Hydrogen sulfide and ethylene regulate photosynthesis, sugar metabolism, and tolerance to heat stress in the presence of sulfur in rice
Abstract
Heat stress impacts photosynthesis and carbohydrate metabolism, challenging food security. To comprehend the mechanisms of thermotolerance, we examined the role of ethylene (ET) and hydrogen sulfide (H2S) with or without sulfur (S) in rice (Oryza sativa L.). Both ET and H2S promoted heat stress tolerance more conspicuously in the presence of S, restoring the balance between carbon assimilation and utilization. The enhanced photosynthesis in ET and H2S-treated plants under heat stress was linked with increased relative expression of Rubisco subunits rbcS and rbcL and carbohydrate metabolizing, including Sucrose Synthase 2 (SuSy2) and Sucrose transport 1 (SUT1). Notably, the H2S application showed the highest increase of 2.3, 3.2, 3.0, and 2.4-fold expression of the rbcS, rbcL, SuSy2, and SUT1, respectively, compared to the heat stress alone. The application of H2S with S more prominently increased starch content, total soluble sugar, and soluble invertase activity by 59.3%, 35.7%, and 25.9%, and also activity of soluble starch synthase and granule-bound starch synthase by 47.2% and 32.8%, respectively, compared to heat-stressed plants. The treatment (H2S plus S) elevated cysteine and GSH content and the activity of the antioxidant enzymes to maintain cellular redox potential under heat stress. These observed tolerance responses were less pronounced in plants treated with hypotaurine (HT; H2S scavenger) than those treated with norbornadiene (NBD; ET inhibitor), underscoring the superior role of H2S over ET in mitigating heat stress. The present study's findings explain that H2S is crucial for the ET-mediated response in augmenting photosynthesis and heat stress tolerance in rice.
1 INTRODUCTION
Climate change has become a burning issue around the world. There have been efforts to maintain a balance between economic development and environmental health under the changing climate conditions. The rate of environmental degradation through anthropogenic activities is far higher than that of sustainable development (Clarke et al., 2022). Frequent heat waves, uneven rainfall, drought, and shifting seasons are some of the major consequences of climate change (Clarke et al., 2022). Consequently, the agricultural sector faces excessive crop losses, lower grain quality, and issues related to food security. Long-term elevated temperatures trigger a range of morphological, anatomical, and physiochemical changes that hamper crop productivity (Wahid et al., 2007).
Heat stress devastates plant physiology by reducing stomatal conductance, resulting in less CO2 absorption and water loss (Gautam et al., 2022). Decreased CO2 levels in leaves diminish photosynthesis and increase photorespiration, resulting in inferior plant biomass and production (Alvi et al., 2024). Moreover, heat stress alters the activity of key enzymes and protein structure and the cell membrane permeability, down-regulates photochemical quenching, carbon assimilation, sucrose metabolism, and carbon assimilation, reducing photosynthesis (Kim et al. 2021; Moore et al. 2021; Gautam et al. 2022). Plants reallocate carbohydrates for energy and defense mechanisms to survive in high temperatures (Mishra et al., 2022).
Sulfur is a critical mineral nutrient that regulates plant performance, metabolic functioning, and stress mitigation (Shah et al., 2022). Under heat stress, S-containing metabolites interact with different biological compounds, such as enzymes, mineral nutrients, and signaling molecules, and produce reduced S compounds essential for thermotolerance defense (Hasanuzzaman et al. 2018). Foliar application of S improved heat tolerance in Brassica napus by improving physiological and yield attributes (Waraich et al. 2022). It has been reported that the potential decrease in S absorption under heat stress is associated with reduced root growth and biomass, limited water supply, and the degeneration of transporter proteins (Vu et al. 2020; Gautam et al. 2021; Mishra et al. 2023).
Ethylene (ET) is connected to S-assimilation through S-adenosylmethionine and influences plant growth and development and abiotic stress adaptation (Chen et al. 2021; Fatma et al. 2022; Khan et al. 2024). Heat stress influences ET production, changing the plant resource allocation (Savada et al. 2017). Ethylene supplementation has been found to alleviate abiotic stress responses via increased S-assimilation, antioxidant enzyme activation, higher photosynthetic efficiency, and plant growth (Asgher et al., 2018). It reduces glucose sensitivity, resulting in the increased reduced glutathione (GSH) production and expression of psbA and psbB genes for PSII protection under salt stress (Sehar et al. 2022). In addition to that, ethylene plays a crucial role in thermotolerance by activating various stress-related proteins (Jegadeesan et al. 2018) and redox homeostasis (Alvi et al. 2024) involved in preserving plant functionality and cellular stability. Furthermore, H2S is a potent gasotransmitter derived from S-assimilation that regulates various plant functions under normal and stressed conditions, such as germination, development, senescence, photosynthesis, and reactive oxygen species (ROS) scavenging (Arif et al. 2021). Hydrogen sulfide has been reported to modulate the content of chlorophyll and carotenoids and improve photosynthesis (Liu et al. 2019). The heat tolerance responses induced by H2S include regulation of gene expression of antioxidant enzymes, sugar levels, chloroplast biogenesis, and transcriptional activation of photosynthetic enzymes (Chen et al. 2011; Yang et al. 2016).
The modulation of ET, abscisic acid, and auxin actions through H2S signaling has effectively alleviated stress (Hou et al. 2013; Jia et al. 2016; Scuffi et al. 2014). The metabolite cysteine (Cys) plays a major role in ET and H2S-mediated actions. The molecular mechanism of the interdependent action of ET and H2S is not precise, but the persulfide formation of specific proteins, such as ascorbate peroxidase 1 and glyceraldehyde, play an essential role (Aroca et al. 2015, 2017).
Rice (Oryza sativa L.) is a staple crop in Asian countries, and being a C3 crop, it is highly susceptible to temperature change. About 90% of rice is grown and consumed in countries such as India and China and is considered a critical dietary crop for food security (Muthayya et al. 2014). Considering the importance of ET and H2S in stress mitigation responses, we tested the potential of ET and H2S in maintaining equilibrium between carbon assimilation and carbohydrate utilization, as well as photosynthetic activity in rice under heat stress. We speculated that ET and H2S would boost photosynthetic S-use efficiency through S metabolic pathways, resulting in higher synthesis of S-reduced metabolites and, thus, thermotolerance in rice. The improved photosynthetic S-use efficiency would enhance Rubisco activity, photosynthesis and sugar metabolism, and establish a defense under heat stress. Using norbornadiene (NBD; ET inhibitor) and hypotaurine (HT; H2S scavenger), we have substantiated the integrative relationship between ET and H2S in the presence of S for augmenting photosynthesis and heat stress adaptation.
2 MATERIALS AND METHODS
2.1 Plant growth conditions and treatment
Rice (Oryza sativa L.) cultivar PS 2511 seeds were obtained from the Division of Agronomy, Indian Agricultural Research Institute (IARI), New Delhi, India. The seeds were surface sterilized with 0.01% HgCl2 for 2 minutes and rinsed with double-distilled water. The seeds were immersed in distilled water for 12 h and were then randomly placed on Petri dishes for germination within an incubator set at 30°C, with deionized water kept at saturation. After germination, the seedlings were transferred into 23-cm earthen pots containing a mixture of sandy loam soil and compost (6:1, v/v). Before seedling transplantation, each pot was supplied with the appropriate concentration of nitrogen (N), phosphorus (P), and potassium (K). The plants were watered to uphold optimal soil moisture levels. These plants were grown in a growth chamber maintained at day: night temperatures of 28°C: 22°C (± 3°C), light and dark cycle of 14/10 h, photosynthetically active radiation (PAR) of 430 μmol photons m−2 s−1, and a relative humidity of 65 ± 5%. Ethylene treatment was given as 200 μL l−1 ethephon (2-chloroethyl phosphonic acid) and H2S as 200 μM NaHS on the foliage of plants in the presence or absence of soil-applied S (2.0 mM SO42−) in the form of MgSO4 under control (28°C) and heat-stressed conditions (40°C). To compare the relative influence of ethylene and H2S on the studied plant variables, we used the ethylene action inhibitor, norbornadiene (NBD), with H2S treatment, and the H2S scavenger, hypotaurine (HT) with ethylene treatment, at 100 μM concentration each along with S under heat stress condition at 15 d after germination (DAG).
The concentrations of S, NBD, HT, ethephon, and H2S were selected based on prior studies done by Alvi et al. (2024), Khan et al. (2022), and Iqbal et al. (2021b) respectively. Teepol (0.05%) was used as a surfactant along with the foliar-applied treatments. The treatments were arranged in a complete randomized block design (CRBD), each with four replicates. The sampling of plants to record different parameters was done on 30 DAG.
2.2 Assessment of photosynthetic traits and growth metrics
Net photosynthesis, stomatal conductance, and intercellular CO2 concentration were measured in the second topmost fully expanded leaves using an Infrared Gas Analyzer (CID-340, Photosynthesis System, Bioscience). The measurements were done between 10:00 a.m. and 11:30 a.m. under light-saturating conditions (PAR) of 800 μmol m−2 s−1 at atmospheric CO2 ~ 380 ± 5 μmol mol−1 and with a relative humidity of around 70%.
The chlorophyll content was quantified employing a SPAD chlorophyll meter (502 DL PLUS, Spectrum Technologies).
Plants were dried at 80°C in a hot air oven to measure dry weight until reaching a consistent weight.
2.3 Determination of oxidative stress biomarkers and histochemical detection of hydrogen peroxide and superoxide anion
Hydrogen peroxide (H2O2) content in leaves was quantified using the methodology described by Okuda et al. (1991), while lipid peroxidation levels were calculated by measuring thiobarbituric acid reactive substances (TBARS), following the procedure given by Dhindsa et al. (1981). The details on the methodology are provided in the Supplementary File 1.
Histochemical staining was done to detect H2O2 and superoxide anions (O2.−) in leaves using the method of Kaur et al. (2016). The leaves were immersed in 100% ethanol, heated at 100°C to remove chlorophyll, and left for cooling. The leaf samples were then stained for 6 h at room temperature with 3,3-diaminobenzidine (DAB) solution and nitro blue tetrazolium (NBT) for H2O2 and O2.− detection, respectively. The samples were transferred to a 20% glycerol solution, and the samples were then photographed.
2.4 Assay of the activity of antioxidant enzymes
The antioxidant activity was assayed using the protocol given by Fatma et al. (2014) and Gautam et al. (2022) for superoxide dismutase (SOD), ascorbate peroxidase (APX) and catalase (CAT). In a pre-chilled mortar and pestle 1 g fresh leaf sample was homogenized in a reaction mixture solution containing 70 mM phosphate buffer (pH 7.0), 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM phenylmethanesulfonyl fluoride (PMSF), 0.5% (v/v) Triton X-100, and 2% (w/v) polyvinyl pyrrolidone (PVP). The homogenate was subsequently centrifuged at 12 000 g for 20 minutes at 4°C, and the resultant supernatant was used for measuring antioxidant enzyme activity. The details are included in Supplementary File S1.
2.5 Determination of photosynthetic-sulfur use efficiency, the content of cysteine, reduced glutathione, and redox potential
The ratio of net photosynthesis to S content per unit leaf area was used to calculate photosynthetic-sulfur use efficiency (p-SUE).
The content of Cys in leaves was determined spectrophotometrically, adopting the methodology of Gaitonde (1967). The details are given in Supplementary File 1. Anderson's (1985) method was used to quantify the reduced glutathione (GSH) content and redox state. The details of the methods are included in Supplementary File 1.
2.6 Determination of total soluble sugars, starch, invertase, and sucrose content
The soluble sugar content in the fully developed top leaves was determined spectrophotometrically using an anthrone reagent following the methodology outlined by Xu et al. (2015). The soluble sugar was extracted from 100 mg oven-dried finely ground leaves powder by mixing with 10 mL of 80% ethanol, which was heated in a water bath at 80–85°C for 30 minutes. The supernatant was collected in a 100 mL volumetric flask after centrifugation. The alcohol extract was evaporated in a water bath at 80–85°C, then diluting the supernatant with 100 mL distilled water.
Starch was estimated following the method of Kuai et al. (2014). The oven-dried leaves were ground and sieved (1 mm), and 10 mg of leaf powder was mixed with 5 mL of 80% ethanol. The reaction mixture was incubated for 30 minutes at 80°C in a water bath shaker and centrifuged at 4000 g for 5 minutes. The resulting pellets were further extracted with 80% ethanol, and the ethanol was evaporated. The starch in the residue was removed after boiling with 2 mL of distilled water for 15 minutes and then cooled to room temperature. Leaf starch was hydrolyzed with 2 mL of 9.2 mol l−1 HClO4 for 15 minutes. The samples were centrifuged at 4000 g after adding 4 mL of distilled water. Subsequently, a second residue extraction was performed using 2 mL of 4.6 mol l−1 HClO4. The resulting supernatants were pooled and diluted with distilled water to reach a final volume of 25 mL.
The soluble acid invertase activity was assayed using the method used by Iqbal et al. (2021a). Sucrose content was determined using the method used by Xu et al. (2015). The reaction mixture contained 50 mM UDP-glucose, 10 mM MgCl2, 50 mM extraction buffer, and 200 μL of extract, and the final volume was 550 μL. The enzyme extract was incubated at 30°C for 30 minutes to initiate the reaction. To stop the reaction, 100 μL of 2 mol l−1 NaOH was added, and the solution was heated at 100°C for 10 minutes to deactivate any remaining hexoses and hexose phosphates. After cooling, the solution was combined with 1 mL of 0.1% (w/v) resorcin in 95% (v/v) ethanol and 3.5 mL of 30% (w/v) HCl, and then incubated for 10 minutes at 80°C, and the sucrose content was determined.
2.7 Determination of activity of soluble starch synthase and granule-bound starch synthase
The activity of both soluble starch synthase and granule-bound starch synthase was assessed by adopting the method used by Sumesh et al. (2008) by measuring the production of adenosine diphosphate (ADP) from adenosine diphosphate glucose (ADPG). The levels of ADP were determined using pyruvate kinase, which transfers phosphate from phosphoenol pyruvate to ADP, with the released pyruvate quantified. The activity of soluble starch synthase was also measured at various concentrations of ADPG (0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, and 1.6 mM) in grains subjected to temperatures of 28 and 40°C. The enzyme's kinetic parameters, Vmax and Km (ADPG), were derived from the double reciprocal plot of ADPG saturation curves.
2.8 qRT-PCR
DNA extraction from both treated and control leaves was done per the protocol described by Gautam et al. (2022). The detailed procedure for isolating DNA and synthesizing cDNA from plants using Trizol reagent (Invitrogen) can be found in Supplementary file S1. The relative amount of the target gene expression was determined by the 2−ΔΔCT method. Table S1 of Supplementary File 1 presents the specifics regarding the primer utilized.
2.9 Statistical analysis
Data analysis was performed using one-way ANOVA with SPSS software version 17.0 for Windows. Treatment means were reported as mean ± standard error (n = 4). The least significant difference (LSD) was computed for the significant data with a significance level of p < 0.05. Bars sharing the same letter indicate no significant differences at p < 0.05, as per the LSD test. Heat map analysis was performed using Origin software.
3 RESULTS
Ethylene (200 μL l −1 ethephon) and H2S (200 μM NaHS) were applied to plants under normal and heat stress conditions to determine their effect on physiological, biochemical and molecular changes with particular emphasis on photosynthesis, carbohydrate assimilation and metabolism as well as antioxidant system. Using ethylene and H2S inhibitors, we compared their effectiveness in mitigating heat stress effects on the performance of rice plants.
3.1 Effect on photosynthetic and growth parameters on rice plants
Heat stress significantly reduced the photosynthetic rate, internal CO2 concentration, stomatal conductance, and plant dry mass equally by about 50%, while chlorophyll content and p-SUE by 39.8% and 29.3%, respectively, compared to the control. Individual treatment of ET and H2S enhanced the photosynthetic parameters, but the effect was more pronounced with the addition of S in ET and H2S treatments. The ET + S and H2S + S increased chlorophyll content by 27.8%, 42.2%, and p-SUE by 32.4% and 55.4%, respectively, compared to the control. Adding S to ET or H2S treatment more effectively reduced heat stress effects on photosynthetic parameters. The treatment ET + S + HS promoted photosynthesis and plant dry mass by 47.7% and 80.4%, while the treatment H2S + S + HS increased the parameters by 74.7% and 97.5%, respectively, compared to heat stress.
Further, adding NBD in H2S + S and HT in ET treatment reduced photosynthetic and growth parameters, and a more conspicuous reduction was observed in ET + S + HT + heat stress compared to plants receiving H2S + S + NBD + heat stress (Table 1). This indicates an interaction between ET and H2S and implies the requirement of H2S in ET-mediated photosynthetic responses. The plant phenotype under different treatments is shown in Figure 1.
| Treatments | Plant dry mass (g plant−1) | Net photosynthesis (μmol CO2 m−2 s−1) | Chlorophyll content (SPAD value) | Internal CO2 concentration (μmol CO2 mol−1) | Stomatal conductance (mol CO2 m−2 s−1) | p-SUE (gm−2) | H2O2 content | TBARS content |
|---|---|---|---|---|---|---|---|---|
| (nmol g−1FW) | ||||||||
| Control | 0.83 ± 0.05e | 21.2 ± 1.1c | 34.1 ± 2.4d | 271.5 ± 10.3d | 0.42 ± 0.023b | 19.1 ± 0.98e | 14.1 ± 2.5bc | 6.2 ± 2.2cdef |
| Heat Stress | 0.41 ± 0.025 g | 11.1 ± 1.6 f | 20.5 ± 1.5 g | 148.2 ± 8.4 g | 0.21 ± 0.012e | 13.5 ± 0.75 g | 43.2 ± 5.3a | 16.5 ± 4.8a |
| ET | 1.17 ± 0.06d | 22.6 ± 1.2 b | 42.5 ± 2.1c | 352.4 ± 10.9c | 0.52 ± 0.026a | 22.4 ± 1.5d | 10.2 ± 1.6 cd | 5.3 ± 1.4def |
| ET + S | 1.62 ± 0.064b | 24.3 ± 1.8 b | 43.6 ± 3.3bc | 384.6 ± 12.2b | 0.54 ± 0.028 a | 25.3 ± 2.0c | 9.5 ± 1.2d | 4.5 ± 1.0 f |
| ET + Heat Stress | 0.70 ± 0.04 efg | 13.5 ± 2.1 ef | 25.1 ± 1.8f | 183.0 ± 9.7f | 0.31 ± 0.014d | 16.4 ± 0.8f | 17.4 ± 1.8b | 9.1 ± 1.6 cd |
| ET + S + Heat Stress | 0.74 ± 0.057efg | 16.4 ± 1.19de | 28.3 ± 1.4ef | 223.4 ± 9.2e | 0.34 ± 0.018 cd | 17.2 ± 0.78ef | 16.1 ± 1.56b | 8.3 ± 2.9cde |
| ET + S + HT + Heat Stress | 0.66 ± 0.034f | 15.2 ± 2.2de | 26.5 ± 1.2f | 198.2 ± 7.4f | 0.28 ± 0.015 d | 16.1 ± 0.81f | 18.1 ± 1.5b | 10.6 ± 2.4 b |
| H2S | 1.35 ± 0.06c | 25.3 ± 1.8a | 45.1 ± 2.5b | 380.7 ± 11.1b | 0.56 ± 0.034 a | 26.5 ± 1.8b | 9.0 ± 1.1d | 3.5 ± 0.9 ef |
| H2S + S | 1.87 ± 0.07a | 29.2 ± 2.9a | 48.5 ± 3.7a | 395.3 ± 11.5a | 0.58 ± 0.038 a | 29.7 ± 1.9a | 8.7 ± 0.9d | 3.1 ± 0.7 f |
| H2S + Heat Stress | 0.78 ± 0.06ef | 17.5 ± 2.2d | 31.6 ± 1.9de | 232.0 ± 9.9e | 0.35 ± 0.016 cd | 17.2 ± 0.76ef | 15.2 ± 1.7b | 7.4 ± 1.4 cdef |
| H2S + S + Heat Stress | 0.81 ± 0.053e | 19.4 ± 2.4 cd | 33.8 ± 1.4d | 241.1 ± 10.1d | 0.39 ± 0.018bc | 18.5 ± 0.85ef | 14.5 ± 1.7bc | 6.3 ± 1.5cdef |
| H2S + S + NBD + Heat Stress | 0.80 ± 0.048e | 16.1 ± 1.9de | 29.1 ± 2.1 ef | 215.7 ± 9.2 e | 0.37 ± 0.023 bc | 18.3 ± 0.73ef | 15.8 ± 2.4 c | 9.5 ± 2.2bc |

3.2 Effect of ethylene and H2S on oxidative stress markers and the antioxidant system
The content of H2O2 and TBARS was recorded as oxidative stress markers. Heat stress increased H2O2 and TBARS content by 206.3% and 166.1%, respectively, compared to the control (Table 1). Application of ET or H2S reduced the oxidative stress markers under heat stress. The plants receiving ET + S or H2S + S in the presence of heat stress exhibited 62.7% and 66.4% reduction in H2O2 content, respectively, compared to heat-stressed plants. The decrease in TBARS content for similar treatments in the presence of heat stress was 49.6% and 61.8%, respectively, compared to heat stress. Further, the addition of HT to ET + S in the presence of heat stress resulted in a greater increase in oxidative stress than NBD addition to H2S + S in the presence of heat stress (Figure 1).
The histochemical detection of superoxide anion and H2O2 (Figure 2) suggests that heat stress increases the free radical content in the cell and increases cellular toxicity. The color intensity of the NBT (Figure 2A) and DAB (Figure 2B) corresponds to the amount of superoxide anion and H2O2, respectively, present in the leaves exposed to different treatments.
For antioxidant enzyme activity, we estimated the activity of CAT, APX, and SOD activity (Figure 3). The increase in the activity of CAT, APX, and SOD was 60.1%, 75.1%, and 63.8%, respectively, in plants subjected to heat stress compared to the control. Plants receiving ET and H2S in the presence of heat stress showed further increments in the activity of CAT, APX, and SOD compared to heat stress. Moreover, treatment ET + S or H2S + S in the presence of heat stress showed an increase in CAT activity by 58.0%, 67.9%, APX by 70.2%, 100.4%, and SOD by 54.2%, 89.8%, respectively, compared to plants subjected to heat stress. The higher decrease in activity of CAT, APX, and SOD in plants under heat stress was observed when plants were treated with ET + S + HT in the presence of heat stress compared to plants treated with H2S + S + NBD in the presence of heat stress. This suggested that H2S and ET along with S are essential for maintaining redox homeostasis through the antioxidant system and ET-mediated defense is derived through H2S.
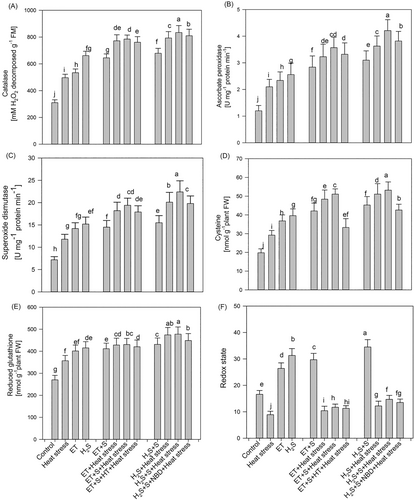
3.3 Effect of ethylene and H2S application on thiol content and redox state
Heat stress increased Cys and GSH levels by 47.4% and 32.1%, respectively, compared to the control. In addition, heat stress resulted in a pronounced imbalance in the cellular redox state, with a decrease of 46.3% compared to the control. Application of ET and H2S increased thiol content and redox state in plants subjected to heat stress compared to control. The application of ET + S and H2S + S under heat stress conditions increased the content of Cys by 75.1% and 82.1%, GSH by 63.2% and 92.6%, and cellular redox state by 31.4% and 66.1%, respectively, relative to plants subjected to heat stress.
The results emphasized the positive regulation of Cys, GSH, and cellular redox state by both ET and H2S (Figure 3). Plants treated with ET + S + HT in the presence of heat stress exhibited a weaker resistance response than those treated with H2S + S + NBD in the presence of heat stress. Thus, H2S was crucial in regulating Cys and GSH levels while maintaining cellular homeostasis.
3.4 Effect of ethylene and H2S on carbohydrate metabolism
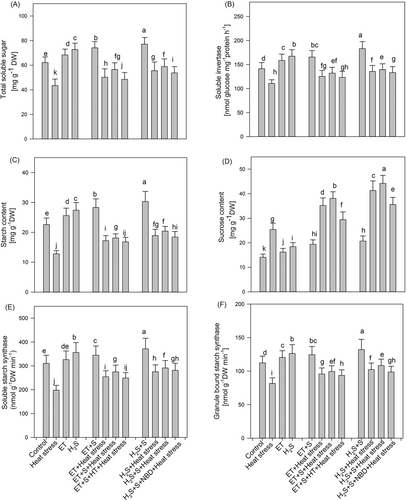
High temperature causes disturbances in carbohydrate metabolism, resulting in its accumulation. The decrease in starch content, total soluble sugars content, and soluble invertase activity under heat stress was 43.3%, 30.1%, and 21.5%, respectively, compared to the control plants (Figure 4). Applying ET and H2S under heat stress increased the values of the attributes mentioned above compared to heat-stressed plants. Further, supplementation of S with ET improved starch content by 41.4%, total soluble sugars by 29.9%, and soluble invertase by 19.2%, while S with H2S increased these parameters by 59.3%, 35.7%, and 25.9%, respectively, compared to the plants subjected to heat stress. In contrast, heat stress increased sucrose content by 80.1% compared to the control. Applying ET and H2S increased sucrose accumulation, more prominently with S supplementation under heat stress. We also observed the activity of soluble starch synthase and granule-bound starch synthase to ensure proper sink activity. The findings indicated that heat stress reduced the activity of both enzymes by 36.2% and 27.2%, respectively, compared to the control. However, ET and H2S application improved their activity under heat stress, more conspicuously with S addition under heat stress. Supplementation of S with ET enhanced the activity of soluble starch synthase by 38.8% and granule-bound starch synthase by 21.8%, while H2S combined with S increased the activity of soluble starch synthase by 47.2% and granule-bound starch synthase by 32.8%, compared to plants subjected to heat stress. Furthermore, the heat stress mitigation response concerning the above parameters was significantly reduced in the combined application of ET + S + HT + heat stress compared to plants treated with H2S + S + NBD + heat stress (Figure 4). The results highlighted the possibility that incorporating HT in the ET + S + heat stress treatment might have disturbed the synergistic interaction between ET and H2S, thereby compromising the carbohydrate metabolism of plants under heat stress.
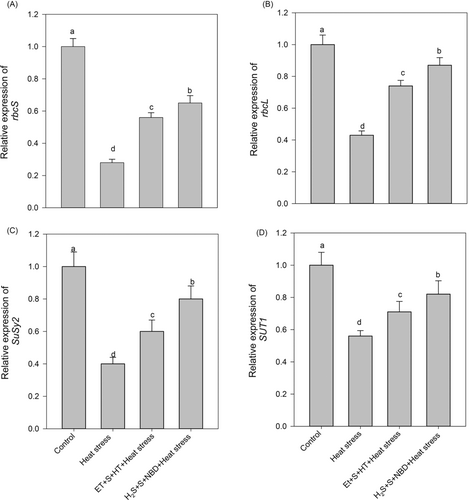
3.5 Gene expression analysis
To ensure the relationship between applied ET and H2S in carbohydrate assimilation and metabolism, we examined the gene expression pattern of necessary carboxylation enzymes, namely ribulose bisphosphate carboxylase/oxygenase (Rubisco, rbcL and rbcS), and carbohydrate metabolizing enzymes SuSy2 and SUT1 (Figure 5). The results indicated that heat stress caused significant damage to carbon assimilation and reduced the transport. The presence of H2S, ET, and S stimulated and maintained the equilibrium between photo-assimilation and sugar utilization between source and sink.
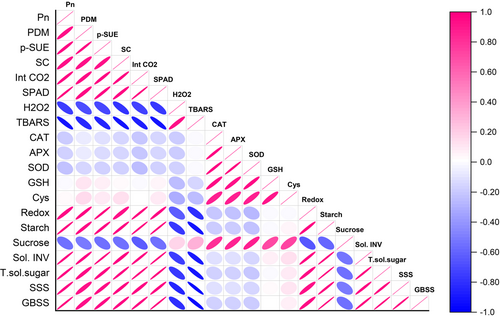
The key observations of the correlation matrix heatmap state the variables and their dependency on one another. The intense pink colour with an elongated eclipse showed a strong positive correlation, whereas the blue colour with an elongated eclipse showed a strong negative correlation. The reference bar represents the range of the correlation matrix (Figure 6). Photosynthetic and growth parameters negatively correlated with the oxidative stress markers (TBARS and H₂O₂) and sucrose accumulation, suggesting that heat-induced oxidative stress and sucrose accumulation adversely impacted photosynthesis and growth. Conversely, antioxidant enzymes, GSH, cysteine content, and redox potential negatively correlated with the oxidative stress markers (H₂O₂ and TBARS), indicating their role in mitigating oxidative damage. Furthermore, soluble invertase, total soluble sugars, soluble starch synthase and granule-bound starch synthase were negatively correlated with sucrose accumulation, reflecting a shift towards enhanced sucrose utilization. These findings implied that by boosting the antioxidant defense system, oxidative stress was reduced, leading to decreased sucrose accumulation, increased sucrose utilization, and ultimately, improved photosynthesis and growth under heat stress conditions.
4 DISCUSSION
Plant development and productivity rely substantially on temperature sensitive mechanisms (Lohani et al. 2020). Fundamental processes like electron transport, Rubisco activation in photosynthesis, as well as dehiscence and seed formation in reproductive development, are highly sensitive to temperature changes (Parthasarathi et al. 2022). Disorderliness in these processes ultimately contributes to yield loss under heat stress conditions. Heat stress impairs photosynthesis through chlorophyll degeneration, Rubisco inactivation, reduced stomatal conductance, altered electron transport and cellular water potential (Alvi et al. 2024). Understanding the reactions of carbon dynamics under heat stress and the mechanisms that regulate the imbalance is crucial for optimal crop growth and development. There are many instances where heat stress disturbed the steadiness between photosynthesis and photorespiration, which decreased the carbon pool, followed by a decline in crop yield (Prasad et al. 2017; Gautam et al. 2022). To gain a better understanding of carbon dynamics from carbon fixation to assimilation, as well as to identify the potential link in maintaining this equilibrium for better development and crop productivity, we studied rice responses to the exogenous application of ET and H2S in the presence of S at normal and heat stressed conditions. In our study increase in temperature caused chlorophyll degradation, decreased photosynthetic rate, reduced stomatal conductance and internal CO2 concentration which led to lower the carbon assimilation (Table 1; Figure 1). Earlier, similar reports on thermal stress have been published in wheat (Triticum aestivum), tomato (Solanum lycopersicum) and tobacco (Nicotiana tabacum) where inactivation of reaction centre and pigment breakdown led to reduced photosynthetic vigour in plants (Tan et al. 2020; Jahan et al. 2021; Sehar et al. 2023b). However, supplementation of exogenous ET and H2S separately and in the presence of S restored photosynthetic efficiency in rice plants under heat stress. The heat stress mitigation strategy of ET and H2S involved increase in chlorophyll content, net photosynthesis, stomatal conductance and restoration of Rubisco activity.
The gene expression analysis of both smaller and larger Rubisco subunits indicated the positive co-relation with exogenously-applied ET and H2S under heat stress and results were more profound in the presence of S. Possibly, ET and H2S have provided thermostability to Rubisco activase (Rac), which in turn removed the inhibitory sugar-phosphate derivatives from the active site of Rubisco and restored its activity (Wachter and Henderson 2015). Further, methionine (S-containing amino acid) and its derivatives are known for thermo- tolerance responses. According to Heckathorn et al., (1998), methionine rich chloroplast heat shock proteins were significantly involved in photosynthetic thermotolerance in tomato. We reported higher S assimilation which was directly proportional to higher thioredoxin protein availability. These heat-stable thioredoxin proteins are heat stable and impart heat tolerance (Chae et al. 2013).
The enzymes of the Calvin-Benson cycle are regulated through post-translational modification involving different states of Cys residue oxidation (Buchanan and Balmer 2005). Thus, ET and H2S in the presence of S improved Cys biosynthesis through the S-assimilation pathway and provided a thiol component and redox state for protecting important photosynthetic enzymes, leading to thermostability in rice. Under salt stress, the positive correlation between S and ET has been reported, where ET precursor aminocyclopropane-1-carboxylic acid (ACC) increased adenosine phosphosulfate reductase (APR) activity by increasing mRNA level of APR1 and APR3 isoforms (Koprivova et al. 2008). Moreover, the modulation of certain aspects of the sulfate starvation response in tobacco has been linked to Ethylene insensitive 3-like 2 (EIL2; Wawrzynska et al. 2010). Hence, it proved that a positive interaction between S and ET was involved in stress alleviation responses. In another study, the inclusion of the H2S scavenger HT in a growth medium containing NO validated the role of H2S in S-assimilation by withdrawing the activity of the enzymes involved in S-assimilation, APR, and ATP-sulfurylase under Cr (VI) toxicity (Alamri et al. 2020). During salt stress in cucumbers (Cucumis sativaus L.), the transcriptome analysis revealed that many differentially expressed genes related to the metabolism of S-containing compounds, such as methionine S-methyltransferase, Cys-rich repeat secretory protein 3, sulfate transporter 3.5, responded to H2S application. The proteomic data also confirm that H2S significantly enriched differentially expressed genes related to plant-pathogen interaction, sulfur-containing metabolism, cell defense, and signal transduction pathways (Jiang et al. 2020). In the present study, we approached an integrative mechanism of action among S, ET, and H2S. According to the results obtained, the application of S positively regulated S assimilation, and in parallel, the application of ET and H2S strengthened the thermotolerance in rice.
Moreover, the salient feedback regulation of H2S on ET limited the excessive ET generation under heat stress, accelerating thermal defense. The regulation of the chloroplastic thioredoxin machinery for persulfidation reaction has been maintained by the exogenous application of ET, H2S, and S, thus maintaining photosynthesis under heat stress. However, the reduction in values for all the above photosynthetic attributes in the presence of ET and H2S inhibitors (NBD and HT) with the lowest value in plants treated with ET + S + HT + heat stress predicted the profound role of H2S in high-temperature tolerance in rice.
Heat stress led to excessive generation of ROS, as depicted by the increased H2O2 and TBARS content in our experiments. These observations are in agreement with Tonhati et al. (2020), Tripathi et al. (2021), and Sehar et al. (2023a). The ROS generation under heat stress causes membrane leakage, degeneration of proteins, enzyme inactivation, damage to the reaction center, and increased respiratory bursts, leading to loss of growth and development (Werner et al. 2020; Bamagoos et al. 2021). Our observations suggested that exogenous application of ET and H2S in the presence of S showed a higher value of antioxidant enzyme activity (CAT, APX, and SOD) under heat stress, which resulted in reduced generation of ROS with lower cellular toxicity (Figure 3). Ethylene and H2S in the presence of S upregulated the antioxidant system and glutathione generation as well as Cys content. The availability of these compounds strengthened the antioxidant defense responses of rice plants under heat stress and, thereby, showed improved overall growth and development of plants. Moreover, ET upregulated the antioxidant and photosynthetic efficacy in wheat (Triticum aestivum) under high-temperature conditions (Sehar et al. 2023a).
The possible mechanism of H2S regulation under heat stress could be the reduced ROS production, lipid peroxidation, and electrolyte leakage, as reported in barley (Hordeum vulgare) under the combined effect of heat and drought stress conditions (Naz et al. 2022). The present study noted that the antioxidant defense responses were significantly hampered without H2S. The enzymatic activity of SOD, CAT, and APX was lowest in plants treated with ET + S + HT in the presence of heat stress. However, plants subjected to H2S + S + NBD in heat stress showed better tolerance symptoms, indicating that H2S acts as a connecting link and is necessary for ET and S-mediated antioxidant responses in rice under heat stress.
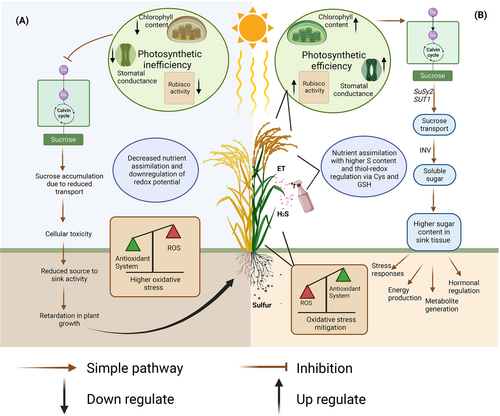
The increase in soluble sugar content is one of the tolerance responses under abiotic stress conditions. However, prolonged exposure to these stresses causes reduced plant growth and development. The carbon fixed through photosynthesis is then assimilated in the form of various structural and non-structural carbohydrates and in the form of transportable sugars, which are then carried from source to sink (Lal et al., 2022). Reduction in photosynthesis under heat stress is associated with a decreased production of photosynthates. An efficient carbohydrate metabolism provides energy and a stronger carbon skeleton as a survival strategy for plants subjected to heat stress. Many structural and non-structural carbohydrates can act as an osmoprotectant and osmolyte, avoiding cellular toxicity, stabilizing membrane proteins, and maintaining cellular turgidity (Stobrawa and Lorenc-Plucińska 2007). The osmoprotectant properties of these structural and non-structural carbohydrates help avoid cellular toxicity, stabilize protein and cell membranes, and maintain cellular turgidity (Bhattacharya and Kundu 2020). Considering the importance of carbohydrate metabolism, we investigated the role of ET and H2S separately and in combination with S under normal and heat-stressed conditions. The onset of heat stress increased sucrose content but decreased starch content and soluble invertase activity, followed by a decrease in total soluble sugars. The impairment in the invertase activity resulted in sucrose accumulation and prevented its conversion to other soluble sugar forms. The possible mechanisms of thermotolerance derived from our findings are summarized in Figure 7.
The source and sink activity must be monitored appropriately for appropriate plant growth and yield. In our study, applying ET and H2S helped maintain grain quality through increased activity of soluble starch synthase and granule-bound starch synthase under heat-stress conditions. Furthermore, the lowest values for both the parameters in plants treated with ET + S + HT + heat stress compared to H2S + S + NBD + heat stress suggested that H2S facilitated soluble starch synthase and granule-bound starch synthase-mediated thermotolerance responses in rice. The decrease in soluble starch synthase and granule-bound starch synthase under heat stress may be associated with a decrease in relative transcriptional expression of soluble starch synthase and granule-bound starch synthase genes followed by inefficient conversion of sucrose to starch and lower grain quality (Lu et al. 2019). However, the exogenous application of ET and H2S might have provided thermostability for these enzymes, followed by higher catalytic efficiency. The gene expression analysis for Rubisco subunits, sucrose transport, and sucrose synthase gene suggested that heat stress damaged Rubisco protein.
In contrast, the presence of ET and H2S significantly helped in overcoming their relative gene expression. Exogenous application of ET has been characterized by enhanced sugar metabolism through upregulation of α-amylase 3 (AMY3) and β-amylase 1(BAM1) followed by increased expression of sucrose transporters, SUT1, SUT4, and SWEET11 in Brassica napus (Lee et al. 2021). Moreover, H2S played an important role in regulating gene expression of Rubisco subunits and sucrose synthesis enzymes during cadmium stress in wheat (Zheng et al., 2023). Likewise, applying H2S and ET separately in the presence of S under heat stress upregulated the relative expression of Rubisco subunits, SuSy2 and SUT1. The integrative role of ET and H2S homeostasis was validated using NBD and HT in plants, observing lower values in the combined treatment of ET + S + HT + heat stress than in plants treated with H2S + S + NBD + heat stress. We found that the synergy among S, ET and H2S profoundly affected critical enzyme activities, gene expressions involved in carbon fixation, transport and metabolism in rice under heat stress.
5 CONCLUSIONS
Our study demonstrated that heat stress severely damages carbon assimilation and sugar metabolism. The applied ET or H2S in the presence of S more prominently enhanced photosynthetic efficiency, sugar metabolism, and the antioxidant defense system. The plants treated with ET showed comparatively lower heat tolerance response than H2S, underscoring the critical role of H2S as a mediator in interactions between S and ET signaling. This also suggests that H2S somewhere acts in feedback regulation of excessive ET generation and thus prevents plant damage. Furthermore, sucrose accumulation typically inhibits growth by impeding sugar transporters and applying H2S with S maximally upregulated sugar metabolism, mitigating the adverse effects of heat stress. This work provides a sustainable approach to increasing rice yield with grain quality in the face of climate change, focusing on balanced photosynthesis and efficient photosynthate use. Future research on heat stress in rice plants needs a multidisciplinary and collaborative approach, integrating advances in genetics, physiology, agronomy, and digital technologies to create climate-resilient rice production systems capable of meeting the global food demand and environmental sustainability.
AUTHOR CONTRIBUTIONS
A.F.A. performed the experiment, analyzed the data, and wrote the first draft of the paper. S.K. prepared the visualizations. N.A.K. conceived the manuscript idea, supervised the research, and wrote the final draft. All authors agreed to the final version of the paper.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open Research
DATA AVAILABILITY STATEMENT
The data and findings of the present study are available upon request.




