Grafting with non-suckering rootstock increases drought tolerance in Corylus avellana L. through physiological and biochemical adjustments
Abstract
Physiological and molecular mechanisms underpinning plant water stress responses still need deeper investigation. Particularly, the analysis of rootstock-mediated signals represents a complex research field, offering potential applicative perspectives for improving the adaptation of fruit crops to environmental stresses. Nonetheless, fundamental knowledge on this subject needs to be widened, especially in some woody species, including European hazelnut (Corylus avellana L).
To fill these gaps, we inspected dynamic changes in gas exchanges and stem water potential of two hazelnut genotypes, the ‘San Giovanni’ cultivar (SG), the non-suckering rootstock ‘Dundee’ (D), and their heterograft (SG/D), during a drought stress treatment followed by recovery. Biometric and anatomical traits were measured at the beginning and end of water stress imposition. Additionally, differences in abscisic acid and proline contents were analysed in leaves and roots taken from well-irrigated, stressed and recovered plants, in combination with expression profiles of candidate genes.
Grafting with ‘Dundee’ rootstock positively affected the ability of ‘San Giovanni’ plants to endure drought by increasing their intrinsic water use efficiency and facilitating post-rehydration recovery. Although anatomical adjustments occurred, we showed that the improved stress adaptation of grafted plants rather depended on biochemical modifications, resulting in increased root proline concentrations and leaf ABA accumulation both during water stress and recovery. We also proved that those metabolic changes were controlled by a differential reprogramming of genes involved in hormone metabolism and stress defence.
Grafting with non-suckering rootstocks could therefore represent a promising and environmentally-friendly strategy for improving the adaptability of hazelnut to water deficit.
1 INTRODUCTION
The European hazelnut (Corylus avellana L.) is a diploid (2n = 2x = 22), monoecious, dichogamous, wind-pollinated species and has sporophytic incompatibility that enforces cross-pollination. This species is characterised by wide geographical distribution (Thompson et al., 1996), which underlies its large genetic variability and climatic adaptation; about 500 cultivars have been described and are available from several ex-situ germplasm collections (Botta et al., 2019). Nevertheless, its optimal cultivation area is characterized by mild winters, cool summers, and sufficient precipitation (800–1000 mm/year) (Botta et al., 2019).
According to the Fifth Assessment Report (AR5) of the Intergovernmental Panel on Climate Change (IPCC), changes in climate and soil conditions seriously altered the productivity and quality of crops (Porter et al., 2014). This assumes great importance in perennial fruit species due to their high sensitivity to environmental conditions, particularly during the stages of floral differentiation, blossom, pollination, fruit set and growth (Cabo et al., 2020). Climatic projections for Europe indicate an increase in radiation and temperatures and a decrease in rainfall (Kröner et al., 2016), and their potential impact on C. avellana has been reported by some authors (An et al., 2020; Jha et al., 2021). A recent analysis of climate alterations occurring in central Italy during the period 1974–2021 highlighted dramatic increases in temperature and water requirements of hazelnut orchards, parallel to a significant decrease in chilling accumulation (Vinci et al., 2023). High minimum winter temperatures could inevitably limit the number of chilling hours required to break dormancy of vegetative buds, and to trigger pollen shed and female anthesis (Mehlenbacher 1991). On the contrary, an increase in the number of days with maximum temperature (Tmax) higher than 35°C and relative humidity (RH) lower than 70% led to severe water stress conditions that affected nut production and quality by determining early cessation of fruit growth, early leaf fall, blank nut increase, kernel decrease and a higher susceptibility to diseases (Girona et al., 1994; Tombesi 1994; Bignami et al., 2000, 2009, 2011; Dias et al., 2005; Cristofori et al., 2014). C. avellana is particularly sensitive to water scarcity, a feature that could mainly rely on the shallow root system typical of this species and on its poor ability to regulate stomatal closure under such conditions (Girona et al., 1994; Cristofori et al., 2014).
The natural habit of C. avellana is a large multi-stemmed shrub, which annually produces suckers from buds located at the basis of the trunk. The suckers are the natural replacement for old, dead, or diseased stems and are used in vegetative propagation. Nonetheless, they compete with the plant for water and nutrient uptake, negatively influencing its growth and yield, and the modern and mechanized hazelnut cultivation requires their annual removal. Currently, there are several techniques for hazelnut sucker management, and their application depends on several factors, such as farm size, cultivation type (conventional or organic), orography, and plant cultivation methods, as reported by Pacchiarelli et al. (2022). Among them, manual or mechanical controls are time-consuming and expensive (about 20 work hours per hectare in multi-stemmed bush orchards at a planting density of 400–500 plants/ha), while mulching and physical control (water steam and fire) find application in organic farms and only in orchards trained as a single trunk. Disbudding procedure, based on the removal of meristematic tissue at the basis of the rooted layer in young nursery-grown plants, shows the risk of leaving viable buds in the portion of sucker subjected to the treatment, thus making further checks necessary in the orchards. On the contrary, chemical control offers speed of execution and cost-effectiveness but is subject annually to a progressive reduction (especially in Europe) in the number of available active ingredients due to their high environmental impact, as well as a reduction in their related effectiveness. The use of non-suckering clonal rootstocks and the introduction of precision agriculture applications, based on calibrated amounts of herbicides, seem to be the most promising methods driving sustainable intensification of the hazelnut orchards (Pacchiarelli et al., 2022).
To date, three different non-suckering hazelnut rootstocks have been tested: i) seeds from selected Turkish tree hazel (C. colurna), widespread in the Balkans, Turkey and the Caucasus (Rovira, 2021; Bijelić et al., 2021); ii) two clonal selections (‘Dundee‘ and ‘Newberg’) obtained from open-pollinated C. colurna × C. avellana (Lagerstedt 1993; Rovira et al., 2014, 2021, 2022); and iii) selected C. avellana cultivars that are vigorous and non-suckering (Mehlenbacher and Molnar 2021; Rovira 2021). Among them, seedlings of C. colurna and hybrids of C. colurna × C. avellana are reported to be drought-tolerant (Mehlenbacher & Molnar 2021; Rovira, 2021). Studies conducted in other woody species, such as grapevine, poplar, prunus and almond, demonstrated that grafting with drought-tolerant rootstocks represents a cost-effective and environmentally friendly strategy for improving the plant's adaptability to water deficit (Tramontini et al., 2013; Lovisolo et al., 2016, Han et al., 2019, Chen et al., 2020, Opazo et al., 2020, Ranjbar et al., 2022). Nonetheless, experimental evidence on this theme is really limited in hazelnut and, to the best of our knowledge, the few research works dealing with grafting practice were addressed to establish its effect on agronomic and physiological traits of the scion, mainly considering impact on fruit quality and production yields (Tous et al., 2009; Miletić et al., 2009; Rovira et al., 2014, 2022, Portarena et al., 2023). Conversely, rootstock-dependent stress adaptation mechanisms have been rarely investigated (Portarena et al., 2022), and significant gaps remain in our understanding of the biochemical and molecular signals controlling stress tolerance in this species.
The present study was addressed to determine whether grafting with a clonal non-suckering rootstock could improve the resilience of hazelnut trees to water deprivation. Therefore, physiological responses of the Italian hazelnut cultivar ‘San Giovanni’ grafted onto the ‘Dundee’ rootstock were characterised during a severe water stress and recovery treatment time-course, making comparisons with data collected from own-rooted trees of the same cultivar and rootstock. Beside morphometric and anatomical modifications, changes in key biochemical and molecular components were inspected in search of endogenous signals underpinning stress adaptation phenomena associated with the rootstock-scion interaction.
2 MATERIALS AND METHODS
2.1 Plant material and microsatellite genotyping
The study was conducted on 2-year-old hazelnut trees provided by the nurseries “Verde Molise” (Termoli, CB, Italy) and “Vivai Nicola” (Mombercelli, AT, Italy), and consisting of i) own-rooted plants of C. avellana cultivar ‘San Giovanni’ (SG); ii) own-rooted plants of the non-suckering rootstock ‘Dundee’ (D); and iii) plants of ‘San Giovanni’ grafted onto ‘Dundee’ (SG/D).
The plant material was first checked through DNA fingerprinting. Briefly, total genomic DNA was extracted starting from 50–70 mg of immature catkins or young leaves (for all plants) and 100 mg of roots (for grafted plants only) using the Plant/Fungi DNA isolation kit (Norgen Biotech Corp). Then, a total of six microsatellite or simple sequence repeats (SSRs) markers, proposed as hazelnut molecular descriptors by Biodiversity International (2008), were analysed: CaT-B107, CaT-B504, CaT-B505, CaT-B507 (Boccacci et al., 2005), CaC-B020, and CaC-B028 (Bassil et al., 2005). Single-locus PCR amplifications and SSR analysis were performed as described in Boccacci et al. (2021). Only those plants that corresponded to the target genotypes (‘San Giovanni’ and ‘Dundee’) were used for the trials.
2.2 Experimental setup and sampling
A total of 45 plants (15 for each group, namely SG, D and SG/D) were used for the experiment. Each plant grew in a 9-L pot filled with a substrate composed of a mixture of 36% peat, 27% Irish peat, 14% pumice (3–8 mm) and 23% coconut fibre (Completo®, Vigorplant). Plants were fertilized once a month with 10 g of fertilizer NPK(SO3) 12.12.17(16.5) with iron and zinc. Potted plants were located in a greenhouse under natural light and photoperiod conditions. The maximum photosynthetic photon flux density in the greenhouse ranged between 1,450 and 1,600 μmol photons m−2 s−1. Temperature (T, °C) and relative humidity (RH, %) changes within the greenhouse were monitored daily for the entire duration of the experiment using Temp/RH data loggers (HOBO® MX2300 series data logger). From bud break (March) to the beginning of the experimental period, plants were irrigated in the morning (at 8:00 a.m.) 2–3 times per week by using an automatic watering system, which provided an established amount of water to the soil. Irrigation was managed to maintain water container capacity, which was previously determined as described in Lovisolo and Schubert (1998). Briefly, water was allowed to flow from the holes at the bottom of the pot and the weight of three reference pots [with one type (D, SG, SG/D) of plant each], not included in the experiment, was checked gravimetrically.
The study was conducted in summer between July and August, in a period characterized by high atmospheric evaporative water demand (VPD), with daily greenhouse temperature and RH values averaging around 28°C and 60%, respectively (Figure S1). The pot location within the greenhouse was randomly changed every day to guarantee exposure of the plants to uniform light conditions. For each group of plants, 7 individuals were maintained irrigated (well-watered, WW) for the whole experiment duration, while the remaining 8 plants were exposed to a severe water stress treatment (WS) by withholding irrigation for 8 days until the stem water potential (Ψstem) dropped below −2 MPa. Once severe water stress levels were reached, the plants were re-watered to container capacity and allowed to recover (REC). Physiological parameters [i.e., Ψstem, stomatal conductance (gs), leaf transpiration (E) and assimilation (AN)] were measured throughout the entire experiment (i.e., from the beginning of stress imposition until complete re-establishment of physiological functions) in stressed (n = 8 in each group) and control plants (n = 7 in each group).
At the end of the water stress treatment, leaf and root samples were collected from WW and WS plants of each plant group, flash-frozen in liquid nitrogen and then stored at −80°C until biochemical and molecular analyses. Further leaf and root samples were taken during the recovery phase at two time points, respectively when stem water potential (RECΨ) and stomatal conductance (REC) returned to pre-stress conditions. In total, 72 samples were obtained (i.e., 3 biological replicates × 2 tissues × 3 plant groups × 4 conditions) and processed for biochemical and molecular assays.
2.3 Measurements of leaf gas exchanges and xylem pressure
Every day, the rates of stomatal conductance (gs), assimilation (AN) and leaf transpiration (E) were measured in the morning between 9:00 and 12:00 h a.m. using a portable infrared gas analyzer (LCpro-SD system, ADC BioScientific Ltd). Measurements were performed on three randomly selected, fully expanded, non-senescing leaves per plant (thus corresponding to 3 technical replicates × 15 plants in each group), exposed to direct sunlight, using a 6.25 cm2 leaf chamber with artificial irradiation and a chamber temperature of 25°C to prevent leaf overheating. CO2 values were maintained at ambient conditions (400–450 ppm) for the whole duration of the experiment. Intrinsic water use efficiency (iWUE, as the ratio between AN and gs) was also calculated on WW, WS and REC plants of each group.
A few days before water stress imposition, light response curves were run and the specific correlation between AN and photosynthetic photon flux density (PPFD, μmol photon m−2 s−1) was inspected in each group of plants (SG, D, SG/D). Effects of photosystem II (PSII) saturation were observed around 1400–1500 PPFD in D (Figure S2A) and SG (Figure S2B), and around 1600 PPFD in SG/D (Figure S2C). We therefore set up the infrared gas analyser artificial irradiation with a PPFD of 1400, as it corresponded to the maximum unsaturation value suitable for analysing all groups of plants.
For each plant, the stem water potential (Ψstem) was measured on equilibrated non-transpiring leaves. For this purpose, fully expanded leaves were placed in humidified plastic bags wrapped with aluminium foil for 30 minutes before removal. After excision, the leaves were allowed to equilibrate in the dark for at least 20 minutes; then, Ψstem was measured using a portable pressure chamber (1505D PMS Instrument Company).
2.4 Stomatal phenotyping, morphometric analyses, and chlorophyll content index
Before stress imposition, stomatal density and stomatal size were analysed on all plant groups (D, SG, SG/D). Stomatal density (number of stomata mm−2) was measured according to Hopper et al. (2014) on a total of ten leaves for each group (n = 10) by collecting the leaf imprints from the abaxial lamina and by analysing them with an optical microscope at 40X magnification. The acquired images were processed by Image J software (1.46r, NIH, https://imagej.nih.gov) and four counts for each imprint were done. Afterwards, the stomatal size (calculated as the product of length by width) of ten randomly selected stomata for each image was determined according to Kardiman and Ræbild (2017). The stomatal pore area per leaf area index (SPI = stomatal density × pore length2) was also obtained according to McKown et al. (2014).
At the beginning of the trial (T0) and at the end of the WS treatment (Tf), the plant height and the stem diameter 20 cm above the ground were measured on all plants (n = 15). For analysing the stem diameter, a digital precision caliper was used, and the measurement was taken from both sides of the stem (so that the stem diameter value of a given plant was calculated as the mean of those two measurements). Additionally, a sign on the plant stem was made to allow identification of the measurement point easily. At the same time points, the root surface area was also measured on four plants (n = 4), randomly chosen from each plant group. Moreover, at the beginning of the experiment, the number of leaves and leaf area were determined in each plant group (T0, n = 15). Images of leaves and roots were acquired with a digital camera (Panasonic Lumix DMC-ZS1 10 MP Digital Camera, Panasonic Canada Inc.), then analysed with the ImageJ software (1.46r, NIH, https://imagej.nih.gov) to calculate the corresponding leaf and root surface areas.
Concomitantly, the chlorophyll content index (CCI) was determined on all plants in each group (SG, D and SG/D) by using a portable chlorophyll content meter SPAD 502 (CCM-200 plus; Opti-Sciences), analysing four leaves per each plant as biological replicates and taking three measurements for each leaf (i.e., 4 leaves × 15 individuals at T0 and 4 leaves × 7 WW/8 WS individuals at Tf).
2.5 Total RNA isolation and quantitative real-time PCR analysis
For each biological replicate, total RNA was extracted starting from 150 mg of leaf and 200 mg of root material, using a rapid CTAB method described in Gambino et al. (2008). RNA quantity and purity were checked using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific), while RNA integrity was inspected by electrophoresis gel analysis. All RNA samples were treated with DNase I (Invitrogen/Thermo Fisher Scientific) to eliminate DNA contamination, and first-strand cDNA was synthesized starting from 250 ng of total RNA using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems/Thermo Fisher Scientific) according to the manufacturer's instructions. Real-time PCR reactions and data elaboration were carried out as described in Moine et al. (2023). Briefly, Real-Time PCR assays were performed in a CFX Connect Real-Time PCR system (Bio-Rad Laboratories), using the SYBR Green (SensiFAST SYBR No-ROX Kit; Meridian Bioscience) method for quantifying amplification results. Thermal cycling conditions were as follows: an initial denaturation phase at 95°C for 2 min, followed by 40 cycles at 95°C for 10 s and 60°C for 30 s. Specific annealing of primers was inspected by checking the dissociation kinetics at the end of each PCR run. Relative expression levels of target transcripts were calculated using the geometric mean of the expression ratios of two housekeeping genes [18 s and ACTIN (ACT, Table S1), both serving as internal controls] as the normalization factor in all samples. All real time PCR assays were carried out using three biological replicates per treatment, and three technical replicates for each of the three biological replicates were run (n = 3). Gene-specific primers used in the Real-Time PCR experiments were designed using the Primer3web software (https://primer3.ut.ee/, version 4.1.0) and are listed in Table S1.
2.6 Quantification of abscisic acid and proline in leaf and root tissues
The content of abscisic acid was determined by HPLC according to Pagliarani et al. (2020). For quantifying the hormone concentration, the external standard method was used with calibration curves made with ABA (Sigma Aldrich; purity 98.5%). The HPLC apparatus (Agilent 1220 Infinity LC system model G4290B, Agilent®) was equipped with gradient pump, autosampler and column oven set at 30°C. A 170 Diode Array Detector (Gilson) set at 265 nm was employed, using a Nucleodur C18 analytical column (250 x 4.6 mm i.d., 5 μm, Macherey Nagel). The mobile phases were water acidified with 0.1% formic acid (A) and acetonitrile (B) at a flow rate of 0.600 mL min−1 in gradient mode, 0–6 min: from 10% to 30% of B, 6–16 min: from 30% to 100% B, 16–21 min: 100% B. Twenty μL per sample were injected, testing three biological replicates for each treatment (n = 3).
Proline content was quantified in leaf and root tissues by the acid ninhydrin colorimetric assay according to Mannino et al. (2020).
2.7 Statistical analysis
Significant differences among treatments were analysed by applying a one-way or two-way analysis of variance (ANOVA) based on the specific data elaboration. Normality of data and homogeneity of variance were checked according to the Shapiro–Wilk and the Levene tests, respectively. When the ANOVA test indicated that either the plant group (SG, D, SG/D) or treatment (WW, SS, REC) or their interaction (G × T) was significant, the Tukey's honestly significant difference (HSD) post-hoc test was applied to separate the means (p < 0.05). The standard error (± SE) of all means was calculated. Fitting of data reported in Figure 1D (i.e., gs response to stem water pressure in the three plant groups) was done using a four-parameter logistic curve (dose–response curve) as previously described by Secchi and Zwieniecki (2014). Significant differences in terms of EC50gs among the fitted curves were also checked (F test, p < 0.05). The GraphPad Prism software (GraphPad Software v.6.01) was used to elaborate figure charts and perform statistical analyses.
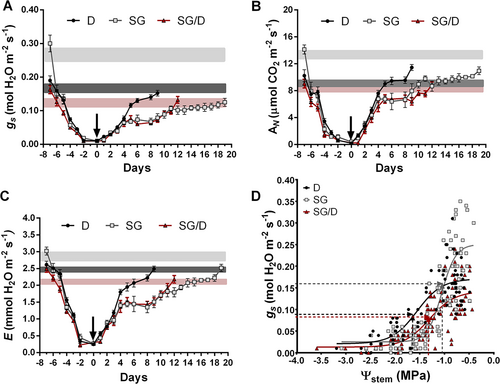
3 RESULTS AND DISCUSSION
3.1 Grafting with the ‘Dundee’ rootstock modifies the stomatal response of the ‘San Giovanni’ cultivar to water stress
At the beginning of the experimental trial (day −7 in Figure 1A-C), rates of stomatal conductance (gs), assimilation (AN) and transpiration (E) were almost two folds higher in SG plants than in SG/D and D plants (Figure 1A-C).
Notably, these differences in gas exchange trends were maintained in WW controls of the three plant groups for the whole duration of the experiment. After one week of water withholding (day 0 in Figure 1A-C), all plant groups reached gs values lower than 0.05 mol m−2 s−1, typical of a severe water stress condition in woody plants (Medrano et al., 2002), including pot-grown hazelnut trees (Catoni et al., 2017). Already during the first two days of WS treatment, SG plants experienced a steep drop in gs (i.e., corresponding to a 55.3% decrease, Figure 1A), accompanied by a decrease of 49.25% and 23.84% respectively in AN (Figure 1B) and E (Figure 1C) rates. Such reduction in stomatal opening and net photosynthesis occurred more gradually in SG/D plants, leading to a decrease of 40.62%, 39.22% and 18.41%, respectively in gs, AN and E during the first 2 days of WS (Figure 1A-C). The analysis of gs/Ψstem regression curves further underlined that once grafted onto D, the physiological response of the SG cultivar to water deprivation was reshaped (Figure 1D). Already in WW conditions (i.e. -0.5 < Ψstem < −0.8 MPa) gs rates measured in SG/D plants were almost half of those recorded in SG. However, along with drought stress imposition, corresponding to progressive lower values of Ψstem, gs dropped less and more gradually in SG/D than SG plants (Figure 1D). Additionally, whereas SG/D and D plants had half of the stomata closed at Ψstem of −1.32 MPa and − 1.57 MPa, respectively, SG plants reached this condition earlier, at Ψstem slightly higher than −1 MPa. The experiment was conducted in summer during a period of high atmospheric evaporative water demand, characterised by average daily temperatures >30°C, average daily RH < 40% and vapour pressure deficit (VPD) values >4 KPa (Figure S1). This condition could have significantly altered the physiological performances of target plants, particularly of the non-grafted ones. Serious stomatal limitation in response to seasonal VPD increases can occur in hazelnut even in the presence of non-limiting water availability conditions (Hogg et al., 2000). Although i) physiological susceptibility of hazelnut to air VPD can vary based on the genotype (Cincera et al., 2019), and ii) the ‘San Giovanni’ cultivar, originating from Southern Italy, should tolerate hot and dry environmental conditions, C. avellana is a water-saving species that overall prefers low VPD values to optimise stomatal conductance and carbon assimilation (Pasqualotto et al., 2018; Cincera et al., 2019). Interestingly, Pasqualotto et al. (2018) demonstrated that, in different hazelnut cultivars and multiple experimental orchards, the stomatal activity of hazelnut plants was quickly and seriously compromised when air VPD exceeds 1 KPa, regardless of the soil water content. This strict correlation between gs patterns and air VPD is therefore considered the basis of the ability of this species to tightly regulate leaf gas exchanges even under moderate water stress conditions and it also explains why gs represents a reliable indicator of early stress symptoms in hazelnut (Altieri et al., 2024). During the WS treatment, early stomatal limitation was observed exclusively in SG plants (Figure 1A-D). In fact, once grafted onto D, the stress susceptibility of this cultivar was modified and, despite showing lower leaf gas exchanges in WW conditions (Figure 1A-C), these plants were able to preserve gs rates along with the onset of stress (Figure 1D). Despite the effect of rootstock on the scion water stress adaptation has been widely documented in other species (e.g., Tramontini et al., 2013; Han et al., 2019), knowledge on this subject is still scarce in hazelnut. Consistently with our findings, Pacchiarelli et al. (2023) observed a significant reduction in the seasonal gs trends of grafted ‘Tonda di Giffoni’/’Dundee’ plants, whereas Rovira et al. (2022) found the highest gs rates in the ‘Negret-N9’/’Dundee’ grafting combination. However, such discrepancy in the analysed physiological traits could rely on the adopted experimental setup [e.g., field-grown plants in Rovira et al. (2022) vs potted plants in Pacchiarelli et al., 2023 and our study], as well as on the use of different scion genotypes.
Furthermore, a novel finding emerging from our study is that grafting significantly affected the ability of hazelnut plants to recover gas exchanges, especially the timing of recovery (Figure 1A-C). In D, the recovery of gs, E and AN was completed in 8–9 days, while in SG, gas exchange never reached the pre-stress levels, even after 18 days from rehydration. However, when SG was grafted onto D, gas exchange recovery was faster, and a complete restoration of transpiration and photosynthesis rates did occur in 12 days (Figure 1A-C), although they had reached the lowest Ψstem levels (around −3 MPa) at the end of WS imposition (Figure 2A). Additionally, both D and SG/D plants displayed a significantly higher intrinsic water use efficiency (iWUE) than SG, both in WW and WS conditions, implying that these plants were able to control water loss more efficiently (Figure 2B), as observed by Han et al. (2019) in grafted poplar plants exposed to water shortage.
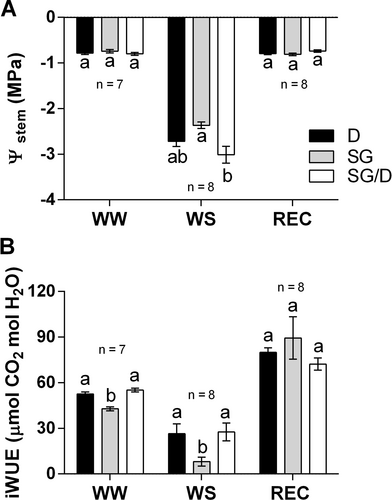
3.2 Biometric and anatomical adjustments underlie the drought tolerance of the ‘Dundee’ rootstock but not that of grafted ‘San Giovanni’ plants
At the beginning of the experimental trial (WW T0), both own-rooted and grafted SG plants showed a similar size (Figure 3A), indicating that grafting did not affect the architecture of the SG cultivar in terms of height. Moreover, measurements taken at the end of the experiment in WW conditions (WW Tf) showed only a slight increase in the height of D plants (of about 4.8%), while increases in the growth rate of both SG and SG/D plants were more evident (respectively of 12.4% and 7.7%). Following water deprivation (WS Tf), the height of D plants did not change and only a slight, but not significant, decrease in plant size was observed in SG/D trees. Conversely, a significant reduction (of about 10.5%) was noticed in the height of SG plants in comparison to the corresponding WW controls (WW Tf vs WS Tf, Figure 3A). These responses could be associated with the fact that, unlike D and SG/D, SG plants encountered serious stomatal limitation already at mild WS conditions (Figure 1), which might have negatively impacted the energy metabolism and hence the plant growth.
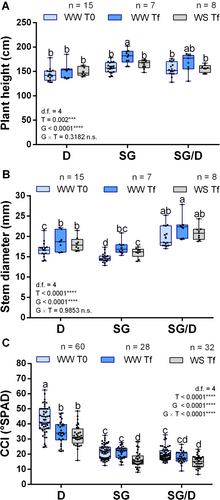
Independently of WS, grafted plants had a significantly bigger stem diameter with respect to D and SG plants (Figure 3B). Although no significant variations emerged from the analysis of ‘Tonda di Giffoni’/’Dundee’ heterografts (Pacchiarelli et al., 2023), Rovira et al. (2022) reported a higher tree diameter in the ‘Negret-N9’/’Dundee’ grafting combination. Changes in this trait could control the improved ability of grafted plants to endure drought (Zhang et al., 2016). Furthermore, since the root system architecture affects water uptake and C. colurna × C. avellana hybrids were shown to have a deeper root apparatus (Botta et al., 2019), roots of WW and WS plants were analysed. However, both at T0 and Tf, SG, SG/D and D plants did not significantly differ in terms of root surface area, regardless of the treatment (Figure S3).
In parallel, WS effects on chlorophyll content were inspected. Prior to WS imposition (WW T0), chlorophyll content (CCI) was two folds higher in D plants in comparison with SG trees, in which the presence or absence of grafting did not significantly affect this parameter (Figure 3C). Significantly higher CCI values were also observed in D leaves at the end of the trial (WW Tf and WS Tf; Figure 3C) both in WW and WS conditions, a response that could be ascribed to the drought-tolerant behaviour of ‘Dundee’. Conversely, WS exposure significantly decreased chlorophyll content both in own-rooted and grafted SG plants (WS Tf; Figure 3C). This variation was not statistically significant in grafted plants, suggesting a positive influence of the drought-tolerant rootstock (Jiao et al., 2023).
Changes in stomatal features can affect the plant's physiological performances in terms of photosynthetic processes, iWUE and plant growth (Franks and Farquhar, 2007). Thus, stomatal density and size were analysed to inspect whether peculiar anatomical adjustments could be at the basis of the improved drought tolerance of grafted plants. Microscope observations of the abaxial leaf surface (Figure S4A-C) showed that D and SG plants had similar stomatal density, though stomata from SG leaves were significantly wider (Table 1). This finding likely supported the physiological differences observed in WW conditions between SG and D plants, attesting a substantial reduction of gas exchange rates in D trees (Figure 1A-C). Strikingly, in SG/D grafts, a significantly higher stomatal density (up to 1/3 more) was found with respect to all own-rooted plants (Table 1). Nevertheless, such a feature could be compensated by the fact that leaves of SG/D plants had significantly smaller stomata than D and SG trees (Table 1). This hypothesis was further confirmed by calculating the stomatal pore index (SPI), which can be considered a good indicator of the plant's transpiring surface (McKown et al., 2019). While SG and SG/D plants had similar transpiring surfaces, significant lower SPI values were obtained for D plants (Table 1). Furthermore, D plants had more leaves but smaller leaf area than SG and SG/D (Table 1). It is therefore conceivable that the reduced basal transpiration rates of the ‘Dundee’ rootstock and its enhanced capacity to counteract WS mainly rely on these peculiar anatomical and morphological features. Changes in leaf morphological traits and stomatal anatomy are strictly associated with the regulation of leaf water and carbon fluxes (Hetherington and Woodward, 2003) and could highly influence hazelnut acclimation to high temperature and water deficit conditions (Catoni et al., 2017). However, grafted plants had only a slight reduction in the leaf size with respect to SG trees, which could have been balanced by the high number of leaves (Table 1).
| D | SG | SG/D | |
|---|---|---|---|
| Stomatal density (stomata mm−2) | 143.52 ± 7.2a | 144.55 ± 5.7a | 219.37 ± 8.5b |
| Stomatal area (mm2) | 0.00049 ± 0.00002b | 0.00060 ± 0.00002a | 0.00041 ± 0.00001c |
| Stomatal Pore Index (SPI) | 0.07086 ± 0.00231b | 0.08657 ± 0.00243a | 0.09100 ± 0.00349a |
| Leaf area (cm2) | 70.70 ± 4.85b | 148.98 ± 13.43a | 100.28 ± 11.91b |
| Leaf number | 64.2 ± 4.47 a | 47.16 ± 2.50b | 52.5 ± 2.87ab |
3.3 Abscisic acid and proline accumulation in grafted plants is associated with physiological changes during drought and recovery
Differences in the accumulation of two key stress-associated metabolites, the hormone abscisic acid (ABA) and the osmoprotectant solute proline (Yoshiba et al., 1997; Raghavendra et al., 2010; Kuromori et al., 2018), were quantified in leaves and roots collected from all plant groups in WW, WS and REC conditions. Our results showed a surge of ABA in leaves from all plant groups upon WS (Figure 4A). The highest levels were observed in SG plants, while in SG/D ABA concentrations were 50% lower of those measured in SG. However, ABA content in SG/D plants was still high 24 h following rehydration (RECΨ) and, although to a lesser extent, at recovery completion (REC), while was not detectable in D and SG samples. These data could explain why i) with respect to D trees, grafted plants experienced a delay in stomatal opening post-stress relief, and ii) unlike own-rooted SG trees, SG/D plants were able to restore gas exchange rates successfully (Figure 1A-D). ABA residual signals in leaves of rehydrated plants were previously reported to constitute a biochemical strategy for facilitating the physiological recovery following WS release (Lovisolo et al., 2008; Martorell et al., 2014). In roots, ABA levels were overall up to 10-fold higher than those quantified in leaves, already in WW conditions, but significant increases due to WS exclusively occurred in SG plants (Figure 4B).
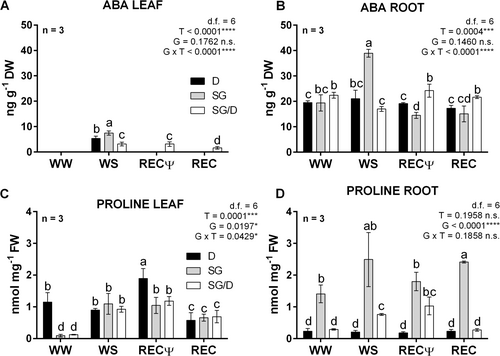
In the roots of grafted plants, ABA significantly decreased following WS imposition; then, the hormone levels returned to pre-stress concentrations already one day after rehydration (RECΨ). Additionally, in the roots of D trees, the hormone concentrations did not significantly change (Figure 4B). The analysis of ABA accumulation profiles hence highlighted different biochemical responses to WS: i) the surge of ABA both in leaves and roots from own-rooted SG plants underlined a strong defence reaction to stress, suggesting a prominent role of this hormone in regulating stomatal closure in these plants (Figure 1D); ii) the persistence of an ABA signal exclusively in the leaves of SG/D plants during the whole recovery period, and occurring concomitantly with an increased accumulation of the hormone in the roots, could underlay improved stress adaptability (Ruehr et al., 2019); iii) the drought-tolerant behaviour of D plants seemed less dependent on variations in ABA concentrations.
Further evidence in support of these findings emerged from the analysis of proline. A steep increase in proline accumulation occurred in WS leaves of both own-rooted and grafted SG plants. The metabolite levels were still high in these plants at the beginning of recovery (RECΨ), then decreased at the end of trial (REC; Figure 4C). Notably, proline concentrations in D leaves only changed in relation to recovery (RECΨ; Figure 4C), pointing to a different biochemical signature that could have improved the osmoregulatory capacity of these plants, in turn facilitating their faster recovery dynamics (Figure 1A-C). Roots of the same plants did not accumulate proline regardless of treatment, and overall showed significantly lower concentrations of this metabolite compared with roots of SG/D and SG plants (Figure 4D), mirroring the patterns observed for ABA (Figure 4B). In parallel, the roots of non-grafted SG plants accumulated the highest levels of proline overall (up to three folds). Nevertheless, a significant increase in the metabolite amounts was exclusively detected in the roots of grafted SG plants upon WS and REC (Figure 4D), showing a pattern similar to that of leaves. Very little information is available about the role of proline in the regulation of hazelnut cultivars and rootstocks' responses to drought. However, it was reported in other woody species, including rootstocks and grafted plants, that alterations in proline amounts can control adaptation strategies to severe drought (Jiménez et al., 2013; Ghosh et al., 2022) and affect recovery (Ruehr et al., 2019). Considering that grafted plants were also those reaching the lowest Ψstem (Figure 2A) and that, unlike own-rooted SG trees, did not show wilting symptoms, such differences in leaf and root proline contents could have triggered specific osmotic adjustments that helped them to limit the negative effects of WS by increasing the plant osmoregulation capacity (Bartlett et al., 2014), ultimately improving their iWUE (Figure 2B).
3.4 ABA metabolism is differently regulated at the molecular level in grafted plants upon drought
Upon WS, the ABA biosynthetic gene 9-cis-epoxycarotenoid dioxygenase (NCED1) was significantly overexpressed in leaves of SG plants, and only slightly induced in SG/D and D leaves with respect to WW controls (Figure 5A). Independently of grafting, NCED1 transcripts strongly decreased in SG leaves already early after rehydration (RECΨ). Compared with WW and WS samples, NCED1 transcriptional rates were also significantly lower in D leaves at RECΨ; subsequently, they returned to pre-stress levels at the end of recovery (REC; Figure 5A). This expression pattern resembled that emerging from the analysis of roots from the rootstock plants (Figure 5B). In SG/D roots, NCED1 transcript amounts were significantly induced upon drought, then they rapidly decreased during recovery. However, NCED expression profiles were not accompanied in D and SG/D plants by significant changes in the root hormonal content (Figure 4B). This could reveal a sustained delivery of root-synthesised-ABA to the leaf, which might represent the main source of the higher hormone amounts measured in this tissue in both plants upon WS. Consistently, only a slight and not significant up-regulation of NCED1 in the leaf of SG/D and D plants was observed (Figure 5A). In parallel, despite WS SG plants being the only ones that accumulated ABA in the root, NCED1 transcription was not affected in this tissue, regardless of the treatment (Figure 5B). Considering that ABA levels in SG plants were double during WS with respect to the others, this result could rely on the rate-limiting nature of NCED1, which tightly regulates ABA biosynthesis, especially upon severe drought (Qin et al., 1999; Iuchi et al., 2001).
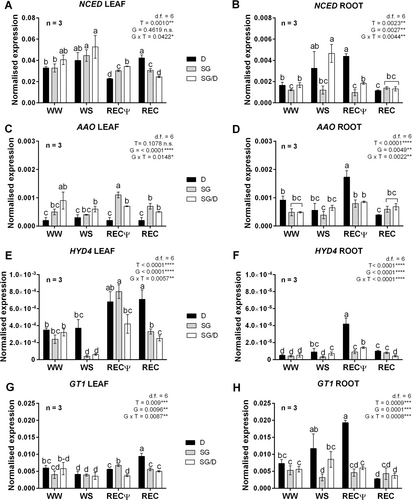
Transcriptional patterns of an aldehyde oxidase-encoding gene (AAO), homologue to AAO3 in Arabidopsis and involved in the last step of ABA biosynthesis (Seo et al., 2000), were also checked. Its expression did not significantly differ among treatments in D and SG/D leaves, while was induced during the early phase of recovery in SG leaves (Figure 5C). The AAO expression profiles in the root further confirmed the effect of the rootstock × scion interaction (Figure 5D). Unlike D leaves, the gene was strongly induced in non-grafted D roots during the first recovery phase, while in grafted SG/D roots its transcription did not significantly vary, regardless of treatment, resembling the expression patterns observed in the roots of own-rooted SG plants (Figure 5D). The fact that AAO transcriptional rates were not affected by WS, regardless of genotype or grafting conditions, could be associated to the notion that upon drought the ABA biosynthetic pathway is predominantly regulated by NCED (Iuchi et al., 2001) and that the role of other enzymes, including AAO, is marginal (Seo et al., 2000). Since this is the first time that ABA metabolic genes were investigated in hazelnut under WS, effects due to intensity and duration of the stress imposition could not be excluded.
Beside biosynthesis, ABA concentration can be regulated by ABA catabolism, which can encompass both oxidation and conjugation pathways (Nambara and Marion-Poll, 2005). Therefore, the expression of two genes, respectively involved in ABA hydroxylation (HYD4) and ABA conjugation (GT1), was checked. Transcriptional levels of the ABA 8′ hydroxylase gene (HYD4) underwent a steep increase in the leaves of both own-rooted and grafted SG plants during recovery, with values respectively up to 4 and 3 folds higher than those measured during WS. The up-regulation of the gene during recovery was also noticed in D leaves, at the last sampling point (REC; Figure 5E). The recovery-dependent over-expression of HYD4 was also evident in roots from SG plants one day from rehydration (RECΨ). Conversely, in the roots of SG/D and D plants, HYD4 was more expressed both during WS and at a higher extent early during recovery. Particularly, D plants displayed the highest transcriptional rates with respect to SG and SG/D roots during recovery (Figure 5E). The ABA-UDPG glycosyl transferase-encoding gene (GT1) was also significantly up-regulated during recovery in the leaf, though with different timing based on the grafting condition (i.e., at RECΨ for SG vs at REC for SG/D plants; Figure 5F). The analysis of GT1 expression in the roots revealed changes mirroring those noticed for HYD4. Particularly, the fact that also this gene was slightly up-regulated upon WS both in D and SG/D plants (Figure 5H) supported the hypothesis that, beside a predominant delivery to the shoot, ABA levels in the root of these plants were predominantly affected by the hormone catabolism (Nambara & Pollon, 2005; Wilkinson & Davies, 2002; Sauter et al., 2002). Particularly, in the case of D plants, the root expression profiles of both ABA biosynthetic and catabolic genes were overall the same (Figure 5 B,D,F,H), hence suggesting a metabolic compensation between ABA anabolism and catabolism. The same did not occur in own-rooted SG plants, for which the ABA degradation routes were either exclusively activated post-rehydration treatment (Figure 5E-G) or did not significantly varied (Figure 5H).
3.5 Key molecular signatures associated with the improved drought adaptation of grafted plants
Leaf transcriptional profiles of the proline biosynthetic gene Δ1-pyrroline-carboxylate synthetase (P5CS1) were similar in own-rooted plants (SG or D), revealing a recovery-specific induction. Also, in grafted plants, the gene was overexpressed only at the end of recovery (REC; Figure 6A). The P5CS1 expression in the roots did not highlight significant changes based on grafting condition or genotype × treatment (G × T) interaction but confirmed that transcription of this gene was mainly induced by REC rather than WS (Figure 6B). Besides, the proline dehydrogenase-encoding gene PDH was strongly activated upon WS in SG leaves. Nonetheless, this transcriptional signature was specific to grafted plants, as in leaves taken from own-rooted trees, PDH transcripts significantly increased either during recovery (REC, SG and D) or in WW conditions (D) (Figure 6C). In the root, PDH expression profiles were reversed with respect to the leaf: a WS-dependent overexpression of the gene occurred in SG roots, whereas the analysis of SG/D roots revealed a peak of transcription at the end of recovery (REC). No statistically significant differences emerged in roots from D plants (Figure 6D).
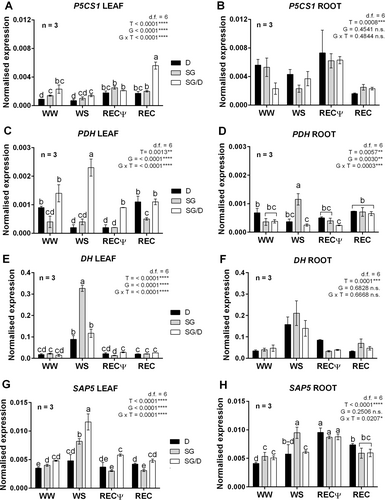
These results apparently contradicted the data of proline in leaves and roots (Figure 4 C,D). It should be considered, however, that proline accumulation following water deprivation can depend on different biochemical processes, such as inhibition of protein production, reduction in proline utilization, decreased proline catabolism and/or increased proline biosynthesis and hydrolysis of proteins (Rizzi et al., 2015; Forlani et al., 2019). Remarkably, proline synthesis, as well as its degradation, is tightly regulated by a negative feedback mechanism, in which WS-based increased accumulation of the metabolite acts as rate limiting factor for the transcription inhibition of the corresponding biosynthetic genes (Adamipour et al., 2020). Stress timing and intensity and plant genotype could also influence those responses (Rizzi et al., 2015; Adamipour et al., 2020). Additionally, the proline metabolic route at the P5CS1 level can either or not be controlled by ABA signalling (Yoshiba et al., 1997), likely supporting the fact that proline concentration profiles observed for a specific group of samples (e.g., D leaves or SG roots) did not always follow those of ABA quantities.
Finally, to gain more information on molecular signals potentially controlling the different stress perceptions of own-rooted and grafted plants, two drought-associated genes were considered. Dehydrin-encoding genes (DH) are up-regulated during drought to prevent cellular damages due to water deprivation (Riyazuddin et al., 2022; Karas et al., 2024), suggesting they can function as reliable biomarkers of plant stress conditions (Aina et al., 2024). The analysis of DH transcripts in both leaves and roots confirmed that the expression of this gene was WS-dependent (Figure 6E,F). However, the up-regulation of DH was up to three times higher in the leaves of SG plants in comparison to D and SG/D samples. This further suggested that, in absence of grafting, SG plants mounted a stronger defence reaction/had a higher stress perception to WS (Figure 6E,F). Notably, this finding was consistent with the differences observed at the level of ABA accumulation patterns in SG, SG/D and D plants (Figure 4B), thereby in agreement with the ABA-mediated activation of DH transcription (Hanin et al., 2011; Zhang et al., 2019).
The second gene analysed (CoravSAP5) encodes a stress-associated protein (SAP) belonging to a class of zinc-finger proteins acting as pivotal regulators of abiotic stress responses in several plant species (Giri et al., 2013; Priya et al., 2019), including woody crops (Lloret et al., 2017). CoravSAP5 is homologue to AtSAP5 of Arabidopsis (Kang et al., 2011) and was previously identified by Boccacci et al. (2015). Leaves from both SG and SG/D plants shared similar SAP5 transcriptional profiles, highlighting a peak of expression upon WS, though more pronounced in SG/D samples (Figure 6G). Conversely, only a slight but significant activation of the gene was observed in WS D leaves compared with WW controls. Moreover, unlike SG and SG/D plants, SAP5 transcript levels in D samples one day after rehydration (RECΨ) remained as higher as in WS conditions (Figure 6G). These results provide evidence that, in the leaf, SAP5 was up-regulated by WS, as reported for AtSAP5 in dehydrated Arabidopsis plants, where its elevated expression level was associated with increased tolerance to WS by reducing transpiration (Kang et al., 2011). Conversely, in the root, SAP5 transcription increased during the first phase of recovery, apart for SG samples where the gene was significantly activated already during WS (Figure 6H). Although the mechanism of action of SAPs is still poorly understood, these results suggested that the transcriptional reprogramming of these genes was differently tuned based on treatment, tissue type, genotype, and rootstock × scion interaction.
4 CONCLUSION
Our study was addressed to gain novel insights into the physiological effects of grafting with non-suckering rootstocks on the adaptability of hazelnut to water deprivation. The collected results attested that grafting with the ‘Dundee’ rootstock significantly improves the ability of the ‘San Giovanni’ cultivar to regulate leaf gas exchanges in response to water withholding. Grafted trees showed increased iWUE and recovered faster following stress relief with respect to own-rooted plants. We proved that this physiological adaptation encompasses specific biochemical adjustments, mainly relying on increased root proline concentrations and leaf ABA accumulation both during water stress and recovery. We also demonstrated that grafting with ‘Dundee’ can mitigate the negative effects of stress despite the high atmospheric water demand period (VPD >4 KPa). This is important, considering that, regardless of soil water availability (> 60%), C. avellana varieties typically encounter severe stomatal limitation in response to high atmospheric VPD values (Pasqualotto et al., 2018; Altieri et al., 2024). Nonetheless, since hazelnut cultivars can display a diverse sensitivity to high VPD and/or water stress conditions (Cincera et al., 2019), further experiments are needed to disentangle the biological mechanisms controlling rootstock-scion interactions. For instance, the potential of the ‘Dundee’ rootstock should be tested in grafting combination with different hazelnut cultivars and considering interactions with other abiotic stresses. Exploitation of the rootstock effect in the control of scion transpiration could offer important ecological advantages to hazelnut cultivars in response to multiple environments and pedoclimatic changes (Portarena et al., 2023). In this frame, further trials conducted in open fields will be necessary to confirm the biological and molecular responses here observed in greenhouse conditions and to collect agronomic data providing information on fruit production and quality of grafted plants.
AUTHOR CONTRIBUTIONS
P.B. conceived the study. C.P. and A.M. planned and designed the research. A.M., C.A., L.N., W.C., F.S., C.P. and P.B. performed experiments. A.M. and C.P analysed data. C.P. wrote the manuscript. A.M. and P.B. collaborated in writing the manuscript. P.B. and G.G. acquired funding. P.B. coordinated and C.P. co-coordinated the INNOCORE and BREEDINCORE projects. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
This work was funded by Fondazione Cassa di Risparmio di Cuneo (CRC) project “INNOCORE: innovazioni vivaistiche e di tracciabilità per la corilicoltura piemontese”, and by Fondazione Cassa di Risparmio di Torino (CRT) project “BREEDINCORE: breeding ed innovazioni a supporto della corilicoltura piemontese”. The authors are grateful to Mr. Luca Bordone for his support in plant management. Open access publishing facilitated by Consiglio Nazionale delle Ricerche, as part of the Wiley - CRUI-CARE agreement.
FUNDING INFORMATION
This work was funded by Fondazione Cassa di Risparmio di Cuneo (CRC) project “INNOCORE: innovazioni vivaistiche e di tracciabilità per la corilicoltura piemontese”, and by Fondazione Cassa di Risparmio di Torino (CRT) project “BREEDINCORE: breeding ed innovazioni a supporto della corilicoltura piemontese”.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




