Comparison of early transcriptomic changes to diverse microbial volatiles in Arabidopsis thaliana
Abstract
Microbial volatiles organic compounds (mVOCs) play diverse roles in modulating plant growth and stress tolerance. However, the molecular responses of plants to mVOCs are largely undescribed. In this study, we examined the early transcriptomic response of Arabidopsis thaliana to two plant growth-promoting mVOCs (PGPVs) and one plant growth-inhibiting mVOC (PGIV). Our phenotype analysis showed that PGPVs from Fusarium verticillioides and Simplicillium sympodiophorum promote plant growth by affecting different organs. In particular, F. verticillioides mVOCs promote plant growth in whole seedlings, while S. sympodiophorum mVOCs increase leaf surface area. Moreover, Arabidopsis treated with the two PGPVs exhibited different growth-associated molecular responses, which corresponded to the phenotype analysis results. For instance, the FAR1 family (regulates light-associated plant development) was upregulated by F. verticillioides mVOCs, while the LBD family (regulates leaf size and shape) was enriched among S. sympodiophorum mVOC-upregulated genes. Hierarchical clustering analysis further indicated that PGPVs induced expression of growth-associated genes and suppressed expression of defense-associated genes. In contrast to the PGPV-induced transcriptional effects, PGIVs caused downregulation of growth-associated genes with coincident upregulation of defense-associated genes. Furthermore, a transcription factor (TF) enrichment analysis suggested that HSFs, NACs and WRKYs might be core regulators in the plant response towards mVOCs. In particular, WRKYs might serve as integrating nodes to regulate salicylic acid- and jasmonic acid-mediated defense responses and growth-defense trade-offs. Overall, this study provides insights into the early molecular responses of plants after mVOC exposure and suggests that these molecular responses contribute to different phenotypic responses.
1 INTRODUCTION
As sessile organisms, plants have evolved intricate signaling networks that mediate specific responses to environmental stimuli and signals from other organisms. Among these responses, plant reactions to different microbes are essential determinants of plant resilience in the face of environmental stresses (De Zelicourt et al., 2013). A major mechanism of microbe sensing by plants involves microbial volatile compounds (mVOCs), and plant responses to mVOCs have been widely characterized in recent decades. Nevertheless, it still remains largely unknown how different plant cells respond to different mVOCs at a molecular level.
mVOCs are microbial secondary metabolites emitted in a gaseous form, which often serve as signals in plant-microbe and/or microbe-microbe interactions (Ryu et al., 2003; Schulz-Bohm et al., 2017). The blend of mVOCs emitted by a single microbe may contain a variety of metabolites, including alkenes, alcohols, sulfur compounds, terpenoids, and other types of molecules (Kanchiswamy et al., 2015). Different mVOCs are known to stimulate different physiological responses in plants, such as growth modulation, enhancement of nutrient uptake and promotion of stress resilience (Schulz-Bohm et al., 2017; Sharifi et al., 2022). mVOCs may also induce systemic resistance and prime a plant's tolerance to biotic and abiotic stress (Zamioudis et al., 2015; Cappellari and Banchio 2020; Yasmin et al., 2021; Pescador et al., 2022; Chang et al., 2023; Chiang et al., 2023).
Based on their functions, mVOCs may be classified as plant growth-promoting mVOCs (PGPVs) or plant growth-inhibiting mVOCs (PGIVs). These designations are often assigned by evaluating plant growth at different ages after exposure to mVOCs. Some PGPVs are known to promote plant growth by modulating signaling of phytohormones, such as gibberellins (GB), cytokinins (CK), brassinosteroids (BR), auxins (IAA), ethylene (ET) and abscisic acid (ABA; Bhattacharyya et al., 2015; Cappellari and Banchio, 2020; Gao et al., 2022; García-Gómez et al., 2020; Kamaruzzaman et al., 2021; Ryu et al., 2003). In addition, PGPVs may promote plant growth by enhancing the uptake of nutrients (e.g., iron and sulfur) or by increasing photosynthesis activity (Castulo-Rubio et al., 2015; Esparza-Reynoso et al., 2021; Meldau et al., 2013; Zhang et al., 2009). In contrast to the range of reports on PGPVs, studies on PGIV-mediated suppression of plant growth are comparatively rare. Nevertheless, WRKY18 has been identified as a key modulator of Arabidopsis growth after PGIV exposure (Wenke et al., 2012). Moreover, previous work also showed that auxin response and the activities of auxin transporters may be suppressed by PGIV exposure (Chang et al., 2023).
Both PGPVs and PGIVs have been shown to prime plant tolerance to biotic and abiotic stresses (Cappellari and Banchio, 2020; Chang et al., 2023; Chiang et al., 2023; Pescador et al., 2022; Yasmin et al., 2021; Zamioudis et al., 2015). For instance, the combination of PGPVs from Pseudomonas fluorescens WCS417 with photosynthesis-associated signals induces systemic resistance in Arabidopsis through MYB72 (Zamioudis et al., 2015). Defense-associated phytohormones like jasmonic acid (JA), salicylic acid (SA) and ethylene (ET) are also known to be involved in different mVOC-induced priming effects (Cho et al., 2008; Rudrappa et al., 2010; Ryu et al., 2004). As such, the mVOC 2,3-butanediol primes Arabidopsis disease resistance via actions involving JA and SA, while SA and ET are essential to acetoin-induced priming in Arabidopsis (Rudrappa et al., 2010; Ryu et al., 2004). In addition, MAPK cascades are crucial immune-related signaling pathways (Ngou et al., 2024), and our previous work showed that MKK1 and MKK3 may act as central regulators of a PGIV-induced defense response (Chang et al., 2023). Furthermore, volatiles from Pseudomonas chlororaphis O6 improve drought stress tolerance via SA, JA and ET signaling (Cho et al., 2008).
Although recent studies have begun to reveal the molecular mechanisms of various plant responses to mVOCs, the mechanisms underlying many known phenotypic responses remain unclear. In this study, we aimed to determine how plants respond at a molecular level after exposure to different mVOCs. To accomplish this goal, we measured transcriptomic changes in Arabidopsis after PGPV and PGIV exposure. Using hierarchical clustering analysis, we found that PGPVs upregulated genes associated with growth and development but suppressed expressions of defense-associated genes. In contrast, PGIVs downregulated the expression of genes related to growth and development but induced the expression of genes associated with defense. In addition, our data suggested that WRKYs might serve as convergence nodes in SA-JA-mediated defense responses and growth-defense trade-offs. Importantly, our results revealed that PGPVs can promote plant growth by stimulating molecular pathways that correspond to different growth-associated processes.
2 MATERIALS AND METHODS
2.1 Plant growth condition and mVOCs exposure experiment
Seeds of A. thaliana ecotype Columbia-0 (Col-0) were surface sterilized by 1.6% NaClO (w/v) containing 0.067% tween20 (v/v) for 8 mins. After sterilization, the seeds were rinsed in sterile distilled water five times and vernalized for two days at 4°C in the dark. After vernalization, A. thaliana seeds were grown for five to six days on 1/4 Murashige and Skoog (MS) medium with 0.01 g ml−1 sucrose (pH 5.7) in a 22°C growth chamber with a 16/8-h light/dark photoperiod.
The mVOCs exposure experiments were conducted as described previously (Chang et al., 2023). One 9-cm Petri dish with 2-days-old F. verticillioides and six 5.5-cm Petri dishes with 2-days-old S. sympodiophorum were used for the mVOC exposure experiments. TSB and PDA medium without microbes were used as the control treatment for mVOCs exposure experiments. During the mVOC exposure experiment, MS medium containing five to six-day-old Arabidopsis seedlings and TSB or PDA medium containing microbes were transferred individually to the sealed co-culture container and cultured in a 22°C growth chamber with a 16-h photoperiod, avoiding direct contact between the seedlings and microbes. For phenotype observation, Arabidopsis seedlings were treated with F. verticillioides mVOCs and S. sympodiophorum mVOCs for four and six days respectively. For transcriptomic analysis, Arabidopsis seedlings were treated with mVOCs for 24 hr. and then used for RNA extraction.
2.2 Microbial Identification and Cultural Condition
F. verticillioides (BCRC number FU31352) is an endophytic fungi in Miscanthus floridulus and was isolated from the seeds of M. floridulus. S. sympodiophorum (BCRC number FU31351) is an environmental fungus. A Forward primer ITS1 (5’-TCCGTAGGTGAACCTGCGG-3′) and reverse primer ITS4 (5’-TCCTCCGCTTATTGATATGC-3′) were used for PCR-based species identification. Amplified fragments were identified as F. verticillioides or S. sympodiophorum according to NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) with the sequenced PCR product. A pphylogenetic analysis of ITS fragments of Fusarium spp. and Simplicillium spp. was performed to confirm the result (Figure S1). The sequences of the ITS fragments of Fusarium spp. and Simplicillium spp. were obtained from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/). A phylogenetic tree was constructed in MEGA 11 with neighbor joining method.
A 0.9-cm diameter agar plaque containing F. verticillioides was cultured in a 9-cm Petri dish containing 20 mL TSB medium. The dish was maintained in a 28°C growth chamber for two days in the dark. S. sympodiophorum was grown on PDA medium, and spores were collected with 10% glycerol. The spores were diluted to 12 000 spores μl−1 and stored at −80°C until further use. A volume of 2 μL of the spore suspension was cultured on 5.5-cm Petri dish containing 10 mL PDA medium and maintained at 28°C in a growth chamber for two days in dark.
2.3 Volatile compound analysis of PGIVs and PGPVs
Methods of volatile compound analysis are described in Chen et al., (2023). For sample preparation, F. verticillioides was grown on TSB medium for two days. S. sympodiophorum was grown on PDA medium for two days. E. aerogenes was grown on LB medium for one day. The samples were used for volatile collection. TSB, PDA and LB medium were used as controls.
2.4 RNA extraction and library construction
Total RNA was extracted from Arabidopsis seedlings with or without F. verticillioides and S. sympodiophorum mVOCs exposure using an RNeasy Plant Mini kit (Qiagen, Hilden, German). DNase I recombinant (Roche) was used for DNA degradation. RNeasy MInElute Cleanup kit (Qiagen) was used for total RNA purification. A NanoDrop™ 2000c Spectrophotometers (Thermo Scientific) was used for RNA Quantitation.
Total RNA from Arabidopsis seedlings with or without F. verticillioides and S. sympodiophorum mVOCs treatment was used for RNA-seq library construction. Two biological replicates were included for each treatment. Construction of RNA-seq libraries was performed according to the instructions provided with the Illumina NovaSeq platform, which generates 150-bp paired-end reads. Illumina sequencing was performed by Genewiz, Inc.
2.5 RNA-seq analysis
Raw data for Enterobacter aerogenes mVOCs-treated Arabidopsis was obtained from a previous study (Chang et al., 2023). Adaptor sequences and poor-quality bases were removed by Trimmomatic v0.36 (Bolger et al., 2014); a quality score threshold was set to 30. Transcript abundance was quantified by Kallisto (Trapnell et al., 2012). DESeq2 was used for differentially expressed gene identification (Love et al., 2014). Genes with | log2(FC) | ≥ 1, p-adj <0.9 were considered responsive genes. Genes identified as either PGPVs- or PGIVs-responsive genes were defined as mVOCs-responsive genes. Z-score based on transcript per million (TPM) values were used for Euclidean distance-based cluster analysis. Gene Ontology enrichment (GO) analysis was performed with BiNGO v3.0.3 (Maere et al., 2005). The significance of enriched categories was analyzed using a Hypergeometric test. The false discovery rate was determined by the Benjamini & Hochberg method (Benjamini and Yekutieli, 2001). TF enrichment analysis was performed by GenFam (Bedre and Mandadi, 2019). The significance of TF enrichment analysis results was tested with Fisher's exact test. HRPE motif analysis of the promoter region was performed in PlantPan 4.0 with the sequence ranging from 1000 bp upstream to 500 bp downstream of the transcription start site (Chow et al., 2019).
2.6 Microarray analysis
The transcriptomic data of Arabidopsis seedlings treated with PGIVs Serratia plymuthica HRO-C48 mVOCs were utilized to validate the findings in this study. The microarray data in Wenke et al., (2012) were analyzed to extract the transcriptomic changes induced by Serratia plymuthica HRO-C48 mVOCs in Arabidopsis seedlings. The Affymetrix CEL files of Serratia plymuthica HRO-C48 mVOCs-induced transcriptome data were downloaded from the National Center for Biotechnology Information Gene Expression Omnibus with the accession number GSE35325 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE35325). Normalization and differential expressed gene analysis were performed on transcriptomic data from Arabidopsis exposed to S. plymuthica HRO-C48 mVOCs for 24 hr. using the R package ‘limma’ and ‘oligo’ (Carvalho and Irizarry, 2010; Ritchie et al., 2015). Genes with | log2(FC) | ≥ 1, p-adj <0.9 were considered to be S. plymuthica HRO-C48 mVOCs-regulated genes.
2.7 Quantitative real-time PCR for relative gene expression analysis
For each sample, 1000 ng RNA was used for cDNA preparation by ImProm-II™ Reverse Transcriptase (Promega). RT-qPCR was performed using the GoTaq®qPCR Master Mix (Promega) on StepOnePlus Real-Time PCR Systems (Applied Biosystems). ACT8 was used as the reference gene. A list of primer sequences used for RT-qPCR is provided in Supplementary Table 1. Relative gene expression was calculated with the 2−ΔΔCT method (Schmittgen and Livak, 2008).
2.8 Statistical analysis
Statistically significant differences in plant phenotypes and RT-qPCR results were assessed with Student's t-test. p < 0.05 was defined as statistically significant.
3 RESULTS
3.1 F. verticillioides mVOCs and S. sympodiophorum mVOCs promote plant growth
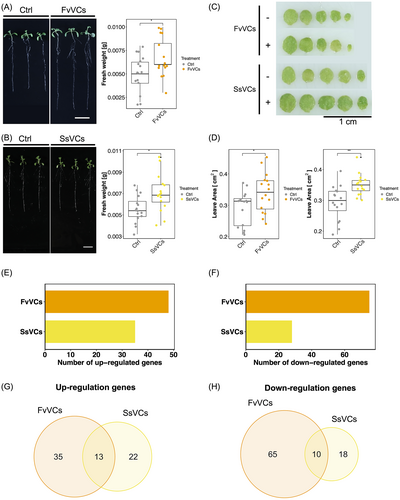
To examine whether F. verticillioides mVOCs and S. sympodiophorum mVOCs promote Arabidopsis growth, we grew Arabidopsis seedlings in the presence of F. verticillioides or S. sympodiophorum mVOCs. The Arabidopsis plants and microbes were grown on separate Petri dishes in a sealed environment, and Arabidopsis growth was monitored. Promotion of whole seedling growth was observed in Arabidopsis after exposure to F. verticillioides mVOCs for four days (Figure 1A, C-D). In the seedlings with S. sympodiophorum mVOCs exposure, significant promotion of the leaf surface area was observed after six days (Figure 1B, C-D). However, there was no observed promotion of root growth in S. sympodiophorum mVOCs-exposed plants (Figure 1B). These results indicate that both F. verticillioides and S. sympodiophorum mVOCs are PGPVs, though the induced growth phenotypes differ.
3.2 Identification of PGPVs and PGIVs
Although the phenotypic effects of different PGPVs and PGIVs have been well documented, the underlying regulatory mechanisms induced by PGPVs and PGIVs are still largely unknown (Cappellari and Banchio, 2020; Chang et al., 2023; Chiang et al., 2023; Pescador et al., 2022; Schulz-Bohm et al., 2017; Sharifi et al., 2022; Yasmin et al., 2021; Zamioudis et al., 2015). Since the effects of E. aerogenes mVOCs on plants growth and stress resilience have been well characterized (Chang et al., 2023; D'Alessandro et al., 2014), E. aerogenes mVOCs were used as a PGIV in this study. To investigate the chemical diversity of the tested PGPVs and PGIVs, GC–MS analysis was performed on the two PGPVs (F. verticillioides mVOCs and S. sympodiophorum mVOCs) and one PGIVs (E. aerogenes mVOCs). In the E. aerogenes mVOC samples, 3-methyl-1-Butanol, 2-Phenylethanol were identified as the key bioactive candidates. In the F. verticillioides mVOCs, dimethyl disulfide, 2,3-dimethyl-pyrazine and 1-ethyl-4-methoxy-benzene were identified as bioactive candidates. In the S. sympodiophorum mVOCs, acetoin, 3-methyl-1-butanol and phenylethyl alcohol were identified as bioactive candidates (Table 1).
| Emitting microbe | Compound name |
|---|---|
E. aerogenes, S. sympodiophorum |
1-Butanol, 3-methyl- |
| E. aerogenes | 2-Phenylethanol |
| F. verticillioides | Disulfide, dimethyl |
| F. verticillioides | Pyrazine, 2,3-dimethyl- |
| F. verticillioides | Benzene, 1-ethyl-4-methoxy- |
| S. sympodiophorum | Acetoin |
| S. sympodiophorum | Phenylethyl Alcohol |
3.3 PGPVs- and PGIVs-induced transcriptional changes in Arabidopsis thaliana
To understand the mechanisms of the Arabidopsis response to different mVOCs, the mVOCs-responsive genes were probed by conducting RNA-seq analysis of Arabidopsis plants exposed to two PGPVs (F. verticillioides mVOCs and S. sympodiophorum mVOCs) and one PGIVs (E. aerogenes mVOCs). A total of 123 genes were identified as F. verticillioides mVOC-responsive genes (Figure 1E, F). Among these genes, 48 were upregulated and 75 were downregulated. Furthermore, a total of 63 Arabidopsis genes were identified as S. sympodiophorum mVOC-responsive genes. Among the S. sympodiophorum mVOC-responsive genes, 35 were upregulated, and 28 were downregulated (Figure 1E, F). A total of 3813 genes were identified as E. aerogenes mVOC-responsive genes. Among these responsive genes, 2110 were upregulated, and 1703 were downregulated (Figure 1E, F). Comparing the list of F. verticillioides mVOC- and S. sympodiophorum mVOC-responsive genes revealed that only a small proportion were commonly upregulated (18.6%) or downregulated (10.8%) by both types of mVOCs (Figure 1G, H).
GO analysis revealed that genes related to anaerobic respiration were commonly upregulated in response to the PGPVs and PGIV (Figure 2A, B, S3). Futhermore, genes upregulated by F. verticillioides mVOCs were enriched in functions associated with post-transcriptional regulation and centromere complex assembly. For genes upregulated by S. sympodiophorum mVOCs, no significant GO terms were identified. Meanwhile, the PGIV-upregulated genes were commonly associated with defense and immunity (Figure 2A). No significant GO terms were identified for genes downregulated by either F. verticillioides mVOCs or S. sympodiophorum mVOCs. Genes with functions related to development and stress response were enriched in the E. aerogenes mVOC-downregulated gene list (Figure 2B). To determine if any general regulatory mechanisms were transcriptionally affected by PGPVs, we examined the functions of the 13 common PGPV-upregulated genes and 10 common PGPV-downregulated genes. Intriguingly, core hypoxia-responsive genes (HRGs) accounted for 84.6% of the common PGPV-upregulated genes (11 out of 13; Mustroph et al., 2009; Table S2). In previous work, the hypoxia-responsive promoter element (HRPE) was identified as a key regulatory motif (interacts with ERF VII) in about half of the 49 core hypoxia-responsive genes (Gasch et al., 2016; Mustroph et al., 2009). Thus, we next checked if the two PGPV-upregulated genes associated with hypoxia response might contain HPREs in their promoter regions (Table S2). However, the two non-hypoxia-associated PGPV-upregulated genes do not contain the HEPE motif, suggesting there may be an alternative regulatory mechanism that upregulates gene expression in response to PGPV exposure.
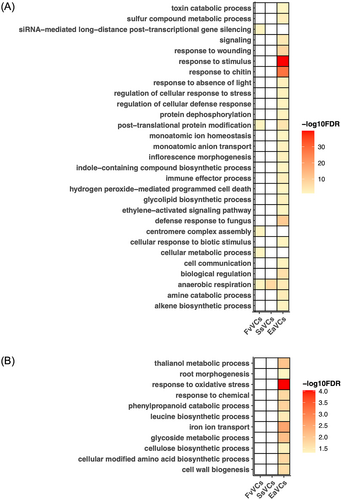
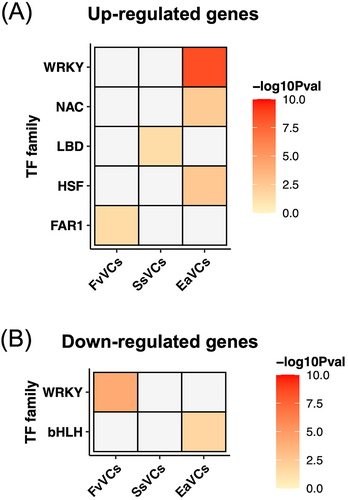
TFs are key regulators of transcriptional responses in plants and other organisms. To explore potential regulatory components that may contribute to phenotypes of growth promotion or inhibition, TF enrichment analysis was performed. The results showed that the FAR1 and LBD families were respectively enriched in the gene sets upregulated by F. verticillioides and S. sympodiophorum mVOCs (Figure 3A, Table S3-S6). In the set of PGIV (E. aerogenes mVOC)-upregulated genes, HSFs, NACs and WRKYs (modulate stress resilience) were enriched (Figure 3A, Table S3-S6). Intriguingly, WRKYs were also enriched in F. verticillioides mVOC-downregulated genes, while bHLHs were enriched in E. aerogenes mVOC-downregulated genes (Figure 3B, Table S3-S6).
3.4 Hierarchical clustering reveals transcriptomic features of Arabidopsis response to PGPVs and PGIVs
To explore the difference in Arabidopsis transcriptomic responses to different mVOCs, we performed hierarchical clustering of mVOCs-responsive genes. The mVOCs-responsive genes were sorted into 10 clusters (c1-c10) using R package pheatmap with default parameter settings. The analysis results showed that c1, c2, c7 and c9 were induced by the PGIVs, whereas c3, c5, c6 and c8 were suppressed by the PGIVs (Figure 4). GO enrichment analysis showed that PGIV-induced clusters (c2) were enriched in functions related to immune response, abscisic acid (ABA) response, JA signaling, SA signaling and ET biosynthesis. Functions of genes in the PGIV-suppressed cluster were enriched in cell wall organization and root morphogenesis (Figure 4). Genes related to immunity were enriched in c4, including MYB51, WRKY33, FRK1, CBP60g, SARD1 (Figure 4). No identified clusters were induced or suppressed by PGPVs (Figure 4).
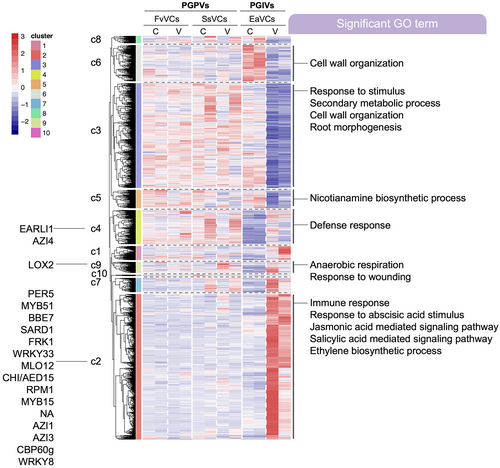
Interestingly, expression levels of c3 and c5 genes were consistently higher after PGPV treatment, as compared with the PGIVs-treated samples. In contrast, c2 genes were upregulated by PGIV exposure, but the transcript levels were lower in the presence of PGPVs. GO term analysis also revealed that c3 is enriched in genes with functions related to cell wall organization and development. Meanwhile, c5 is enriched in genes with functions related to nicotianamine biosynthesis (Figure 4). By comparing the gene expression pattern between PGPV-exposed plants and PGIV-exposed plants, the result suggest that genes associated with growth and development have higher expressions after PGPV treatment. Moreover, lower expression of genes associated with defense and immunity was observed after PGPV exposure, as compared to PGIV exposure (Figure 4). To extend this idea, we examined the previously reported transcriptomic changes induced by S. plymuthica HRO-C48 mVOCs in Arabidopsis. S. plymuthica HRO-C48 is a rhizobacteria (Kalbe et al., 1996), as its mVOCs inhibit Arabidopsis leaf and root growth after 24 h of exposure (Wenke et al., 2012). GO analysis indicates that S. plymuthica HRO-C48 mVOC-upregulated genes are enriched with in functions associated with defense and immunity response. Meanwhile, S. plymuthica HRO-C48 mVOC-downregulated genes are enriched with functions associated with stress response and auxin response (Figure S2).
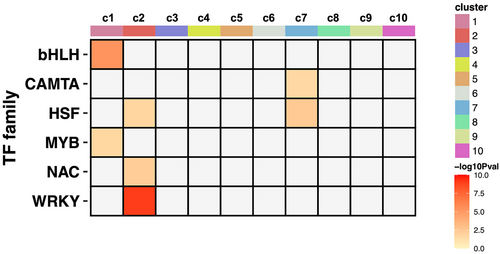
TF enrichment analysis on gene clusters further showed that MYBs and bHLHs were enriched in c1. In c2, enriched TF families included HSFs, NACs and WRKYs, which modulate plant stress resilience. Finally, HSFs and CAMTAs were enriched in c7 (Figure 5).
3.5 Plant immune systems respond to diverse mVOCs in different ways
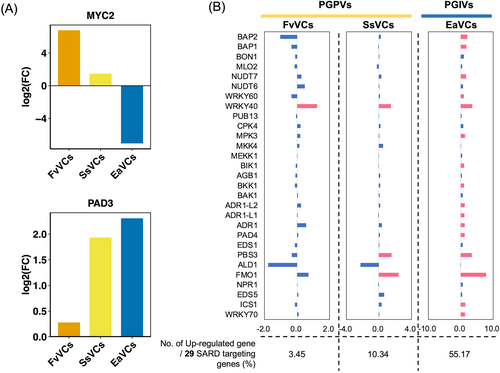
In a previous study, we found that mVOCs prime plant disease resistance (Chang et al., 2023; Chiang et al., 2023). Therefore, we wondered whether mVOCs might increase the expression of genes associated with plant disease resistance and thereby improve stress resilience. In plants, the immune systems can be subdivided into two categories, induced systemic resistance (ISR) and systemic acquired resistance (SAR). These two types of responses are respectively induced by beneficial microbes and pathogens (Vlot et al., 2021). To clarify which type of response is induced by the tested mVOCs, we examined ISR- and SAR-associated gene expression profiles in Arabidopsis after PGPVs and PGIVs exposure. In ISR responses, MYC2 typically serves as a central regulator. For instance, MYC2 negatively regulates camalexin biosynthesis genes in the context of JA-mediated defense responses (Millet et al., 2010). Our data further showed that the level of MYC2 was lower after E. aerogenes mVOCs treatment than after F. verticillioides and S. sympodiophorum mVOC treatments. In contrast, the levels of camalexin biosynthesis gene PAD3 were higher after E. aerogenes mVOC treatment than after F. verticillioides and S. sympodiophorum mVOC treatments (Figure 6A). It is known that SARD1 is a key TF that drives SA-mediated SAR responses. Thus, we next investigate the SARD1-targeting gene expression profiles (Sun et al., 2015). The result demonstrates that F. verticillioides and S. sympodiophorum mVOCs respectively upregulated 3.45% and 10.34% of SARD1-targeting genes. However, 55.17% of SARD1-targeting genes were upregulated by E. aerogenes mVOCs. These results suggest that ISR responses are induced by both F. verticillioides and S. sympodiophorum mVOCs. Meanwhile, SAR response plays a central role in the effects of E. aerogenes mVOCs (Figure 6B).
4 DISCUSSION
In this study, we showed that mVOCs of both F. verticillioides and S. sympodiophorum increase plant growth by affecting different organs in the seedlings. mVOCs of F. verticillioides promoted Arabidopsis growth of whole seedlings, while mVOCs of S. sympodiophorum only increased the leaf surface area (Figure 1A-D). Due to the diversity of the microbial metabolic profiles among different species, the chemical components of mVOCs emitted by each species can be extremely varied. Thus, our results imply that the different phenotypes in Arabidopsis after exposure to different mVOCs could be due to the different constituent compounds in F. verticillioides and S. sympodiophorum mVOCs.
In addition, the comparison of F. verticillioides mVOC- and S. sympodiophorum mVOC-responsive genes showed that less than 40% of upregulated and downregulated genes were commonly regulated by both PGPVs. The result implies that Arabidopsis might respond to F. verticillioides mVOCs and S. sympodiophorum mVOCs in a different manner at the molecular level (Figure 1G, H). In support of this idea, TF enrichment analysis of mVOC-responsive genes showed that the FAR1 family is enriched in the F. verticillioides mVOC-upregulated genes, while LBD family is enriched in the S. sympodiophorum mVOC-upregulated genes (Figure 3). Both the FAR1 and LBD families are known to mediate plant growth and development. FAR1 family members are involved in the light-mediated regulation of plant development (Lin and Wang, 2004), while LBDs are essential to the regulation of leaf size and shape (Shuai et al., 2002). Thus, the results suggest that F. verticillioides mVOCs might increase the size of whole Arabidopsis seedlings by stimulating FAR1 activity. However, S. sympodiophorum mVOCs affect LBDs to increase the leaf surface area in Arabidopsis.
Interestingly, we found that WRKYs were enriched in F. verticillioides mVOCs downregulated genes (Figure 3). WRKYs constitute a large family of TFs and have diverse roles in growth, development and stress responses in plants (F. Chen et al., 2017; J. Chen et al., 2017; Chi et al., 2013). Thus, we closely examined the list of identified WRKY TFs enriched in F. verticillioides mVOC-downregulated genes. The results indicated that WRKY38, WRKY51, WRKY54 and WRKY62 were downregulated by F. verticillioides mVOCs. Each of these TFs is associated with defense and immunity regulation (Table S4). WRKY51 is known to be an activator of SA-mediating responses and a repressor of JA-mediating responses (Gao et al., 2011). Additionally, both WRKY38 and WRKY62 are the downstream regulators of SA signaling (Xie et al., 2010). WRKY62 is a repressor of JA-mediating responses (Mao et al., 2007). WRKY54 is a homolog of WRKY70 (Wang et al., 2006), and both of these proteins act as negative regulators of cell wall-associated defenses against necrotrophic pathogens (Li et al., 2017). In addition, WRKY70 serves as a central node for activation of SA-mediating defense responses, but it represses JA-mediating defense responses (Li et al., 2006, 2004). Collectively, our expression data and these known functions of different WRKYs suggests that F. verticillioides mVOCs might repress SA-mediated defense responses and promote JA-mediated defense responses. In line with this idea, we also found that the central regulator of JA signaling, MYC2 was upregulated by F. verticillioides mVOCs.
SA-mediated signaling and JA/ET-mediated signaling respectively constitute crucial regulatory mechanisms in plant defense against hemibiotrophic/biotrophic pathogens and necrotrophic pathogens (Glazebrook, 2005). F. verticillioides (formerly F. moniliforme) was reported to grow within its host either as a hemibiotrophic pathogen or as an endophyte (Lanubile et al., 2014; Lee et al., 2009; Murillo-Williams and Munkvold, 2008), wth environmental factors modulating its pathogenicity (Murillo-Williams and Munkvold, 2008). In light of our findings, it can be speculated that mVOCs of hemibiotroph F. verticillioides serve as signals to weaken plant resistance via the antagonistic interplay between SA and JA signaling pathways.
Growth-defense trade-offs are important strategies utilized by plants to reallocate resources and prioritize either growth or defense. However, plants exposed to mVOCs can often mount both growth promotion and defense responses at the same time (Blom et al., 2011; Fukada, 2024; Zamioudis et al., 2015, 2013). It was previously shown that E. aerogenes mVOCs are PGIVs, which inhibit plant growth and prime plant disease resistance (Chang et al., 2023). In contrast, we found that F. verticillioides and S. sympodiophorum mVOCs are PGPVs that promote plant growth. In this study, we identified a total of 2682 genes that respond to these three types of mVOCs. According to our hierarchical clustering analysis, the mVOC-responsive genes were classified into 10 clusters based on their expression pattern in response to PGPVs and PGIVs. The largest clusters were c2 and c3. These two clusters exhibited opposite patterns. Genes in c2 are mainly associated with defense and immunity, while genes in c3 are mostly involved in growth and development (Figure 4). Our data demonstrated that genes in c3 exhibited higher mRNA levels in Arabidopsis exposed to PGPVs from F. verticillioides and S. sympodiophorum (Figure 4). In contrast, genes in c2 exhibited low mRNA levels after exposure to PGPVs from F. verticillioides and S. sympodiophorum (Figure 4). Conversely, PGIVs from E. aerogenes largely induced c2 genes and suppressed c3 genes (Figure 4). Collectively, the results show that PGIVs-induced Arabidopsis growth inhibition corresponds to the upregulation of a large set of defense-associated genes. He et al., (2022) proposed that the reallocation of energy in growth-defense trade-offs may be modulated by the intensity of environmental stimuli, suggesting that growth-defense trade-off is not an all-or-nothing type of response. This idea could help to explain why plants may simultaneously exhibit both growth promotion and defense reinforcement under certain conditions of mVOC exposure.
Our findings provide insights into how plants may partition energy resources into growth and defense responses at a molecular level. Comparing the transcriptional changes in PGPVs- and PGIVs-treated plants, we found that the numerous defense-associated genes were upregulated in the transcriptome of PGIVs-treated plants but PGPV-treated plants (Figure 4). It is known that WRKYs play essential roles in diverse stress-induced responses in plants (Chen et al., 2019; Goyal et al., 2023). Furthermore, WRKY18 is inducible after PGIVs treatment and served as a negative regulator of plant growth (Wenke et al., 2012). Our results (Figure 3 and Table S3-S6) demonstrated that WRKYs were enriched among PGPV-downregulated genes and in PGIV-upregulated genes. Overall, the changes in transcript levels of WRKYs seemed to exhibit a similar pattern with the number of mVOCs-induced defense-associated genes. Thus, we postulate that WRKYs might serve as convergence nodes to integrate the intensity, frequency and duration of environmental stress cues. Transcriptional regulation of WRKYs may allow the plants to direct energy into required biological processes, underlying the growth-defense trade-off.
To further explore this idea, we considered stress resilience in Arabidopsis after different PGPV and PGIV exposures. Copper-based antimicrobial compounds (CBACs) have been used as agricultural protectants against pathogens for over a hundred years (Lamichhane et al., 2018), and it is known that copper enhances plant disease resistance by suppressing ABA biosynthesis (Liu et al., 2020). However, suppressing ABA biosynthesis can impair stomatal closure and thus lead to a higher transpiration rate (Liu et al., 2020), making plants more susceptible to drought stress. In our previously published study, we found that mVOCs of Tolypocladium inflatum GT22 promote plant growth and improve both copper stress tolerance and disease resistance (Chiang et al., 2023). Thus, we examined the expression of factors involved in resistance to copper stress in our data. Our results suggested that copper stress tolerance was not improved in Arabidopsis after F. verticillioides and S. sympodiophorum mVOC treatments (Figure S4). However, Arabidopsis treated with E. aerogenes mVOCs exhibited improved copper tolerance (Figure S4). Combining these findings with our previous result in Chiang et al., (2023), we conclude that the number of upregulated WRKYs is well-aligned with stress resilience and growth retardation in plants (Table S7).
The microbiomes can provide a host plant with various advantages, including growth promotion, nutrient uptake and stress resilience (Trivedi et al., 2020). Plant-associated microbiomes do not just exist in modern land plants. In fact, the ancestor of land plants, green algae (Tena, 2020), formed symbiotic relationships with microbes in order to obtain vitamin B12 (Croft et al., 2005). In addition, the earliest land plant, Marchantia (Bowman et al., 2017), had a microbiome, although its functions are still unclear (Alcaraz et al., 2018; Marks et al., 2018; Wicaksono et al., 2023). Since mVOCs are gaseous metabolites emitted by microbes, it is plausible that plants have evolved different strategies to respond to signals from different microbes. In this study, we found that HSFs, NACs and WRKYs are the TF families that are enriched in the defense-associated cluster of mVOC-regulated genes (c2, Figure 5). HSFs, NACs and WRKYs also existed in the early-diverging Marchantia and are crucial in both defense and development in modern land plants (Eulgem et al., 2000; Jin et al., 2014; Scharf et al., 2012; Wani et al., 2021; Yuan et al., 2019). Thus, our results imply that HSFs, NACs and WRKYs might be essential regulators of mVOCs-induced responses and exhibit conserved functions in various land plants exposed to mVOCs.
AUTHOR CONTRIBUTIONS
C.H.C. analyzed the data and wrote the manuscript. C.H.C., C.C.H., P.Y.S., Y.S.C. and C.Y.W. performed the experiments. H.M.T. and Y.Y.Z. performed the volatile compound analysis. I.J.L. and C.C.H. performed the microbe isolation and microbial identification. H.J.H. organized this work.
ACKNOWLEDGMENTS
We would like to thank National Science and Technology Council (NSTC) for offering funding to this project. We thank Technology Commons (TechComm), College of Life Science, National Taiwan University, for the VOCs trapping and GC–MS system.
FUNDING INFORMATION
This work was supported by National Science and Technology Council (NSTC-112-2313-B-006 -005-, MOST-111-2313-B-006-005-, MOST-109-2313-B-006-008-).
Open Research
DATA AVAILABILITY STATEMENT
The data underlying this article are provided as supplemental tables.




