2020). Total RNA (2 μg) was reverse-transcribed with random hexamers and Superscript III (Invitrogen), according to the manufacturer's instructions. Purified cDNA (2 ng) was used as a template for qRT-PCR. The cDNA was amplified using SYBRGreen-PCR Master kit (Applied Biosystems) containing an AmpliTaq Gold polymerase on an iCycler (BioRad) by following the supplier's protocol. Expression levels were determined for genes encoding the serine palmitoyltransferase I (OeSPT/LCB1, Unigene ID: OL002160), LCB kinase (OeLCB Kinase, Unigene ID: OL002715), alkaline dihydroceramidase (OeACER, Unigene ID: OL002183) and glucosylceramidase (OeGlcCerase, Unigene ID: OL002165). qRT-PCR assays were performed with gene-specific primers (Table S1). Samples were subjected to thermal cycling conditions of DNA polymerase activation at 94°C, 45 s at 55°C, 45 s at 72°C, and 45 s at 80°C. A final elongation step of 7 min at 72°C was performed. The melting curve was designed to increase by 0.5°C every 10 s from 62°C. The amplicon was analyzed by electrophoresis and sequenced once for identity confirmation. In addition, qRT-PCR efficiency was estimated via a calibration dilution curve and slope calculation. Expression levels were determined as the number of cycles needed for the amplification to reach a threshold fixed in the exponential phase of the PCR (CT). The data were normalized for the quantity of the Olea europaea UBIQUITIN (OeUb) gene (Gomez-Jimenez et al., 2010b). Duplicates from three biological replicates were used in two independent experiments.
2.5 Plant hormone quantification
The levels of plant hormones abscisic acid (ABA), JA, SA, indole-3-acetic acid (IAA), gibberellin 1 (GA1), gibberellin 4 (GA4), trans-Zeatin (tZ), dihydrozeatin (DHZ) and isopentenyladenine (IP) were quantified as described elsewhere (Camarero et al., 2023a,2023b). A pool of 100 mg fresh weight/sample was used for each measurement, split into three independent biological replicates per sample. Aliquots of lyophilized material were extracted with 80% methanol-1% acetic acid. Deuterium-labeled hormones (purchased from Prof. L Mander- Canberra, OlChemim Ltd-Olomouc, or Cambridge Isotope Lab- Andover): [17,17-2H]GAn, [2H5]IAA, and [2H6]ABA were added as internal standards for quantification of SA and ABA. For the quantification of JA, the compound dhJA was used instead. For collecting the acids fraction containing SA, ABA, and JA, the extracts passed consecutively through HLB (reverse phase), MCX (cationic exchange), and WAX (ionic exchange) columns (Oasis 30 mg, Waters). The final residue was dissolved in 5% acetonitrile-1% acetic acid, and the hormones were separated by reverse phase UPHL chromatography (2.6 μm Accucore RP-MS column, 100 mm length × 2.1 mm i.d., ThermoFisher Scientific) with a 5% to 50% acetonitrile gradient. The hormones were analyzed by electrospray ionization and targeted-SIM using a Q-Exactive spectrometer (Orbitrap detector, ThermoFisher Scientific). The concentrations of hormones in the extracts were determined using embedded calibration curves and the Xcalibur 4.1 SP1 build 48 and TraceFinder programs.
2.6 ROS production measurement
Intracellular H2O2 was detected by staining fruit AZs using DCFH2-DA (dichlorodihydrofluorescein diacetate) as previously described (Gil-Amado and Gomez-Jimenez, 2012). The proximal and distal fracture planes of fresh AZs from olive fruits were stained with 50 μM DCFH2-DA for 10 min, rinsed with distilled water, and then examined under a FV1000 confocal laser scanning microscope (Olympus). Quantitative measurement of fluorescence intensity (excitation, 488 nm; emission, 525 nm) was performed using FV10 4.2 software. All data are presented as the means ± SD of three biological replicates from three independent experiments.
2.7 Cell death estimation
To assess the cell death in olive fruit AZs, the proximal and distal fracture planes of fresh AZs were immersed in 3 μg mL−1 propidium iodide (PI) dissolved in distilled water for 10 min. After washing, the samples were examined with an FV1000 confocal laser scanning microscope (Olympus) using an excitation wavelength of 546 nm to estimate the percentages of PI-positive cells of each side of AZs. A PI-positive nucleus is a strong indication of the loss of membrane integrity (De Cnodder et al., 2005; Xu et al., 2010). Cell death was calculated as the percentage of dead cells to the total number of cells. To detect the PCD in fruit AZs, we performed the method with double staining PI and Hoechst 33342 (Ma et al., 2010). The blue-fluorescent Hoechst 33342 is a cell-permeable nucleic acid binding dye, whereas the red-fluorescent PI is an impermeable cell intercalating DNA dye, which enters the cells with a membrane integrity loss. The proximal and distal fracture planes of fresh AZs were double stained with PI (0.05%) and Hoechst 33342 (10 mg/mL) for 10 min in darkness, washed with PBS 3 times, and observed under an FV1000 confocal laser scanning microscope (Olympus). All data are presented as the means ± SD of three biological replicates from three independent experiments.
2.8 Mitochondrial membrane potential measurement
Mitochondrial membrane potential (ΔΨm) was measured with tetramethylrhodamine methylester (TMRM) staining, which accumulates only in active mitochondria with intact membrane potential (Scaduto and Grotyohann, 1999). The proximal and distal fracture planes of fresh AZs were incubated with 100 nM TMRM in darkness for 10 min and washed 3 times with PBS. Samples were subsequently imaged using a fluorescence confocal microscope (Olympus FV1000). The excitation and emission wavelength for TMRM was 549 nm and 575 nm, respectively. For quantitative analysis, the fluorescence intensity of the TMRM was expressed as mean density by FV10 4.2 software (Olympus). All data are presented as the means ± SD of three biological replicates from three independent experiments.
2.9 Statistical Analysis
All the experiments were performed in triplicate, and all data are presented as mean ± SD. Variables of three biological replicates were compared using Tukey's multiple-range test, and a p-value at 0.05 was considered significant.
3 RESULTS
3.1 Fruit break strength and sphingolipid LCB content during natural fruit abscission
To explore the potential role of sphingolipid in fruit abscission, this study used the table olive cultivar ‘Manzanilla Sevillana’ (Figure 2A), which displayed a high fruit detachment force (FDF) at the commercial harvest stage while undergoing massive natural fruit abscission after ripening under natural conditions (Figure 2B). Indeed, the FDF at the fully ripe fruit stage (169 DPA) significantly decreased compared with values recorded on the day of commercial harvest (140 DPA) (Figure 2B). In contrast, total sphingolipid (determined as free LCBs and bound LCBs in complex sphingolipids) levels proved to be higher in the AZ at the fully ripe fruit stage (169 DPA) as compared to the AZ at the commercial harvest stage (140 DPA) (Figure 2C), indicating a clear correlation between the sphingolipid accumulation and the AZ activation during the abscission process in olive. These results, as previously observed (Parra-Lobato et al., 2017), suggest that the increase of complex sphingolipids associated with the transition from the inactive to active stage of the AZ can play a putative role in reducing the force required for olive fruit detachment.
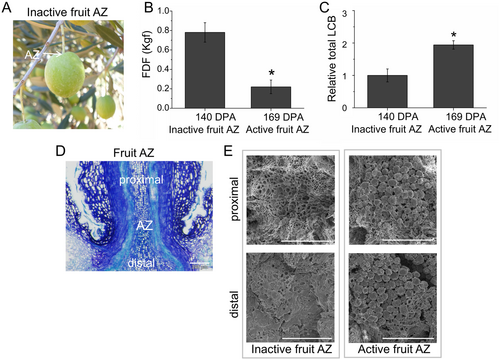
Consistently, the ripe-fruit abscission occurred at the pedicel-fruit AZ (Figure 2D), which was characterized by spherical elongated cells on the proximal and distal sides of the cell separation plane upon spontaneous detachment (the active AZ), whereas the sides of the fracture plane showed no evidence of AZ activation at the commercial harvest stage (the inactive AZ) with cells ruptured after forcible fruit removal (Figure 2E). Thus, these changes in FDF and LCB levels between the two stages were correlated with differences in the morphology of the fruit AZ.
3.2 Contribution of sphingolipids to AZ activation during fruit abscission
Next, to evaluate the role of sphingolipids in fruit abscission in more detail, treatments with exogenous sphingolipids LCBs and inhibitors of sphingolipid biosynthesis in planta were performed on ‘Manzanilla Sevillana’ olive cultivar. Changes in FDF as induced by exogenous LCBs and inhibitors of sphingolipid biosynthesis were monitored 3 and 6 days after treatments at the commercial harvest stage (Figure 3).
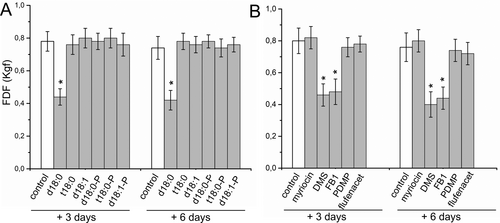
Application of exogenous LCB sphingosine (d18:1) or phytosphingosine (t18:0), as well as their phosphorylated derivatives (d18:1-P or t18:0-P) did not affect FDF compared with the control fruits (Figure 3A). Nevertheless, the application of sphinganine (d18:0), but not d18:0-P (dhS1P), significantly decreased the FDF after 3 and 6 days of treatment in olive fruit (Figure 3A), indicating that exogenous d18:0 accelerates olive fruit abscission.
Regarding sphingolipid inhibitors, we tested the FDF in response to myriocin, an inhibitor of serine palmitoyltransferase (SPT) activity; N,N-dimethylsphingosine (DMS), an inhibitor of LCB kinase in animals (Buehrer and Bell, 1992; Edsall et al., 1998) and plants (Coursol et al., 2003, 2005); fumonisin B1 (FB1), a mycotoxin that inhibits ceramide synthase activity (Spassieva et al., 2002; Guenther et al., 2008) and is associated with the accumulation of free LCBs (Abbas et al., 1994); d-l-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP), an inhibitor of glucosylceramide synthase (GCS), or flufenacet (FFNT), an inhibitor of very long-chain fatty acid (VLCFA) biosynthesis (Figure 1). The results revealed that DMS and FB1 were the only ones that significantly diminished the FDF after 3 and 6 days of treatment compared to control (Figure 3B). Thus, the chemical treatment approach demonstrated that ceramide synthase and LCB kinase activities negatively mediated fruit abscission, suggesting that these two steps of sphingolipid biosynthesis are key regulated components of sphingolipid metabolism in the AZ during fruit abscission. Taken together, these results suggest that over-accumulation of free non-phosphorylated LCBs, particularly of d18:0, positively modulates fruit abscission in olive.
3.3 Regulation of sphingolipid LCB composition and gene expression during fruit abscission
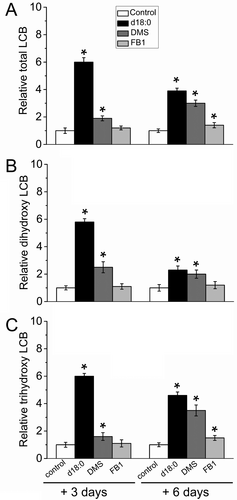
To test how the external application of LCBs or inhibitors of sphingolipid biosynthesis influence sphingolipid metabolism during the AZ activation, we first analyzed the LCB profiles resulting from the fraction of hydrolyzed sphingolipids (which include free LCBs and the LCBs contained in complex sphingolipids) from the fruit AZ in response to all three treatments with fruit abscission induction, exogenous d18:0, DMS or FB1.
As shown in Figure 4, the application of exogenous d18:0 and DMS significantly raised the total LCB levels in the olive fruit AZ after 3 and 6 days of treatment, showing accumulation in both trihydroxy- and dihydroxy-LCBs; however, DMS or d18:0-induced differences in LCB content were more marked and became maximal on day 3 compared with controls. By contrast, the FB1 application, an inhibitor of ceramide synthesis, only slightly raised total LCB levels, primarily due to the increase in trihydroxy-LCBs, and this effect was observed only after 6 days of treatment (Figure 4). As noted above during natural fruit abscission, the total LCB levels were clearly higher in the treated AZs than in the control AZs, indicating a correlation between sphingolipid species, AZ activation, and fruit abscission.
In particular, the exogenous d18:0 or DMS application led to a significant rise of all detected LCB species in the fruit AZ on day 3 or 6 compared with controls, except for 1,4-anhydro-t18:0 and 1,4-anhydro-t18:1(8Z), which did not significantly change in the fruit AZ after any treatment (Figure 5). On the other hand, 18:1(8Z)-Glc, d18:1(4E), d18:1(8Z) and d18:1(8E) were undetectable in any AZ samples following chemical treatments.
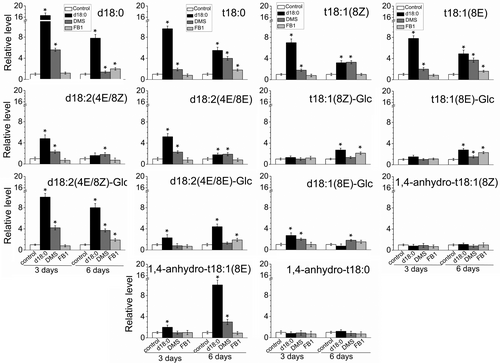
As expected, after 3 days of treatment, large accumulations of saturated LCBs, d18:0 and t18:0, were found in DMS- or d18:0-treated AZs (Figure 5). In particular, the di-hydroxylated LCB (d18:0) increased by 16- and 8-fold in the fruit AZ treated with d18:0 or DMS, respectively, whereas the amount of d18:0 did not significantly change in the AZ treated with FB1 after 3 days of treatment. In addition, only the DMS or the d18:0 application augmented t18:1(8Z), d18:2 (4E/8Z) and d18:2 (4E/8E) levels in the fruit AZ compared with the controls after 3 days of treatment.
After 6 days of treatment, all three treatments significantly raised the levels of di- and tri-hydroxylated LCBs in their saturated and unsaturated forms (8Z, 8E and 4E) in the fruit AZ, such as d18:0, t18:0, t18:1(8E), and d18:2 (4E/8Z)-Glc, but this significant rise also was pronounced in the case of the LCB d18:0 (Figure 5), establishing a close association between the endogenous d18:0 and the AZ activation. Thus, the gradual decrease in FDF observed in d18:0-, FB1- or DMS-treated fruits correlated with the accumulation of sphingolipids containing mainly the LCB d18:0, but an increase of other LCB t18:0, t18:1(8E), and d18:2 (4E/8Z)-Glc was also detected in all treated AZs. These results revealed that the AZ activation affects levels of sphingoid LCBs, showing strengthened accumulation of d18:0 in response to d18:0, DMS or FB1 treatments.
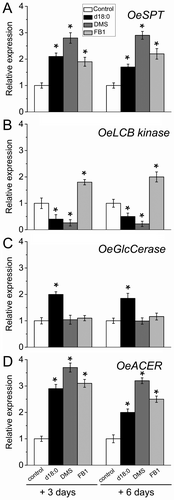
Next, we examined sphingolipid biosynthesis/turnover gene expression in the fruit AZ following chemical treatments. The expression of the sphingolipid metabolism genes, OeSPT, OeLCB Kinase, GLUCOCEREBROSIDASE (OeGlcCerase) and ALKALINE CERAMIDASE (OeACER), was affected by exogenous d18:0 as well as by inhibitors of sphingolipid biosynthesis (Figure 6). Collectively, the four transcripts were differentially regulated in the fruit AZ among the three treatments: OeSPT (up in all exogenous d18:0, LCB Kinase inhibition and ceramide synthase inhibition), OeLCB Kinase (down in exogenous d18:0 and LCB Kinase inhibition, up in ceramide synthase inhibition), OeGlcCerase (up in exogenous d18:0, unaffected in LCB kinase inhibition and ceramide synthase inhibition) and OeACER (up in all exogenous d18:0, LCB Kinase inhibition and ceramide synthase inhibition). Thus, all three treatments were found to induce the expression of OeSPT and OeACER in the AZ, indicating that OeSPT and OeACER expression are involved in fruit abscission in parallel to the greater LCB content. The increase in the transcript accumulation of OeSPT and OeACER is presumably the result of positive regulation due to the increase of the endogenous d18:0 level in the AZ, suggesting that sphingolipid biosynthesis and catabolism regulation by d18:0 at the transcriptional level operate concomitantly in the AZ during fruit abscission. Overall, these results indicate that fruit abscission may be accompanied by the inhibition of ceramide synthase or LCB kinase activity, and the promotion of d18:0 level via up-regulation of OeSPT and OeACER in the AZ, suggesting that endogenous d18:0 may play a key role in the fruit abscission.
3.4 Changes in plant hormonal levels in the fruit AZ during d18:0-, DMS- and FB1-induced abscission
To investigate whether hormone signaling was involved in the decrease of FDF induced by d18:0 DMS or FB1, we monitored changes in plant hormonal levels during d18:0-, DMS- and FB1-induced abscission in the fruit AZ at 3 and 6 days after treatment. In general, all treated AZs showed similar variation patterns of the hormone levels after treatment of d18:0, DMS or FB1 (Figure 7).
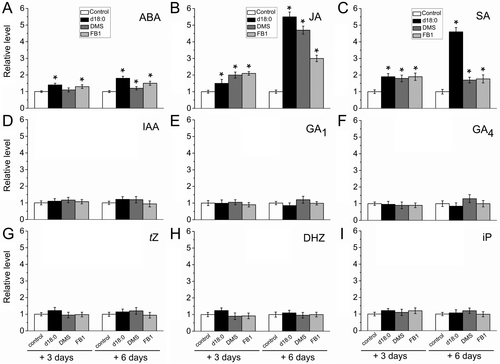
The application of any treatment on the fruit AZ prompted a significant increase in the endogenous levels of JA, SA and ABA after 3 and 6 days of treatment compared to the control AZ (Figure 7A-C). It bears noting that the abundance in the fruit AZ was higher for JA, followed by SA and then by ABA after any treatment. Additionally, the maximal increase of JA, SA and ABA was notably greater in response to exogenous d18:0 after 6 days of the treatment (Figure 7A-C). For the levels of auxin (Indole-3-Acetic Acid, IAA), gibberellins (GA1 and GA4), and cytokinin (CKs), no significant differences were detected in treated-AZs relative to control-AZs (Figure 7D-I). Thus, we found that exogenous d18:0 influenced mainly the JA and SA levels in the AZ, thereby modulating fruit abscission.
3.5 Involvement of sphingoid bases in mediating reactive oxygen species production and cell death during fruit abscission
Since plant organ abscission is linked to ROS production and programmed cell death (PCD), we evaluated whether the changes in sphingolipid metabolism induced by d18:0, DMS, and FB1 affected H2O2 levels and cell death during the induction of fruit abscission at the commercial harvest date. To do this, we examined ROS and cell death levels on both the proximal and distal sides of the AZ (Figure 8).
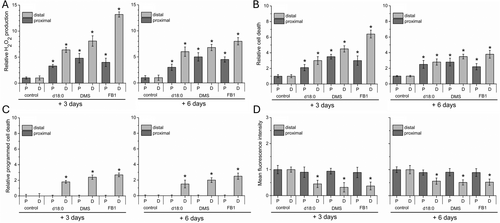
H2O2 production on both sides of fresh AZs was measured using dichlorodihydrofluorescein as described in the Methods section (Figures 8A and S1). All three treatments with fruit abscission induction exhibited a 3- to 13-fold increase in H2O2 levels on both sides of the fruit AZ compared with controls, indicating a positive correlation with the abscission induction recorded (Figure 8A). Subsequently, cell death was detected in the AZ using propidium iodide (PI) staining (Figures 8B and S2). As shown by intracellular H2O2 analysis, treatment with d18:0, DMS or FB1 significantly induced AZ cell death, which was also markedly increased on the distal side of the AZ compared with controls (Figures 8B and S2). In addition, for close monitoring of the correlation of PCD during the abscission induction, we measured PCD on both sides of AZ by double staining with PI and Hoechst 33342 staining as the ratio of both stained cells to total cells (%). This analysis showed that PCD was induced only on the distal side of the AZ by d18:0, DMS or FB1 treatments compared with controls (Figures 8C and S2).
To confirm that the PCD had a distinct spatial profile in the fruit AZ, we analyzed the mitochondrial membrane potential (ΔΨm) using tetramethylrhodamine methylester (TMRM) staining (Figures 8D and S3). TMRM staining and quantitative analysis demonstrated that significant loss of ΔΨm was caused by d18:0-, DMS- or FB1 treatments on the distal side compared with the proximal side of the AZ (Figures 8D and S3), this result is consistent with PI/ Hoechst 33342 staining. Thus, the two sides of the AZ showed similar variation patterns of the levels of H2O2 and cell death when fruit abscission was induced by the application of d18:0, DMS or FB1, displaying spatially regulated induction of PCD in the AZ.
To test whether the oxidative stress and cell death/PCD also occur during natural fruit abscission in the AZ, we measured H2O2 production and cell death on both sides of fresh AZs at 140 and 169 DPA. The H2O2 level significantly rose on both sides of the fruit AZ during abscission under natural conditions (Figures 9A and S4). This significant rise was very pronounced in the case of the distal side of the fruit AZ (Figure 9A). Consistent with the H2O2 accumulation during natural abscission, the results of PI staining showed that abscission led to significant cell death, especially on the distal side of the fruit AZ (Figures 9B and S5), suggesting that accumulated H2O2 and cell death may participate in abscission signaling.
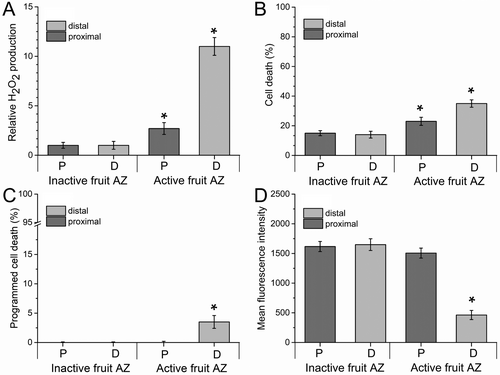
In the case of PCD, natural fruit abscission also induced the PCD exclusively on the distal side of AZ, indicating that PCD is spatially regulated in the fruit AZ. (Figures 9C and S5). Similarly, TMRM fluorescence on the proximal side within the fruit AZ was also significantly higher than the distal TMRM fluorescence during natural fruit abscission (Figures 9D and S6). These data imply that natural fruit abscission induced mitochondrial depolarization, detected by the decrease in TMRM fluorescence intensity, which resulted in the execution of PCD in the AZ activation and abscission. Notably, the same pattern was observed on the distal sides of fruit AZs treated with d18:0, DMS and FB1, indicating the loss of mitochondrial function during the fruit abscission process. Thus, a surge of d18:0, higher levels of endogenous H2O2, and a decrease in mitochondrial inner membrane ΔΨm —factors that accompany or induce PCD— were observed in the AZ during fruit abscission, implying their involvement in the abscission process.
4 DISCUSSION
Although the participation of sphingolipids in several aspects of plant development is well documented (Cassim et al., 2020), their involvement in the abscission process is poorly understood (Gil-Amado and Gomez-Jimenez, 2013; Parra-Lobato et al., 2017). In olive (Olea europaea L.), sphingolipids are a major constituent of fruit AZs during cell separation (Parra-Lobato et al., 2017). Moreover, both sphingolipid content and gene expression increase in the AZ during natural fruit abscission (Gil-Amado and Gomez-Jimenez, 2013; Parra-Lobato et al., 2017). However, whether sphingolipids play a signaling role in the abscission process is unknown, even in the Arabidopsis model plant. To provide a fundamental understanding of the sphingolipid role in the signaling mechanisms that activate abscission, we here investigate how sphingolipids modulate olive fruit abscission, delving into the role that determines cell death in the fruit AZ.
In the present study, treatments with sphingolipid LCBs and biosynthesis inhibitors resulted in differential abscission of olive fruit. We demonstrate induction of fruit abscission in response to exogenous sphinganine (d18:0) but not to t18:0, d18:0 or their phosphorylated derivatives, with a concomitant increase in sphingolipid LCB content, especially in d18:0, in the AZ of olive fruit, as previously observed during natural fruit abscission (Parra-Lobato et al., 2017). Moreover, we demonstrate that ceramide synthase and LCB kinase proteins can act as key mediators of sphingolipid homeostasis in the AZ during fruit abscission. High levels of endogenous sphingoid LCBs in the AZ, particularly d18:0, are also produced by pharmacological inhibition of ceramide synthase activity by FB1 application and by inhibition of LCB kinase activity after DMS application stimulating olive fruit abscission. The observation that the treated AZ has higher levels of LCBs, especially d18:0, relative to the control AZ opens the possibility that a free non-phosphorylated d18:0 or biosynthetic intermediate controls fruit abscission via ceramide synthase or LCB kinase activity in the fruit AZ during abscission. Thus, our data suggest that the free d18:0 serves as a second messenger for abscission factors and, when elevated abnormally, leads to AZ cell separation and abscission.
Sphinganine (d18:0) is a bioactive sphingolipid responsible for regulating diverse cellular functions in plants (Pata et al., 2010; Cassim et al., 2020; Huby et al., 2020), which is synthesized mainly through two pathways. The first pathway is the de novo synthesis pathway that occurs in the endoplasmic reticulum, when the condensation of serine and palmitoyl Co-A proceeds via catalysis by SPT, which is the rate-limiting enzyme of de novo sphingolipid biosynthesis (Hanada, 2003; Cassim et al., 2020). In fact, one of the first studies of sphingolipid homeostasis showed that supplying exogenous LCBs to cultured cells leads to a compensatory reduction in SPT activity (van Echten et al. 1990; Mandon et al. 1991). Here, we found that d18:0-induced abscission of olive fruit was associated with up-regulated OeSPT gene transcript in parallel with a rise of the endogenous d18:0 level in the AZ. Notably, our data reflect that inhibition of ceramide synthase or LCB Kinase boosts the LCB level in the AZ, which can be explained by a blockage in ceramide or LCBP production but also by LCB synthesis up-regulation at the transcriptional level via SPT genes. This supports the contention that high rates of d18:0 biosynthesis occur in the AZ when olive fruit abscission is induced. However, we cannot rule out that SPT might be regulated by translational or post-transcriptional mechanisms, as previously shown (Kimberlin et al., 2016). Furthermore, the coordination between enzymes might provide a powerful means to regulate sphingolipid metabolism in the AZ during olive fruit abscission. This leads us to ask whether the LCB products of OeSPT are simply released into the ER membrane or whether they could be handed off to downstream enzymes to inhibit efficient ceramide or LCBP production in the fruit AZ during abscission.
The second pathway is the catabolic pathway of ceramidase, which generates LCB and fatty acyl side chain by hydrolyzing ceramide present in the plasma membrane or lysosome (Mao and Obeid, 2008; Li et al., 2015; Wu et al., 2015; Zheng et al., 2018). In fact, LCBs produced by ceramide-catalyzed catabolism are involved in a variety of plant stress responses (Huang et al., 2022; Cahoon et al., 2024), including PCD, autophagy, turgor pressure, and oxidative stress responses (Chen et al., 2015; Li et al., 2015; Zheng et al., 2018; Zienkiewicz et al., 2020). In particular, an alkaline ceramidase, ACER, has been identified as a key component that regulates LCB production in plant defense against insects and in response to wounding (Huang et al. 2022). Loss of the Arabidopsis ACER increases ceramides and lowers LCB levels in plants (Li et al., 2015; Wu et al., 2015; Zheng et al., 2018). Here, we found that the expression of OeACER is up-regulated by exogenous d18:0 and by inhibition of ceramide synthase or LCB kinase in the AZ, suggesting that the abscission process may, in turn, up-regulate LCB levels by inducing the catabolic enzyme ACER. This expression pattern is consistent with the observed accumulation of LCBs, particularly d18:0, in d18:0-, DMS- or FB1-treated AZ. Accordingly, we hypothesize that the turnover of ceramide at the plasma membrane in the AZ can result in the accumulation of d18:0 in sphingolipid-rich domains (lipid rafts) and/or as signaling molecules regulating the abscission process. These results confirm that d18:0-regulated d18:0 levels were associated with its induction of ACER expression in AZ tissues, which is crucial to reveal the AZ activation of d18:0 during olive fruit abscission. Indeed, these data fit with those in the AZ during natural fruit abscission in olive (Gil-Amado and Gomez-Jimenez, 2013). Experiments are still needed to elucidate how the protein levels and the enzyme activity of ACER are regulated during fruit abscission. However, we cannot dismiss the idea that other members of the ACER family might be involved in olive fruit abscission. Recent research has shown that mutants with an orm2 background exhibit upregulation of genes encoding enzymes involved in sphingolipid catabolism (Gonzalez-Solis et al., 2020). This suggests a connection between SPT regulation by ORM proteins and the control of the transcription of genes related to sphingolipid metabolism or even related to sphingolipid metabolic intermediaries (Gonzalez-Solis et al., 2020; Liu et al., 2023). On the other hand, the expression of OeLCB kinase is negatively regulated by exogenous d18:0 in the fruit AZ during abscission. Notably, these findings imply that sphingolipid synthesis and catabolism were bolstered in the AZ, while the sphingolipid phosphorylation was downregulated in response to exogenous d18:0 at the level of transcription. Thus, the results provide new data on the sphingolipid homeostasis in the AZ activation and the mechanism by which the balance between the LCB and LCBP/ceramide can be established for manipulating the abscission process. Specifically, we found that d18:0 metabolism is controlled in the AZ cells by feedback mechanisms, including the transcriptional regulation of SPT and ACER as well as the inhibition of ceramide synthase and LCB kinase activities, constituting strong evidence that these enzymes play a key role in regulating sphingolipid levels in the AZ during fruit abscission.
The spontaneous PCD of the knockout mutants loh1, cerk (acd5), acd11, ipcs2 (erh1) with affectation in ceramide synthase, LCB kinase, sphingosine transfer protein, inositol- phosphoryl-ceramide synthase, respectively, provide evidence concerning the role of ceramides and LCBs in the induction of PCD (Brodersen et al., 2002; Liang et al., 2003; Wang et al., 2008; Ternes et al., 2011). The use of FB1, which also induces PCD, further supports this premise since it induces the accumulation of endogenous free LCBs, particularly of d18:0 (Saucedo-García et al., 2011). Additionally, due to the lack of LCB2a, a subunit of the heterodimer of SPT, LCB accumulation and PCD manifestation are reduced but are partially restored by the addition of FB1 or exogenous d18:0 (Saucedo-García et al., 2011). Thus, d18:0 has been pointed out as the inducer of PCD in response to FB1. In this study, sphingolipids containing or derived from d18:0 were found to be involved in olive fruit abscission.
Based on this association between plant sphingolipids and PCD processes (Berkey et al., 2012; Michaelson et al., 2016; Huby et al., 2020), we formulated the hypothesis that, during abscission, PCD involving sphingolipids occurs in the AZ. Our data reveal that d18:0 induces changes in AZ cells consistent with PCD during olive fruit abscission. Furthermore, here we demonstrate the occurrence of cell death with characteristics of PCD on the distal side of d18:0-, FB1- and DMS-treated AZs in parallel to increased endogenous d18:0, higher ROS levels and a weakening of mitochondrial membrane potential (∆Ψm). Thus, d18:0 may participate in mediating ROS/PCD during olive fruit abscission. Likewise, observations in the AZ were similar during natural fruit abscission in olive, where the PCD is also spatially and temporally regulated as well as associated with sphingolipid d18:0 accumulation, suggesting that d18:0 is the main LCB responsible for inducing PCD in the AZ. This result agrees with observations showing that PCD is a key mechanism occuring asymmetrically during the normal progression of abscission (Bar-Dror et al., 2011; Wen et al., 2024). Similarly, the induction of different nuclease and protease activities as well as encoding genes associated with PCD has been reported in AZs during abscission (Helm et al., 2008; Tripathi et al., 2009; Bar-Dror et al., 2011; Corbacho et al., 2013; Gil-Amado and Gomez-Jimenez, 2013; Parra et al., 2013; Glazinska et al., 2017; Briegas et al., 2020; Yu et al., 2023). In line with this, our data reveal that the expression pattern of OeACER is consistent with increased ROS and higher cell death levels observed in treated AZ of olive fruit. Because ceramidases hydrolyze ceramides into LCBs and fatty acids (Okino et al., 1998), we hypothesized that greater LCBs (d18:0) via ACER might be one of the reasons for more ROS and cell death in treated AZ compared with control AZ of olive fruit. However, it is possible that biosynthesis of the abscission-related sphingolipid d18:0 occurs in the AZ. Our observation that the SPT transcript, associated with sphingolipid biosynthesis, is accumulated to a higher level in the AZ supports this possibility. A coupling between the elevated d18:0 biosynthesis or ceramide catabolism in the AZ and the PCD process may exist during abscission. In such a case, a loss of d18:0 biosynthesis or ceramide catabolism would result in the inhibition of PCD in the AZ and a delay in the abscission progress. Experiments are underway to test this hypothesis.
Notably, mitochondria are involved in the ROS production associated to PCD in the AZ during olive fruit abscission. ROS generation has been attributed to NADPH oxidase at the plasma membrane, electron transport chain reactions at the mitochondria (mETC), and photosynthetic processes at the chloroplast (Peer et al., 2011; Zavafer et al., 2021). However, in non-photosynthetic tissues, mitochondria are considered to be the main source of ROS in plant cells (Ye et al., 2021). Mitochondrial ROS (mROS) production in plants has been implicated in the execution of PCD via inhibition of the mETC (Huang et al., 2016). Besides, harmful mROS production could be generated by a decrease in mitochondrial transmembrane potential (∆Ψm), affected by ATP synthesis or changes in mitochondrial permeability (Ye et al., 2021). In animal cells, it is widely reported that the ∆Ψm is reduced during apoptosis, a type of PCD. The reduction in ∆Ψm is thought to be due to the activation of the mitochondrial permeability transition pore (mPTP), a mega-channel that can be activated by mROS, producing oxidative stress in the mitochondrial matrix (Rottenberg, 2023). The results described in this work from the distal side of the active AZ demonstrated a strong correlation between ∆ψm and ROS production. This correlation was also observed in the induction of fruit abscission in inactive AZ treated with d18:0, DMS, and FB1. This suggests that sphingolipids may impact mitochondrial function to promote the induction of PCD in the AZ.
Free LCBs, such as d18:0, t18:0, and d17:1 as well as C2/C6-ceramides trigger ROS and cell death in plants (Yao et al., 2004; Townley et al., 2005; Bi et al., 2011; Peer et al., 2011; Zhang et al., 2020). Several studies have shown the induction of ROS and antioxidative components during the abscission process (Cai and Lashbrook, 2008; Sakamoto et al., 2008; Goldental-Cohen et al., 2017; Meir et al., 2019; Lee et al., 2022). In olive, it has been previously demonstrated that ROS, such as H2O2, can participate in natural fruit abscission as well as in fruit abscission induced by exogenous ethylene (Gil-Amado and Gomez-Jimenez, 2012). In the present study, ROS are linked to the sphingolipid effect on olive fruit abscission. Moreover, we report that the induction of the H2O2 and cell death levels in the AZ are associated with the upregulation of JA, SA and ABA levels in response to exogenous d18:0, but not t18:0, which perhaps plays a role in activating the expression of abscission-related genes for stimulating fruit abscission. Nonetheless, the way in which d18:0 regulates JA-, SA- and ABA-generating enzymes remains to be clarified. Similarly, we found that the inhibition of ceramide synthase and LCB kinase activities increased H2O2 and cell death levels in parallel to d18:0 accumulation in the AZ enriched in JA, SA and ABA. Previous reports have presented evidence of a close functional relationship between sphingolipids and plant hormone-mediated signaling; for example, the regulation of PCD (Greenberg et al., 2000; Bi et al., 2014; Yang et al., 2019; Cassim et al., 2020; Huang et al., 2021). During PCD, the modulation of the ratio between LCB and ceramide quantity impacts JA- and SA-signaling pathways in plant defense responses (Asai et al., 2000; Sánchez-Rangel et al., 2015; Zienkiewicz et al., 2020; Zeng and Yao, 2022). Our results suggest that the typical defense mechanisms may be operational in the AZ during olive fruit abscission. The connections between abscission and other related processes, such as pathogen defense, have been acknowledged in plants (Meir et al., 2011; Patharkar and Walker, 2019), and hence, cross-talk between these processes is almost certain. Several plant hormone pathways, such as that of ethylene, SA, JA and ABA are involved in both abscission and pathogen defense processes (Meir et al., 2010; Corbacho et al., 2013; Estornell et al., 2013; Gil-Amado and Gomez-Jimenez 2013; Tranbarger et al., 2017; Patharkar and Walker, 2018, 2019; Ma et al., 2021). It bears noting that, among the plant hormones studied here, SA and JA showed the most pronounced increase in all treated AZs during fruit abscission, indicating a significant interaction between SA and JA in sphingolipid-induced fruit abscission.
JA and SA are involved in the induction of sphingolipid-derived cell death (Huby et al., 2020). The first evidence of the role of the JA and SA signaling pathways in the PCD triggered by sphingolipids came from the use of FB1 (Asai et al., 2000). Transgenic NahG plants, in which SA is metabolically degraded, and the mutant jar1-1, which is insensitive to JA, exhibited much less susceptibility to FB1. Moreover, other mutants with elevated levels of ceramides and LCBs also underscore the relation between SA and JA. For example, the spontaneous cell-death phenotype of acd5 is attenuated in mutants where SA signaling is partially blocked (Greenberg et al., 2000). Likewise, the lack of isochorismate synthase required for SA biosynthesis suppresses SA accumulation and cell death in acd11 mutant (Brodersen et al., 2005). Here, higher accumulation of SA was observed in response to exogenous d18:0 in the fruit AZ, while lower levels were seen with DMS treatment, suggesting that phosphorylated LCBs are necessary to modulate SA levels during fruit abscission. In fact, the suppression of LCBKs decreases SA production while promoting JA biosynthesis in response to FB1 administration in Arabidopsis (Qin et al., 2017). In the AZ of olive fruit, while the other treatments moderately boosted SA levels, the d18:0 treatment dramatically elevated SA levels (by 4.5-fold) at 6 days of treatment.
In the case of JA, its participation has been reported in controlling plant organs abscission events (Kim et al., 2013; Marasek-Ciolakowska et al., 2020; Dziurka et al., 2022; Fidelibus et al., 2022; Kućko et al., 2022; Liu et al., 2022b). Recently, JA pathway was found to contribute to Arabidopsis petal abscission by JA-induced autophagy (Furuta et al., 2024). Moreover, the addition of methyl JA treatment accelerates ceramide accumulation and cell death in acd5, suggesting that the SA and JA pathways are involved in the cell-death phenotype of the acd5 mutant (Huang et al., 2021), possibly by modulating sphingolipid metabolism and raising ceramide levels. In line with this, further experiments are needed to clarify whether the progression of floral organ abscission is affected in the Arabidopsis mutants, such as acd5 or loh1 (Ternes et al., 2011; Huang et al., 2021). In olive, JA promoted both fruit ripening and abscission when exogenous applications of methyl JA were included in the pre-harvest treatment at the onset of fruit ripening to produce olive fruits enriched in antioxidants (Blanch et al., 2018). However, it remains unknown whether JA contributes to olive fruit abscission by promoting modification of the sphingolipid homeostasis in the AZ. However, it cannot be ruled out that JA may act downstream of d18:0 in abscission signaling. In this context, ABA also can play a key role with sphingolipids in olive fruit abscission. ABA levels are notably elevated in many plants before floral organ or fruit abscission (Li et al., 2023). However, the effects of ABA on abscission seem to depend more on its interactions with other plant hormones than on ABA alone, leading to the activation of the AZ (Eccher et al., 2013; Estornell et al., 2013). In olive fruit, the AZ activation by d18:0, DMS, and FB1 slightly boosted ABA levels compared to SA and JA levels. This may be explained by the antagonistic relationship between ABA and SA, as demonstrated by Yang et al. (2019). These authors found that exogenous ABA suppressed the effect of the SA analog benzo-1,2,3-thiadiazole-7-carbothionic acid-S-methyl ester (BTH), which induces cell death and sphingolipid accumulation in the acd5 mutant, known for spontaneous cell death at late developmental stages (Greenberg et al., 2000). Since the concerted action of multiple hormones is involved in the control of fruit abscission, it would be informative to investigate whether the JA- or d18:0-induction of olive fruit abscission could be counteracted by other plant hormones. In consideration of this scenario, we propose here that d18:0 is a possible link to establish the relationship between ROS/cell death and the underlying regulatory mechanism and execution of fruit abscission, which is accompanied by active JA and SA pathways.
5 CONCLUSION
This study provides first evidence that sphingolipid is an essential component involved in cell death during the plant organ abscission process. Our data show that sphinganine (d18:0) positively regulates olive fruit abscission in mediating sphingolipid LCB homeostasis as well as ROS and cell death levels in the AZ. This control may occur via inhibiting either ceramide synthase or LCB kinase activities, most likely via increases in LCBs, particularly d18:0, within AZs enriched in JA and SA levels. Our results suggest that the rise in LCB levels is also controlled by SPT via de novo biosynthesis of d18:0 and by ACER via hydrolysis of ceramides in AZs. These findings advance our understanding of the complex network of the biochemical and molecular events taking place during olive fruit abscission. In the future, in an effort to determine the extent of these physiological functions of sphingolipids related to plant organ abscission, Arabidopsis mutants with increases or decreases in selected sphingolipids could be tested to assess the relative contribution to AZ activation during floral organ abscission.
AUTHOR CONTRIBUTIONS
M.C.G-J. conceived and designed the experiments. B.B., M.C.C. and J.C. performed the experiments, and J.L., V.S-V., M.S-G., M.G-R and M.C.G-J analyzed the data. M.S-G., M.G-R and M.C.G-J prepared the manuscript. All authors have read and approved the final version of this manuscript.
ACKNOWLEDGEMENTS
The authors are grateful to the Research Support Service of University of Extremadura for its contribution to this work. The authors also thank the Plant Hormone Quantification Service (IBMCP, Spain) and D. Nesbitt for English editing. Maria C. Gomez-Jimenez dedicates this paper to the memory of Miguel A. Paredes (Universidad de Extremadura, Spain).
FUNDING INFORMATION
This work was supported by grants to M.C.G-J (PID2022-138573OB-I00 funded by MCIN/AEI/10.13039/501100011033 and, by “ERDF A way of making Europe”) from the Spanish Ministry of Science, Innovation and Universities.
Open Research
DATA AVAILABILITY STATEMENT
Supporting data can be requested by contacting the corresponding author.




