Heterologous expression of Sesuvium portulacastrum SpCIPK2 confers salt tolerance in transgenic Arabidopsis thaliana
Abstract
Calcineurin B-like interacting protein kinases (CIPKs) play critical roles in plant adaptation to salt stress. However, the biological functions of CIPKs in Sesuvium portulacastrum, a halophyte flourishing in coastal mudflats, remain poorly understood. Here, a highly expressed CIPK gene, SpCIPK2, was identified from transcriptomic analyses of S. portulacastrum root systems under salt stress. Subcellular localization assays confirmed the cytoplasmic presence of SpCIPK2. Arabidopsis thaliana plants overexpressing SpCIPK2 exhibited markedly improved salt tolerance, characterized by increased fresh weight under salt stress. Transgenic plants demonstrated significantly lower levels of O2·− and H2O2 compared to wild-type plants. Furthermore, transgenic plants revealed a reduced relative conductivity and enhanced peroxidase (POD) activity in the leaves. Salt treatment accelerated Na+ efflux while slowing K+ efflux in transgenic plants, resulting in diminished Na+ accumulation and an elevated K+/Na+ ratio during salt stress. This evidence suggests that SpCIPK2 enhances salt tolerance by regulating ion homeostasis, activating antioxidant enzymes activity, and scavenging reactive oxygen species (ROS) in salt-stressed plants.
1 INTRODUCTION
Soil salinization represents a growing global challenge that significantly restricts agricultural productivity (Zhang et al., 2023b). Increased soil salinity generates ionic and osmotic stresses, which subsequently trigger secondary effects such as oxidative stress and nutritional deficiencies (Yang et al., 2009; Hasegawa & Li, 2000). Plants have evolved sophisticated strategies to manage Na+ accumulation in the cytoplasm, including the limitation of Na+ uptake, enhanced Na+ efflux, sequestration of Na+ in vacuoles, and the regulation of Na+ transport from roots to shoots (Shi et al., 2002). Therefore, maintaining a stable K+/Na+ ratio within the cytoplasm is vital for optimal cellular functions in plants, influencing various physiological and developmental processes (Kim et al., 2009).
Ca2+ functions as a widely distributed secondary messenger in plants that governs numerous physiological processes, including growth, development, nutrient absorption, and stress responses (Yu et al., 2014). Environmental stressors can prompt spatial and temporal fluctuations in cytoplasmic Ca2+ concentrations (Ma et al., 2020). Ca2+ sensors, such as Ca2+-dependent protein kinases (CDPKs), calcineurin B-like proteins (CBLs), calmodulin (CaM), and CaM-like proteins (CMLs), identify these fluctuations and engage with downstream target proteins, initiating a series of physiological and metabolic responses to environmental changes (Yu et al., 2014; Ma et al., 2020; Tang et al., 2020; Zhang et al., 2022). CBLs, which lack intrinsic kinase activities, establish a plant-specific Ca2+ signal transduction system through their specific interaction with CBL-interacting protein kinases (CIPKs; Yu et al., 2014; Ma et al., 2020). CIPKs, characterized as plant-specific serine/threonine kinases, consist of an N-terminal kinase domain and a C-terminal regulatory domain. The interaction with CBLs occurs via a conserved C-terminal asparagine-alanine-phenylalanine (NAF) domain, facilitating the formation of complexes that target proteins essential for plant responses to various abiotic stresses (Sheng et al., 2014). Evidence indicates that CIPKs play a significant regulatory role in plant growth, development, and tolerance to abiotic stress. The salt overly sensitive (SOS) signal transduction pathway, driven by the CBL-CIPK complex, is particularly important for sodium transport (Qiu et al., 2002), influencing intracellular Na+ transport and sustaining cytoplasmic Na+/K+ homeostasis, thereby improving plant salt tolerance. In A. thaliana, the AtCBL4 (AtSOS3) protein forms a complex with AtCIPK24 (AtSOS2), promoting the activation of the Na+/H+ antiporter AtNHX7 (AtSOS1) and the tonoplast AtNHX1 protein. This interaction effectively expels excess Na+ from the cytosol and sequesters Na+ into vacuoles, enhancing salt tolerance (Mahajan & Tuteja, 2005; Li et al., 2009). Furthermore, recent investigations indicate that AtCIPK24/AtCIPK8 interacts with AtCBL5 to modulate salt tolerance during the seed germination phase in Arabidopsis (Xie et al., 2024).
CIPK proteins have been thoroughly studied in various species. Overexpression of the Hordeum spontaneum HsCIPK2 gene in A. thaliana reduced the negative impacts of NaCl and Hg2+ on seed germination and root development (Pan et al., 2018). Similarly, the overexpression of Zea mays ZmCIPK42 increased tolerance to elevated salt stress (Chen et al., 2021). HbCIPK2 plays a role in mediating the responses of wild Hordeum brevisubulatum to drought, salinity, and ABA stress, with its overexpression mitigating the salt-sensitive phenotype in the A. thaliana sos2-1 mutant (Li et al., 2012). Furthermore, AtCIPK8 has been shown to enhance both salt tolerance and nitrogen uptake in A. thaliana (Yin et al., 2020; Yasuda et al., 2017). Overexpression of Oryza sativa OsCIPK12 improves tolerance to low temperatures, drought, and salt stress by elevating proline and soluble sugar levels (Xiang et al., 2007). Zheng et al. (2014) demonstrated that the overexpression of Nitraria tangutorum NtCIPK2 in A. thaliana enhanced drought and salt stress resilience via increased antioxidant enzyme activities, minimized oxidative damage to the plasma membrane, and augmented photosynthesis and accumulation of osmoregulatory substances. Furthermore, NtCIPK9 boosts salt tolerance by upregulating K+ transporter protein gene expressions and sustaining cytoplasmic Na+/K+ homeostasis (Lu et al., 2020), while NtCIPK11 improves both salt and drought tolerance through elevated proline levels (Lu et al., 2021).
Sesuvium portulacastrum, a perennial halophyte within the Aizoaceae family, predominantly inhabits tropical coastal mudflats. This species exhibits exceptional salt tolerance and adaptability to varying environmental conditions. In response to salt stress, S. portulacastrum effectively reduces oxidative damage to cellular membranes while preserving the relative stability of K+ and Mg2+ ions (Sun et al., 2009). Multiple salt-tolerance genes have been identified in this organism, with increased expression of fructose-1,6-bisphosphate aldolase (SpFBA) markedly improving salt tolerance (Fan et al., 2009). Overexpression of the SpBADH gene in A. thaliana diminishes H2O2 accumulation, elevates proline levels, and activates antioxidant enzyme pathways, thereby enhancing the scavenging of reactive oxygen species (ROS) and improving drought and salt tolerance (Yang et al., 2015). Research has identified the S. portulacastrum plasma membrane Na+/H+ antiporter gene, SpSOS1, which lowers Na+ levels in transgenic yeast mutants and boosts salt tolerance (Zhou et al., 2015). Co-expression of SpSOS1 with the H+-ATPase gene SpAHA1, further augments salt tolerance in A. thaliana (Fan et al., 2018; Fan et al., 2019). Moreover, the tonoplast Na+/H+ antiporter gene SpNHX1 is integral to the response to salt stress (Zhou et al., 2018a). Under salt stress, the SpCBL10-SpCIPK8 complex enhances the tolerance provided by SpSOS1 (Zhou et al., 2023).
Despite the identification of several salt-tolerance genes, information on CIPK genes in S. portulacastrum is limited. This study identified SpCIPK2 as the most highly expressed gene under salt stress, utilizing full-length transcriptome and RNA-seq data from previous research (Li et al., 2023). Quantitative real-time polymerase chain reaction (qPCR) assessed the expression pattern of SpCIPK2 under salt stress, while subcellular localization studies revealed its cellular distribution. Overexpression of SpCIPK2 in A. thaliana enabled an investigation into its role in salt stress resistance. This study establishes a theoretical framework for leveraging the SpCIPK2 gene to enhance plant salt tolerance through genetic improvement.
2 MATERIALS AND METHODS
2.1 Plant materials and treatment
S. portulacastrum was cultivated at the experimental base of the Hainan University and propagated hydroponically from cuttings, with the conditions as follows: 28°C with 16 h light/25°C with 8 h dark, light intensity of 150 μmol m−2 s−1, and relative humidity of 85% ± 5% (Li et al., 2023). Vigorous and uniformly-sized plants were maintained in Hoagland's nutrient solution under natural environmental conditions for 10 days, with solution replacement every five days. Following this, uniformly grown S. portulacastrum plants were exposed to salt stress using 800 mmol l−1 NaCl, with three biological replicates conducted. Leaves and roots were harvested at 0, 6, 12, 24, 48, and 72 hours post-treatment for RNA extraction.
2.2 Identification and expression analysis of the SpCIPK2 gene
Transcriptome data from S. portulacastrum roots under salt stress (PRJNA930581) revealed a highly expressed SpCIPK2 gene. qPCR assessed the expression profiles of SpCIPK2 in response to salt stress, aligning with findings from prior studies (Li et al., 2023).
A phylogenetic tree was constructed to compare SpCIPK2 with CIPK proteins from A. thaliana using the maximum likelihood (ML) method in the MEGA X software. Multiple sequence alignments of CIPK2 proteins across diverse plant species utilized ClustalW, while conserved domains of CIPK proteins were visualized with WebLogo under default parameters.
2.3 Subcellular localization of SpCIPK2
Total RNA extraction and cDNA synthesis were done using the RNAprep Pure Plant kit (Tiangen Biotech Co., Ltd., DP441) and the PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, RR047A). PCR employed mixed cDNA as a template along with primers SpCIPK2-1300-Sal I-F and SpCIPK2-1300-GFP-Kpn I-R, detailed in Table S1. The resultant PCR product, devoid of a stop codon, was cloned into the pCAMBIA1300-GFP localization vector, at the Sal I and Kpn I sites, generating the pCAMBIA1300-SpCIPK2-GFP recombinant plasmid. This plasmid was subsequently transfected into A. thaliana protoplasts using the method outlined by Yoo et al., (2007). GFP fluorescence was examined via confocal laser scanning microscopy (FV1200; Olympus Corporation), with protoplasts transformed with the empty pCAMBIA1300-GFP vector serving as a control.
2.4 Construction of transgenic A. thaliana
To assess the salt tolerance function of SpCIPK2 in plants, the target gene was amplified using the SpCIPK2-1300-Sal I-F and SpCIPK2-1300-Kpn I-R primers (Table S1), leading to the construction of the recombinant plasmid pCAMBIA1300-SpCIPK2. This plasmid facilitated the genetic modification of A. thaliana with Col-0 background through an Agrobacterium-mediated transformation (Clough & Bent, 1998). Screening of transgenic A. thaliana seeds occurred on 1/2 MS medium supplemented with 50 mg l−1 Hyg B. The seedlings were then positioned in a plant light incubator at 22°C, with a photoperiod of 16 h light/8 h of dark. The relative humidity was maintained at 70%. To identify positive plants, A. thaliana leaf DNA was analyzed using the TransDirect Plant Tissue PCR Kit (Beijing TransGen Biotech Co., Ltd., AD301-01), following the manufacturer's instructions. The screening utilized the same primers, SpCIPK2-1300-Sal I-F and SpCIPK2-1300-Kpn I-R.
2.5 Salt stress treatment
T3 generation transgenic A. thaliana seeds and wild-type (WT) seeds were subjected to sterilization, which involved sequential rinsing with 75% alcohol for three times, 10% sodium hypochlorite for four times, and ddH2O for five times. Subsequently, the seeds underwent vernalization at 4°C for two days before being sown on 1/2 MS solid medium. Following this, the seeds were incubated vertically in a light incubator at 22°C, with a photoperiod of 16 h light/8 h of dark. After seven days, the seedlings were transplanted onto 1/2 MS solid medium supplemented with either 0 or 200 mM NaCl and cultured for an additional seven days. Plant growth was monitored and documented through photography. Concurrently, 10-day-old A. thaliana seedlings were transferred to a substrate composed of vermiculite and nutrient soil (1:1 ratio) for one month, watering it with 1/2 Hoagland solution every five days. WT and transgenic A. thaliana plants exhibiting similar growth characteristics were selected for subsequent salt stress treatments, which included concentrations of 0 and 200 mM NaCl, each replicated three times. After 10 days of treatment, phenotypic observations were conducted, and fresh weights were recorded.
2.6 Physiological indicator measurements
Leaves from WT and transgenic A. thaliana were harvested following exposure to salt stress. Accumulation of hydrogen peroxide (H2O2) and superoxide anion (O2·−) in these leaves was assessed using nitroblue tetrazolium chloride (NBT) and 3,3′-diaminobenzidine (DAB) staining techniques (Zhu et al., 2021; Daudi & O'Brien, 2012). Relative conductivity and peroxidase (POD) activity of both WT and transgenic plants under salt stress were measured according to established protocols from prior studies (Yang et al., 2015; Zhou et al., 2018b).
2.7 Measurement of ion flux rate and ion contents
T3 generation transgenic and WT seeds were cultivated on 1/2 MS medium for seven days before being transferred to 1/2 MS medium supplemented with 200 mM NaCl for 24 hours. Roots were then harvested and immersed in a measuring solution (Na+: 0.5 mM NaCl, pH 5.8; K+: 0.5 mM KCl, pH 5.8) for a 20-minute equilibration period. Ion net fluxes (Na+ and K+) were recorded for 5 minutes from the elongation zone of the roots (3 mm from the root tip) utilizing a non-invasive micro-test (NMT) system, as previously detailed (Liu et al., 2019; Zhang et al., 2022). This procedure was repeated eight times. Flux microsensors (Na+: NMT-HC-02; K+: NMT-HC-03) were positioned 20 μm from the root tip, and flux data were analyzed using the imFluxes 3.0 software (xuyue.net).
Following NaCl treatment, potted plants were collected and dried. A 0.1 g dry sample was weighed, and Na+ and K+ contents were quantified using atomic absorption spectrometry (AA-670, Shimadzu Corp., Kyoto, Japan; Yin et al., 2020). Three biological replicates were conducted.
2.8 Expression analysis of stress-related genes under salt treatment
To investigate the expression patterns of salt stress-related genes in transgenic plants, qPCR analysis was conducted on antioxidant-related genes, including AtP5CS1, AtSOD1, and AtPOD, as well as ion transport-related genes such as AtSOS1, AtSOS2, AtSOS3, AtSCABP8, AtHKT1;1, and AtNHX1. The AtACT2 gene served as an internal control. Relative gene expression was determined following the 2−ΔΔCT technique (Yang et al., 2015). Primer sequences were listed in Table S1.
2.9 Statistical Analysis
All experiments were performed in three biological replicates unless otherwise stated. Statistical analysis was carried out utilizing the SPSS 20 software, and statistical differences were assessed utilizing one way analysis of variance (ANOVA). Data are expressed as means with standard errors, and p < 0.05 was regarded as statistically significant.
3 RESULTS
3.1 SpCIPK2 is a salt-induced gene in S. portulacastrum
Analysis of full-length transcriptome and RNA-seq data from S. portulacastrum under salt stress (Li et al., 2023) identified the gene transcript_HQ_Sp_Three_transcript60151/f6p0/2156, which was significantly upregulated by salt stress, within the S. portulacastrum CIPK gene family (Figure S1). The SpCIPK2 gene comprises of a coding region of 1359 bp, encoding a protein of 451 amino acids. Phylogenetic analysis revealed high similarity between SpCIPK2 and A. thaliana AtCIPK2 (Figure 1A), leading to its classification as SpCIPK2. Sequence comparisons indicated 74.95% similarity with Beta vulgaris BvCIPK2 and 73.22% similarity with Spinacia oleracea SoCIPK2 (Figure 1B). Structural analysis classified SpCIPK2 within the serine/threonine protein kinase family, containing an N-terminal serine/threonine kinase domain, a C-terminal CBL-binding region NAF/FISL domain, and a protein phosphatase binding domain (PP2C-Interact domain) (Figure 1C, D).
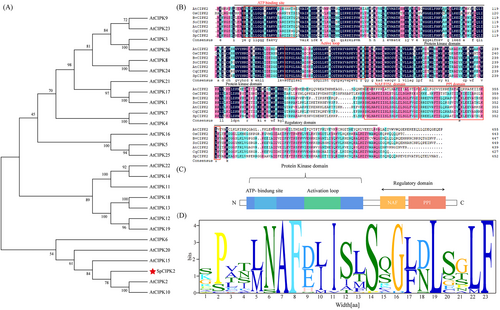
Expression patterns of SpCIPK2 under 800 mM NaCl treatment were evaluated through qPCR, revealing significant upregulation in both roots and leaves. Peaking at 48 hours, the increases in roots were 108-fold, and in leaves, 272-fold compared to baseline levels (Figure 2).
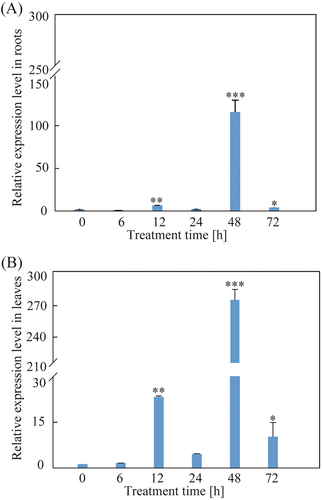
3.2 Subcellular localization of SpCIPK2
To elucidate the localization of SpCIPK2 in plant cells, the SpCIPK2 gene was cloned without its stop codon (Figure S2 A) and subsequently integrated into the pCAMBIA1300-GFP vector (Figure 3A). The resultant recombinant plasmid 1300-SpCIPK2-GFP, along with and the empty pCAMBIA1300-GFP vector, was introduced into A. thaliana protoplasts via an Agrobacterium-mediated approach. Confocal fluorescence microscopy revealed that SpCIPK2 primarily localized in the cytoplasm, whereas GFP fluorescence in the control group was distributed throughout the cell (Figure 3B).
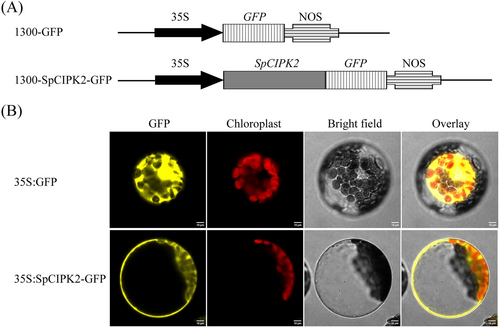
3.3 Heterologous expression of SpCIPK2 enhances salt tolerance in transgenic Arabidopsis thaliana
To investigate the function of SpCIPK2, the pCAMBIA1300-SpCIPK2 recombinant plasmid was constructed and transfected into Arabidopsis via Agrobacterium mediation. PCR amplification of leaf DNA confirmed 22 positive seedlings following HygB selection (Figure S2 B). Both transgenic and WT Arabidopsis seedlings were subsequently transplanted onto 1/2 MS medium containing 0 and 200 mM NaCl for a duration of seven days. Under control conditions, both groups exhibited robust growth (Figure 4A). In contrast, exposure to 200 mM NaCl resulted in stunted growth and leaf yellowing. Notably, transgenic lines demonstrated improved growth and significantly greater fresh weight under salt stress compared to WT plants (Figure 4B).
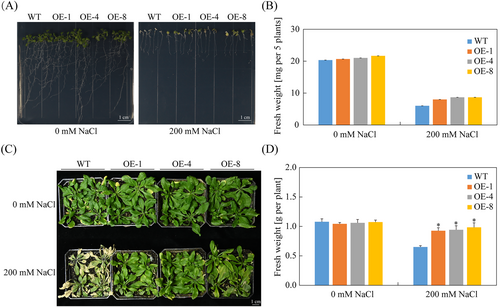
To analyze the response of SpCIPK2 to salt stress, 10-day-old seedlings cultivated in 1/2 MS medium were transplanted into soil and allowed to grow for one month prior to salt treatment. Under 200 mM NaCl, WT plants displayed yellowing and wilting, while transgenic Arabidopsis maintained normal growth and exhibited significantly greater fresh weight (Figure 4C). Under normal conditions, no significant growth differences were noted between transgenic and WT plants (Figure 4D).
3.4 SpCIPK2 improves salt tolerance in Arabidopsis by scavenging ROS
Abiotic stress disrupts ROS homeostasis in plants, resulting in the accumulation of ROS, including H2O2 (Chen et al., 2014). NBT and DAB staining analyzed H2O2 and O2·− accumulation in leaves subjected to salt stress. Under normal conditions, both transgenic and WT plants exhibited minimal and uniform staining (Figure 5A), reflecting low levels of H2O2 and O2·−. However, salt stress induced more pronounced staining in both WT and transgenic plants (Figure 5B), indicating elevated H2O2 and O2·− levels compared to untreated controls. Notably, transgenic plants demonstrated lighter staining than WT plants under salt stress, suggesting that SpCIPK2 can alleviate the accumulation of H2O2 and O2·−.

Relative conductivity and POD activities were measured in leaves (Figure 5C, D). Salt stress elevated relative conductivity in both WT and transgenic Arabidopsis, although levels remained lower in transgenic plants (Figure 5C), indicating reduced leaf membrane damage under salt stress. Additionally, POD activity was greater in transgenic lines than in WT plants (Figure 5D), suggesting that Arabidopsis overexpressing SpCIPK2 demonstrated enhanced antioxidant enzyme activities and accumulated higher levels of osmotic-regulating substances in response to salt stress.
3.5 SpCIPK2 improves salt tolerance by regulating intracellular Na+ and K+ balance
The net flux of Na+ in the elongation zone of the root tip under salt stress was assessed using the NMT technique. Under control conditions, Na+ fluxes in the roots of both transgenic and WT plants remained low and relatively stable (Figure 6A, E). However, after 24 hours of salt treatment, both WT and transgenic Arabidopsis roots exhibited a pronounced tendency to efflux Na+ (Figure 6B). Importantly, the net efflux rate of Na+ from transgenic roots was significantly higher than that of WT plants under salt stress, with increases of 27, 17, and 16 times compared to WT Arabidopsis (Figure 6F).
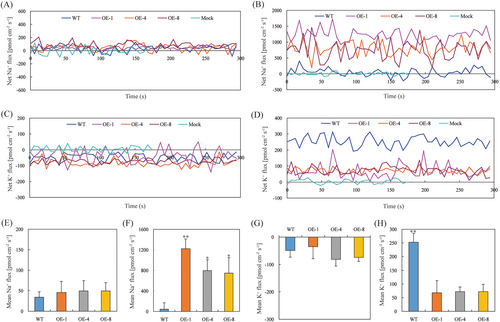
The net flux of K+ in the elongation zone of root tips was monitored before and after salt stress. Prior to the treatment, both WT and transgenic Arabidopsis roots displayed K+ uptake (Figure 6C, G). However, under salt stress both types exhibited K+ efflux, with transgenic roots showing significantly reduced K+ exclusion rates (Figure 6D). The average net efflux of K+ from transgenic lines (OE-1, OE-4, OE-8) was 27%, 28%, and 28% of that from WT Arabidopsis roots, respectively (Figure 6H).
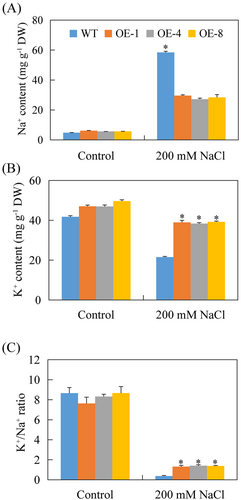
Additionally, Na+ and K+ contents were assessed. Under normal conditions, no significant differences in Na+ and K+ levels were evident between WT and transgenic plants (Figure 7A, B). After exposure to salt stress, Na+ content increased in all plants but was notably higher in WT compared to transgenic Arabidopsis (Figure 7A). Conversely, K+ content declined in all plants, with transgenic Arabidopsis exhibiting significantly less reduction than WT (Figure 7B). Consequently, transgenic A. thaliana sustained a higher K+/Na+ ratio under salt stress, with ratios in the three transgenic lines being 3.58, 3.83, and 3.75 times greater than those in WT plants, respectively (Figure 7C).
3.6 SpCIPK2 overexpression enhances salt stress-related gene expression
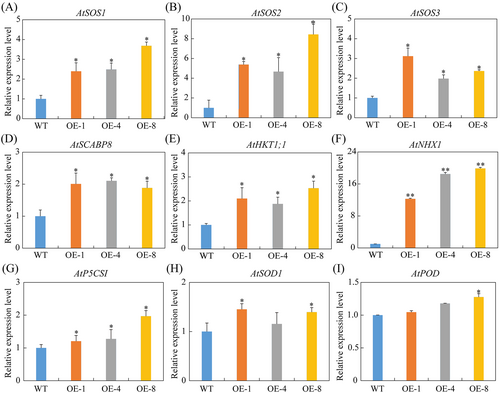
To elucidate the molecular mechanisms underlying the SpCIPK2 response to salt stress, expression levels of various stress-related genes were analyzed using qPCR. Genes involved in the regulation of Na+ and K+ homeostasis, such as AtSOS1 (Figure 8A), AtSOS2 (Figure 8B), AtSOS3 (Figure 8C), AtSCABP8 (Figure 8D), AtHKT1;1 (Figure 8E), and AtNHX1 (Figure 8F), demonstrated upregulation in SpCIPK2 overexpression lines. Among these, AtNHX1 showed markedly higher expression in three transgenic lines, approximately 12-, 18-, and 20-fold greater than in WT. Furthermore, ROS scavenging-related genes, including AtP5CS1 (Figure 8G), AtSOD1 (Figure 8H), and AtPOD (Figure 8I), exhibited elevated expression levels in transgenic lines compared to WT plants under salt stress (Figure 8).
4 DISCISSION
Plant growth and development are affected by multiple factors. Under stress conditions, the expression of stress-responsive genes initiates physiological responses that enable plants to withstand and adapt to environmental challenges. CIPKs, a class of plant-specific protein kinases featuring serine/threonine functional domains, regulate Ca2+ signaling and activate specific physiological and biochemical pathways that enhance plant tolerance to abiotic stresses (Xi et al., 2017; Mo et al., 2018; Ma et al., 2020; Zhang et al., 2023a). Numerous studies have established the essential role of CIPKs in enhancing plant stress tolerance (Bai et al., 2023; Ding et al., 2023; Gu et al., 2023; Li et al., 2022). However, their specific functions within the salt tolerance signaling pathways of S. portulacastrum remain inadequately characterized. This study identified and cloned the SpCIPK2 gene from S. portulacastrum using RNA-seq data. Multiple sequence alignment analyses revealed that SpCIPK2 encoded a protein featuring a conserved N-terminal Ser/Thr protein kinase domain and a variable C-terminal regulatory domain, which contains the NAF/FISL motif that interacts with CBL proteins, aligning with the structural characteristics of CIPK kinases (Figure 1). Moreover, SpCIPK2 expression significantly increased in response to salinity in both roots and leaves (Figure 2). A previous study showed that overexpression of Arabidopsis AtCIPK2 or Hordeum spontaneum HsCIPK2 in transgenic Arabidopsis alleviated toxic effects of salinity and mercury on seed germination and root growth (Pan et al., 2019). It is hypothesized that SpCIPK2 is involved in regulating stress signaling through the CBL-CIPK pathway.
Salt stress leads to a substantial accumulation of ROS in cells, which can irreversibly damage membrane lipids, proteins, and other cellular components, potentially resulting in cell death (Chen et al., 2014; Luo et al., 2017). To counteract ROS-induced damage, plants typically enhance antioxidant enzyme activity through transcriptional and metabolic regulations (Acosta-Moto et al., 2017; Jaspers and Kangasjarvi, 2010; Miller et al., 2010; Pan et al., 2023). H2O2 is a prominent form of ROS (Sun et al., 2015). Recent studies suggest that CIPKs play a critical role in regulating ROS homeostasis during salt stress. For instance, TaCIPK29 boosts catalase (CAT) and POD activities, thereby promoting ROS scavenging in wheat (Triticum aestivum; Deng et al., 2013a). TaCIPK14 enhances CAT activity to mitigate H2O2 levels under salt stress (Deng et al., 2013b). Additionally, GmCIPK21 stimulates the expression of antioxidant-related genes GmPOD10 and GmCAT1, thereby increasing antioxidant enzyme activity to reduce salt-induced ROS accumulation (Li et al., 2022). Luo et al., (2017) demonstrated that overexpression of BdCIPK31 enhanced activities of CAT, POD, and superoxide dismutase (SOD), thereby alleviating salt stress-induced damage in plants. Similarly, TaCIPK24 has been shown to improve the activity of antioxidant enzymes such as SOD, CAT, and POD, promoting the clearance of H2O2 accumulation (Sun et al., 2015). Overexpression of GhCIPK6a also elevates SOD and POD activities in transgenic plants, promoting the scavenging of salt-induced H2O2 (Su et al., 2020). In the present study, overexpression of SpCIPK2 reduced the detrimental effects of salt stress on transgenic Arabidopsis growth, as indicated by increased fresh weight under saline conditions (Figure 4). This outcome likely results from diminished cellular damage and enhanced ROS clearance in the transgenic plants (Figure 5B). Enhanced POD activity in transgenic Arabidopsis surpassed that of WT plants (Figure 5D), reflecting heightened antioxidant enzyme activities in response to salt stress. The overexpression of SpCIPK2 also upregulated antioxidant-related genes, including AtP5CS1, AtSOD1, and AtPOD in transgenic lines (Figure 8). It is proposed that SpCIPK2 expression in transgenic Arabidopsis activates the antioxidant enzyme system under salt stress, promoting the transcription of related genes. Relative conductivity, an indicator of cell membrane permeability and damage under stress (Xu et al., 2021), significantly decreased in transgenic plants compared to WT plants (Figure 5C). This observation suggests that transgenic lines possess a robust ROS scavenging capacity, minimizing membrane damage and preserving cell membrane integrity (Figure 9).
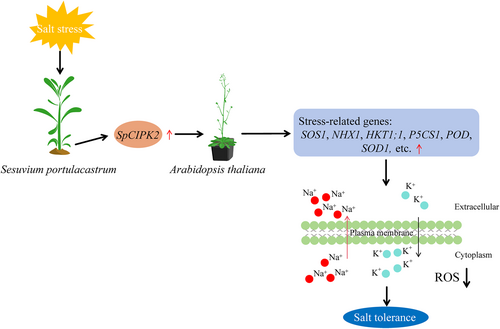
K+ is an essential macronutrient for plant growth and development, significantly influencing physiological processes such as enzymatic reactions and osmotic adjustments (Chen et al., 2017; Li et al., 2021). Salt stress results in elevated Na+ levels in plant cells, impairing K+ uptake, disrupting the Na+/K+ balance, and causing metabolic disorders (Chen et al., 2017; Li et al., 2021). Research indicates that CIPKs play a vital role in regulating Na+/K+ balance in plants. For example, NtCIPK9 activates the expression of HKT1, AKT2, NHX1, and NHX7 genes in transgenic Nicotiana tabacum, which reduce intracellular Na+ accumulation, increase K+ content, and enhance salt tolerance (Lu et al., 2020). Overexpression of TaCIPK29 elevates the expression of SOS1, NHX2, and NHX4 in transgenic plants, improving the cell K+/Na+ ratio and mitigating salt stress-induced cellular damage (Deng et al., 2013a). Likewise, overexpression of ZmCIPK21 in A. thaliana resulted in reduced Na+ accumulation and higher K+ retention, thus improving salt tolerance (Chen et al., 2014). NHX genes encode Na+/H+ antiporter proteins that enhance salt tolerance by either excluding intracellular Na+ or compartmentalizing it into the vacuole (Yang et al., 2017). In transgenic chrysanthemum overexpressing CmCIPK8, Na+ accumulation and K+ leakage were diminished compared to WT plants, leading to elevated K+/Na+ ratios in the transgenic lines (Ding et al., 2023). In this study, transgenic Arabidopsis overexpressing SpCIPK2 demonstrated a reduced net K+ efflux and increased Na+ efflux in roots under salt stress (Figure 6), thus maintaining a higher K+/Na+ ratio in plant cells (Figure 7). Additionally, the expression of genes associated with Na+ and K+ transport, including AtSOS1, AtSOS2, AtSOS3, AtSCABP8, AtHKT1;1, and AtNHX1, was significantly elevated in transgenic A. thaliana compared to WT (Figure 8). These results suggest that SpCIPK2 plays a regulatory role in Na+/K+ homeostasis, mitigates ion toxicity, and enhances salt tolerance in transgenic A. thaliana (Figure 9).
In conclusion, SpCIPK2 from the halophyte S. portulacastrum was upregulated in response to salinity treatment, enhancing salt tolerance in transgenic Arabidopsis plants. This protein positively regulates salt tolerance by preserving the K+/Na+ ratio under salt stress through the induction of AtSOS1, AtSOS2, AtSOS3, AtSCaBP8, AtHKT1;1, and AtNHX1 expressions. Furthermore, SpCIPK2 diminished ROS accumulation in transgenic Arabidopsis leaves by activating the antioxidant defense system.
AUTHOR CONTRIBUTIONS
Y.Z. and Z.S. conceived and designed the experiments. Y.L. and Y.H. performed the experiments. All data were analyzed and interpreted by Y.L., Y.H., W.L., H.X., and Y.L., the manuscript was written by Y.Z. All authors read and approved the manuscript.
ACKNOWLEDGEMENTS
We thank the engineer Longfei Zheng (Bapu (Shanghai) Information Sci. &Tech. Co., Ltd.) for Non-invasive Micro-test. We also thank the reviewers and editors for helpful comments on earlier drafts of the manuscript. This study was supported by Hainan Provincial Natural Science Foundation of China (318QN189), the Education Department of Hainan Province (Hnky2021-19), and Horizontal Project of Hainan University (HD-KYH-2023093).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article and its supplementary information files.




