Iron metabolism acts as a bridge between photosynthesis and red coloration of bud galls induced on Nothofagus obliqua (Nothofagaceae)
Abstract
Color and morphology are some of the most intriguing traits of plant galls, whose patterns resemble fruits and flowers. Many hypotheses were proposed to explain the involvement of anthocyanin accumulation with the development of red gall hues, whose mechanisms seem idiosyncratic. Anthocyanins are related to photoprotective strategies in green tissues and metal accumulation in some flowers. Despite that, the combination of such physiological phenomena has been neglected for galls, which are photosynthetic neoplasms genetically similar to reproductive organs. Here, we integrated different perspectives by measuring photosynthetic pigment and anthocyanin concentration combined with fluorescence quenching analysis, antioxidant activity assays, and histochemical elemental mapping in red and green galls induced by Espinosa nothofagi (Hymenoptera) on Nothofagus obliqua (Nothofagaceae). We found no relationship between high anthocyanin concentrations, light exposure, and red coloration in galls as anthocyanin concentrations were higher in the outermost tissues of green galls than in red galls. Red galls presented higher concentrations of total chlorophyll and lower carotenoid concentrations than green galls and leaves, which correlated with their highest photosynthetic activity and iron accumulation. The red color coincides with the accumulation of aluminum and Fe3+ and the lowest antioxidant capacity in the gall outer tissue. The high antioxidant capacity of N. obliqua galls and the Fe2+ and Fe3+ distribution are related to high photosynthesis, Fe-use efficiency in galls, and the supply of Fe to the inducer diet. Overall, iron metabolism connects the high photosynthesis activity to the red gall color in the presence of low anthocyanin concentrations, like some flowers.
1 INTRODUCTION
Color changes in plant galls, especially from green to red hues, have been observed and are related to the modulation of pigment contents, especially anthocyanins, in different systems (Lev-Yadun, 2016; Guedes et al., 2023). Various hypotheses have been proposed to explain anthocyanin accumulation and the red color of galls. The high cytokinin contents in gall tissues associated with sugar metabolism, as well as the defensive role of anthocyanins, are the most discussed in the literature (Connor et al., 2012; Lev-Yadun and Inbar, 2023). Besides, other studies suggest that gall color can be determined by inducers' genetic factors (Korgaonkar et al., 2021), the influence of host intraspecific chemical variation (Cardoso et al., 2023), or environmental factors, such as light exposure (Bomfim et al., 2019). The evolution of red coloration in land plants has occurred numerous times in response to photosynthetically active and UVB radiations, and among all red pigments, anthocyanins are the most common (Davies et al., 2022). Anthocyanin biosynthesis is modulated by the cross-talk of multiple factors in vegetative and reproductive organs, including hormonal signals, plant age, nitrogen contents, oxidative stress, light, and temperature (LaFountain and Yuan, 2021). For flowers and fruits, color expression also depends on the combined accumulation of anthocyanins with carotenoids, chlorophyll, and phenolic compounds (Yuan et al., 2013; Kalisz et al., 2023). Although less emphasized, flower color modulation has also been historically associated with the complexation of anthocyanin molecules with metal ions, such as aluminum and iron (Chenery, 1946, 1948; Momonoi et al., 2009; Houghton et al., 2021; Bahreini et al., 2024). Both metals can form stable complexes with anthocyanins (Bayer et al., 1966), exhibiting not only red but also blue, purple, violet, green, and yellow colors, depending on the pH, chemical structure of the anthocyanin molecule, hydration, combination of color hues, and specific metal ions (Takeda, 2006; Tanaka et al., 2010; Tang and Gusti, 2020; Davies et al., 2022). Such flower color manipulation has been demonstrated with the application of metal salts to pots with Hydrangea (Hydrangeaceae) flowers (Chenery, 1948).
Aluminum can be considered a beneficial element for some plant species but is toxic for most of them (Ma et al., 2022), demoting the generalized involvement of this metal in the color modulation of reproductive organs and galls. For plant galls, the possible involvement of metal ion accumulation interacting with pigments and altering gall color expression has not been previously studied, although metal accumulation has been demonstrated in different plant-gall systems (Bagatto et al., 1991; Arriola et al., 2020, 2024). Iron, for example, is an essential metal cofactor with the ability to form complexes with anthocyanins (Tanaka et al., 2010; Houghton et al., 2021). It is also related to photosynthesis efficiency, being involved in chloroplast development and chlorophyll synthesis (Cakmak et al., 2022). Iron is the most abundant transition metal of the photosynthetic apparatus, acting in all electron transfer complexes, i.e., photosystem (PS) II and I, the cytochrome b6f complex, and ferredoxin (Vigani et al., 2019). Therefore, chloroplasts are the Fe-richest cellular compartment and the main source of reactive oxygen species (ROS) production (Sági-Kazár et al., 2022) because of the relative ease of change in the Fe oxidation state, which produces superoxide anion radicals (O2•−) and hydroxyl radicals (OH•) (Cakmak et al., 2022). During the developmental processes and cytological events of gall formation, the coordination of ROS accumulation in both the apoplast and symplast requires a high redox potential of gall tissues for the maintenance of photosynthetic rates and the avoidance of hypersensitive reactions (Isaias et al., 2015). The complex coordination of Fe homeostasis and photosynthesis efficiency is dependent on cross-talk with other minerals and the plant's antioxidant capacity (Therby-Vale et al., 2022). As an essential enzymatic cofactor, Fe can act in ROS scavenging as Fe-superoxide dismutase (Alscher et al., 2002). Additionally, the dynamic changes in apoplastic-Fe deserve special attention, as it may function as a storage pool and take part in stress signaling, mainly under nutritional deficiencies (Liu et al., 2023). Overall, the multiple functions of Fe and its importance for many physiological processes in plants highlight the existence of potential bridges between the Fe-use efficiency and photosynthetic performance of host plants toward gall inducer nutrition, as proposed by Arriola et al. (2024).
Although gall formation has variable effects on photosynthesis performance when induced on leaves (Samsone et al., 2012; Oliveira et al., 2017), the results for galls induced in buds have aroused interest in the significance of photosynthesis in the context of the adaptive value of galls for gall inducers (Dorchin et al., 2006; Haiden et al., 2012). The clavate galls induced in the axillary buds of Nothofagus obliqua (Mirb.) Ørsted (Nothofagaceae), by the hymenopteran Espinosa nothofagi Gahan, 1947 (Hymenoptera: Pteromalidae), naturally presents green and red colors and are covered by cone-shaped emergences (i.e., leaf-like emergences). Regardless of color, the galls on N. obliqua have three tissue compartments: the outer compartment (OC), which is composed of a uniseriate epidermis and chlorenchyma; the median compartment (MC), which is composed of the water-storing parenchyma; and the inner compartment (IC), which is delimited by the sclerenchyma layers with interspersed vascular bundles and 2–3 layers of common storage tissue bordering the nutritive cells located around the larval chamber (Aguilera et al., 2022). In addition to exhibiting color differences, these galls have high antioxidant potential (Guedes et al., 2022), which herein we propose to be connected with Fe accumulation, gall color expression (mediated by anthocyanins), and photosynthetic efficiency. Currently, we ask if (i) red galls have higher anthocyanin concentrations than green galls and leaves do; (ii) there is a difference in photosynthetic performance depending on the gall color and tissue compartment; (iii) there is a difference in antioxidant potential between red and green galls; and (iv) does iron accumulation differ between the green and red gall tissue compartments?
2 MATERIALS AND METHODS
2.1 Study site and plant material
The population of Nothofagus obliqua is located on the Concepción campus, Universidad de Concepción (UdeC, Spanish acronym), Chile (36°49′38”S 73°02′06”W, 40 m.a.s.l.). A voucher specimen was deposited in the CONC Herbarium (Department of Botany, UdeC) under accession number 190234. Five different trees (n = 5) were sampled during the gall development peak in September 2022. For each tree, seven branches per tree (n = 35 branches) exposed to the sun were collected, and UV-B and photosynthetically active radiation (PAR) were measured at this level. UV-B radiation was measured with a UV light meter (CCE-UV 34, PCE Instrument), and PAR was measured with a LI-COR quantum PAR sensor (LI-190SA, LI-COR). Both red and green galls were sampled under the UVB value of 1.15 Mw cm−2, and the PAR value of approximately 300 μmol m−2 s−1, which was measured on a spring day between 11 am and 12 pm.
2.2 Photosynthetic pigment and anthocyanin concentrations
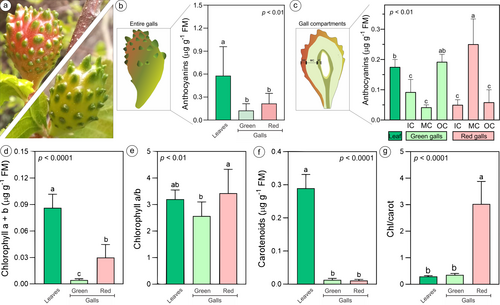
Fresh disks of 10 mm in diameter from the leaves (n = 10) and entire green and red galls (Figure 1a; n = 10 for each color) of three individuals were weighed, macerated in semi-dark using 80% acetone (v:v) for the extraction of photosynthetic pigments, and centrifuged at 825 x g (RCF). The supernatant of the extracts was analyzed with a spectrophotometer (ELX800, Bio-Tek) at 470, 663, and 646 nm. The concentrations of chlorophyll and carotenoids were calculated following the equations proposed by Lichtenthaler and Wellburn (1983) and expressed as μg g−1 of fresh weight. For the first anthocyanin quantification, fresh leaves and entire red and green bud galls (Figure 1b; n = 8 for leaves and each gall color) were collected from three different N. obliqua trees, weighed, and immediately ground in a mortar with liquid nitrogen. The anthocyanins were extracted with 80% acidified methanol (HCl 0.5 N) (1/10 p/v) in an ultrasonic bath (Elma) for 5 min and centrifuged at 3,500 rpm at 4°C for 5 min. The total anthocyanin concentration was determined via the differential pH method described by Giusti and Wrolstad (2001). For this purpose, a supernatant fraction of the samples was mixed with a potassium chloride buffer solution (pH 1.0), and another fraction was mixed with a sodium acetate buffer (pH 4.5). The samples were left in the dark for 15 min, and the absorbance was read at 700 nm against a blank of distilled water. The data were measured with a spectrophotometer (ELX800, Bio-Tek), and the results were expressed as mg of cyanidin-3-glucoside per g of fresh weight. Afterward, the measurements for anthocyanin concentrations were redone using frozen material (−23°C) collected during the same period. This time, the red and green galls (n = 5 per color) were dissected with a scalpel (Guedes et al., 2022) to separate the three tissue compartments according to Aguilera et al. (2022): the outer compartment (OC) (epidermis and chlorenchyma), median compartment (MC) (water-storing parenchyma), and inner compartment (IC) (sclerenchyma, vascular bundles, common storage, and nutritive tissues) (Figure 1c). These compartments were compared with those of frozen non-galled leaves (n = 5). The anthocyanin extraction and quantification were performed following the protocol described above.
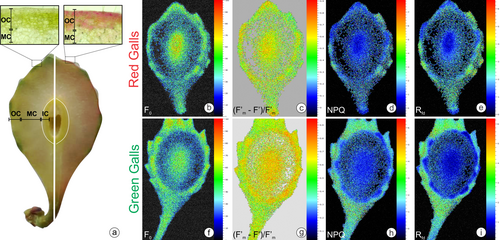
2.3 Photochemical parameters: fluorescence quenching analysis
Fluorescence quenching analysis was performed on leaves and green and red galls (n = 5 for each treatment) using a modulated fluorescence imaging apparatus, Handy Fluorcam PSI (Photo Systems Instrument). The equipment operates with weakly modulated measuring light in combination with saturating light (up to 4,900 μmol m−2 s−1) and actinic light (approximately 1,400 μmol m−2 s−1). This study used the quenching protocol with saturating light at 1,015 μmol m−2 s−1 and actinic light at 350 μmol m−2 s−1. The fluorescence parameters were measured on the surface of the whole green and red galls (not sectioned) at two different sites: leaf-like emergences and the gall surface. For the measure of the photosynthetic yield in the innermost cell layers, the galls were opened longitudinally with a scalpel, and the fluorescence parameters were also measured. The maintenance of the integrity of the electron chains and the capacity of non-galled and galled tissues for photosynthesis were demonstrated after a dark-adapted time (30 min) and exposure to various light treatments following the software protocol Quenching (Photo Systems Instruments, Version 2). The following parameters were measured in this study: F0 (minimum fluorescence of PSII in the dark-adapted state); Fm (maximum fluorescence of PSII in the dark-adapted state); Fv (variable fluorescence in the dark-adapted state); Fp (peak of fluorescence during the initial phase of the Kautsky effect); Fv/Fm (maximum PSII quantum yield in the dark-adapted state, where Fv = Fm–F0); (F'm–F′)/F'm (PSII operating efficiency, where F'm is the fluorescence signal when all PSII centers are closed in the light-adapted state and F′ is the measurement of the light-adapted fluorescence signal); qP (coefficient of photochemical quenching in the steady state); Rfd (instantaneous fluorescence decline ratio in light); and NPQ (steady-state non-photochemical quenching) (Genty et al., 1989; Oxborough, 2004).
2.4 Antioxidant activity
Non-galled leaves and red and green galls were collected from five different trees (n = 25 galls and leaves). For this assay, the three tissue compartments were considered again. The leaves and each separate gall compartment were dried in an oven (Venticell, Spain) at 40°C for six days. The dried tissues were pulverized with a mortar and macerated in 100% methanol (0.5 g of plant material in 5 mL of methanol) (Guedes et al., 2022). The samples were sonicated in an ultrasonic bath (Elma) for 5 min and stored in the dark at 4°C for 24 h. The extraction was repeated twice, and the extracts were combined, filtered, and concentrated under reduced pressure in a rotary evaporator (LabTech).
The antioxidant activity of each sample extract was screened by the DPPH [2,2-diphenyl-1-picrylhydrazyl] (Singh et al., 2016) and ABTS [2,2′-azino-bis3-ethylbenzothiazoline-6-sulfonic acid] (Re et al., 1999) assays with 96-well microtiter plate methods. A Trolox solution [6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid] at a concentration of 10 μg ml−1 was used as a positive control. The determination of the antioxidant activity of methanol extracts of the OC, MC, and IC of the galls and non-galled leaves was performed in triplicate. For the DPPH assay, an aliquot (20 μL) of the extracts was mixed with 180 μL of DPPH solution. After 30 min of incubation at room temperature, the absorbance was measured at 515 nm. The DPPH radical without extracts and Trolox solution was used as a control. For the ABTS assay, the radical was dissolved in ultrapure water at a 3.5 mM concentration. The ABTS radical cation (ABTS•+) was produced by reacting the ABTS stock solution with 1.225 mM potassium persulfate (K2S2O8) in the dark at room temperature for 16 h before use. ABTS•+ was diluted with ultrapure water to obtain an absorbance of 0.70 (± 0.2) nm at 750 nm. Then, an aliquot (20 μL) of the extracts at a concentration of 10 μg ml−1 was mixed with 180 μL of ABTS•+ solution. After 30 min of incubation at room temperature, the absorbance was measured at 750 nm. ABTS•+ without extracts and Trolox solution was used as a control. The antioxidant activity (ABTS and DPPH) was measured in triplicate in a spectrophotometer (ELX800, Bio-Tek).
The capacity to scavenge DPPH+ and ABTS•+ radicals was calculated and expressed as percentage inhibition using the following equation: % inhibition of radical: (Ac- As)/Ac × 100, where Ac is the control absorbance and As is the sample absorbance.
2.5 Semi-quantitative determination of metal accumulation
Fragments (n = 5) of N. obliqua non-galled leaves and stems and hemisections of green and red galls were fixed with buffered glutaraldehyde (pH 7.2) for 48 h (Moss and Rubio-Huertos, 1966). Following, the samples were subsequently dehydrated in ethanol, embedded in Paraplast Plus® (Sigma–Aldrich), and sectioned (14–16 μm) (Johansen, 1940) with a rotary microtome (Leica Jung Biocut). The slides were deparaffinized with butyl acetate at 40°C and subjected to histolocalization and semi-quantification of Fe and Al.
Adapted Prussian blue tests were used for the histolocalization of ferrous (Fe2+) and ferric iron (Fe3+). The hydrated slides containing sections of non-galled leaves and stems and red and green galls were deposited in clean staining jars filled with a 1:1 solution of 4% [v/v] HCl and 4% [w/v] potassium ferricyanide (K3[Fe(CN)6]) for Fe2+ detection. For Fe3+, the potassium ferricyanide was substituted by potassium ferrocyanide (K4[Fe(CN)6]) (Suvarna et al., 2018). The staining jars were stored and protected from light in a chapel for 48 hours. Afterward, the solutions were discarded, and the slides were washed with deionized water and mounted with glycerinated gelatin (Johansen, 1940). Positive reactions are demonstrated by dark Turnbull's blue for Fe2+ and dark Prussian blue for Fe3+ (Suvarna et al., 2018). The entire slide sections were photographed with a Leica S9i stereomicroscope, and the slide details were recorded with a Leica DM2500 light microscope with an ICC50 HP coupled camera (Leica). The intensity of the blue color on brightfield images (40x magnification) for both reactions (Fe2+ and Fe3+) was measured by submitting the images to the color deconvolution method (Ruifrok and Johnston, 2001). After deconvolution, the blue channels of the 8-bit images (n = 5) were measured in quintuplicate areas (150 × 150 pixels) for each group (stems = outer cortex; leaves = palisade parenchyma; galls = outer, median, and inner compartments). In brightfield images, positive results were expressed as values below 255 Gy (Gy = gray value).
Morin reagent [2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one] was used for the determination of the presence of Al in plant tissues (Vitorello and Haug, 1997). The deparaffinized slides were immersed in 95% ethanol for 10 min, stained with 0.2% morin in 0.5% acetic acid and 85% ethanol for 30 min, washed with 95% ethanol, mounted in distilled water (Vitorello and Haug, 1997), and analysed in an epifluorescence microscope (Leica DM2500 Led) with a FITC filter (450–490 nm excitation light). Slides not subjected to morin and mounted with distilled water were analysed as controls. Positive reactions are indicated by green fluorescence. For fluorescence images, the intensities of the morin reaction were evaluated by the gray scale methodology (Gy) (Martini et al., 2021) after the images were converted to 8-bit images. In the fluorescence images, positive results were expressed as values above 0 Gy. The pre-treatment of images, measures of color intensity, and gray values were performed with ImageJ (Fiji version) (Schindelin et al., 2012).
2.6 Statistics
All the data were previously evaluated using the Shapiro–Wilk test for variance normality and the Levene test for homogeneity. Parametric data (chlorophyll a/b ratio) were submitted to One-way ANOVA following the Tukey test, and non-parametric data (chlorophyll and carotenoid concentrations, chlorophyll/carotenoid ratio, anthocyanin concentration, antioxidant activity, and photochemical parameters) were submitted to Kruskal-Wallis test, both of which consider differences at 5% significance. The results for gray values of Fe2+, Fe3+, and Al histochemistry were compared against non-galled leaf results through the Kruskal-Wallis test followed by Dunn's test. The ratio of Fe2+/Fe3+ for the maximum and minimum mean intensities was calculated as indicative of the tissue Fe-reduction capacity. Statistical analysis was performed on JMP 5.0 Software (Sas Institute Inc.) and the InfoStat program (di Rienzo et al., 2020), and graphs were constructed using GraphPad Prism software (version 7.0, San Diego).
3 RESULTS
3.1 Pigment concentrations
The red and green galls (Figure 1a) had a lower concentration of pigments than non-galled leaves (Figure 1b, d-f). The non-galled leaves had significantly higher anthocyanin concentrations than entire galls, but there were no differences between red and green galls (Figure 1b). However, when considering the gall tissue compartments, the higher anthocyanin concentrations were detected in the MC of red galls and the OC of the green galls, which were statistically similar (Figure 1c). The anthocyanin concentrations of the OC from the green galls were similar to those of the non-galled leaves but were higher than the anthocyanin concentrations of the OC from the red galls (Figure 1c). The anthocyanin concentrations in non-galled leaves and the OC of green galls were statistically similar (Figure 1c).
Compared with red galls, green galls had lower chlorophyll concentrations, with proportionally higher investment in chlorophyll b (low levels of chlorophyll a/b; Figure 1d-e). Considering the carotenoid concentration, green and red galls showed similar values, but red galls showed higher chlorophyll/carotenoid rates (Figure 1f-g), an effect observed probably due to the higher values of chlorophylls a and b.
3.2 Photochemical analysis
There were significant differences in photochemical performance between leaves and green and red galls (Table 1). The F0, Fm, Fv, Fv/Fm, and (Fm′ – F′)/Fm′ were lower in galls than in leaves. However, the NPQ and Rfd decreased in the leaf-like emergences on the green galls. Compared with those on the leaves, the leafy emergences on the red galls had higher values of (Fm′ – F′)/Fm′. The leafy emergences on the green galls showed higher values of Fm, Fv, Fv/Fm, and (Fm′ – F′)/Fm′ than the leafy emergences on the red galls (Figures S1 and S2). Nevertheless, the surfaces of both the green and red galls had inverse values for these last parameters, i.e., decreased in red galls (Table S1).
| Photochemical parameters | Non-galled leaves | Leafy on the green galls | Leafy on the red galls | p-value |
|---|---|---|---|---|
| F0 - minimal fluorescence in the dark-adapted state | 64.13 ± 6.81 | 70.31 ± 9.39 | 61.92 ± 10.99 | 0.390 |
| Fm - maximum fluorescence in the dark-adapted state | 290.27 ± 46.44b | 415.58 ± 47.33a | 326.73 ± 43.97a | 0.007 |
| Fv - variable fluorescence in the dark-adapted state | 226.14 ± 41.84b | 361.62 ± 72.07a | 261.83 ± 33.38b | 0.004 |
| Fv/Fm - maximum PSII quantum yield | 0.77 ± 0.02c | 0.83 ± 0.01a | 0.81 ± 0.01b | 0.007 |
| (Fm′ – F′)/Fm′ - PSII operating efficiency in light-adapted steady state | 0.51 ± 0.06b | 0.65 ± 0.05a | 0.57 ± 0.04b | 0.020 |
| NPQ - steady-state non-photochemical quenching | 2.32 ± 0.41a | 1.57 ± 0.38b | 2.28 ± 0.56ab | 0.040 |
| Rfd - instantaneous fluorescence decline ratio in light | 3.23 ± 0.57a | 2.03 ± 0.43b | 3.04 ± 0.74ab | 0.040 |
When we performed the quenching analysis of the hemisected galls (Figure 2), there were some differences among the compartments analysed. The OC of the green galls showed higher values of (Fm′ – F′)/Fm′ than the OC of the red galls. However, NPQ was greater in the OC of red galls than in that of green galls (Table 2). For the IC, the difference occurred only for (Fm′ – F′)/Fm′, which was higher in red galls (Table 2). In an overall analysis, taking off the effects of coloration on galls, F0, Fm, and Fv, especially NPQ and Rfd, were higher in the OC than in the IC. Unexpectedly, there were no differences between OC and IC compartments for (Fm′ – F′)/Fm′ and Fv/Fm (Table 2).
| Photochemical parameters | OC | p-value | IC | p-value | Overall tissue compartments | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Green Gall | Red Gall | Green Gall | Red Gall | OC | IC | ||||
| F0 - minimal fluorescence in the dark-adapted state | 28.22 ± 3.28 | 25.99 ± 2.96 | 0.29 | 62.53 ± 17.79 | 54.30 ± 7.36 | 0.67 | 27.11 ± 3.17 | 58.42 ± 13.55 | 0.002 |
| Fm - maximum fluorescence in the dark-adapted state | 127.76 ± 24.09 | 98.18 ± 23.75 | 0.21 | 269.06 ± 104.30 | 224.32 ± 47.83 | 0.53 | 112.97 ± 27.41 | 246.70 ± 80.05 | 0.003 |
| Fv - variable fluorescence in the dark-adapted state | 99.54 ± 21.62 | 72.19 ± 21.13 | 0.21 | 210.56 ± 81.74 | 170.12 ± 41.57 | 0.53 | 85.86 ± 24.78 | 190.28 ± 64.76 | 0.004 |
| Fv/Fm - maximum PSII quantum yield | 0.77 ± 0.02 | 0.72 ± 0.04 | 0.11 | 0.78 ± 0.01 | 0.75 ± 0.02 | 0.23 | 0.76 ± 0.02 | 0.75 ± 0.04 | 0.30 |
| (Fm′ – F′)/Fm′ - PSII operating efficiency in light-adapted steady state | 0.67 ± 0.04 | 0.58 ± 0.06 | 0.03 | 0.70 ± 0.02 | 0.63 ± 0.06 | 0.03 | 0.62 ± 0.07 | 0.67 ± 0.05 | 0.08 |
| NPQ - steady-state non-photochemical quenching | 0.67 ± 0.14 | 0.91 ± 0.14 | 0.02 | 0.47 ± 0.12 | 0.72 ± 0.27 | 0.14 | 0.79 ± 0.18 | 0.60 ± 0.24 | 0.03 |
| Rfd - instantaneous fluorescence decline ratio in light | 1.06 ± 0.18 | 1.32 ± 0.18 | 0.06 | 0.68 ± 0.23 | 0.98 ± 0.40 | 0.21 | 1.19 ± 0.22 | 1.83 ± 0.34 | 0.01 |
3.3 Metal histolocalization in galls induced on N. obliqua
Both Fe2+ and Fe3+ were detected in the non-galled stems and leaves of N. obliqua (Figure S3). In stems and leaves, both Fe forms were detected in vacuoles and cell walls, but in leaves, they were usually associated with chloroplasts. The comparison of the mean color intensities between non-galled organs revealed differences only for Fe3+ (Table 3). The ratios of Fe2+/Fe3+ were higher for the leaves than for the stems. For red and green galls, both forms of Fe were detected in all gall compartments (Figure 3a, b), with higher intensities in the IC, especially in the vacuoles of nutritive cells and sclerenchyma (Figure 3c-f). However, the color intensities for Fe3+ in the IC of red galls were lower than those for green galls, diminishing the Fe2+/Fe3+ ratio. The MC of both red and green galls presented similar color intensities for Fe, lower overall Fe detection, and higher Fe ratios. In the MC, Fe is usually associated with cell walls. In the OC, the color intensities for Fe3+ were similar for both red and green galls but different for Fe2+, with higher values for the green galls (Figure 3g-j; Table 3). The Fe2+ was intensely detected in the vacuoles and chloroplasts in the chlorenchyma layers, especially in the leaf-like emergences of the green galls. The cell vacuoles and walls also showed positive reactions for both Fe forms in the epidermis, adjacent chlorenchyma, and vascular bundles of red and green galls. The MC of both red and green galls had similar color intensities for Fe, lower overall Fe detection, and higher Fe ratios.
| Organs | Fe2+ | Fe3+ | Fe2+ / Fe3+ | Al | |
|---|---|---|---|---|---|
| Stems | 135.9 ± 18.76c | 218.7 ± 8.48c | 0.56–0.68 | 3.75 ± 0.52d | |
| Leaves | 139.6 ± 23.36c | 109.2 ± 32.31ab | 1.15–1.51 | 8.60 ± 2.60d | |
| Green Gall | IC | 56.23 ± 14.82a | 94.47 ± 22.21a | 0.57–0.61 | 35.48 ± 9.95a |
| MC | 164.1 ± 18.06c | 195.4 ± 9.07b | 0.78–0.89 | 1.76 ± 0.75d | |
| OC | 84.66 ± 10.17b | 125 ± 29.15ab | 0.61–0.77 | 7.09 ± 2.30d | |
| Red Gall | IC | 62.29 ± 15.83ab | 160.3 ± 28.33b | 0.35–0.41 | 20.47 ± 8.64b |
| MC | 157.4 ± 21.40c | 174 ± 12.74b | 0.73–0.96 | 5.29 ± 3.66cd | |
| OC | 110 ± 22.71c | 127.3 ± 17.18ab | 0.79–0.92 | 18.04 ± 2.78b | |
| Overall | (p-value) | < 0.001 | < 0.001 | < 0.001 | |
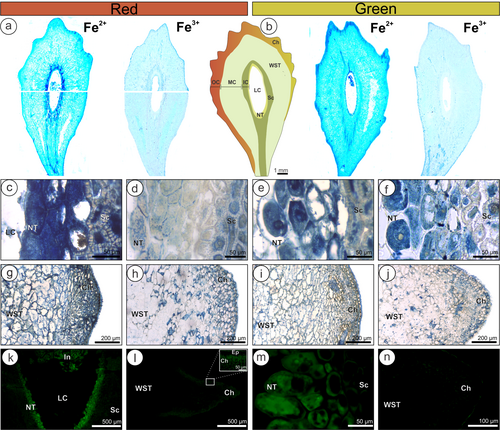
Aluminum was detected mainly in the IC and OC of gall tissues, with very low mean gray values for non-galled organs and the MC of galls (Figure S3; Table 3). The vacuoles of the nutritive cells of both galls had intense labeling for aluminum (Figure 3k and m), which was detected even in the larval body of the galling insect (Figure 3k). The sclerenchyma layers in the IC presented lesser intensities of aluminum labeling. In the OC, aluminum was intensely labeled in the chlorenchyma cells of the leaf-like emergences of red galls associated with chloroplasts (Figure 3l). For green galls, aluminum was also detected in the chlorenchyma (Figure 3n), but with lower intensity than in the red galls (Table 3).
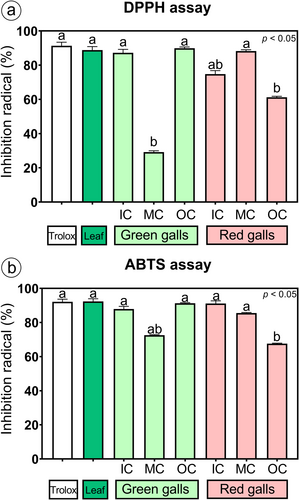
3.4 Antioxidant activity of methanol extracts
The methanol extracts of the N. obliqua green galls had a high capacity to scavenge the DPPH and ABTS radicals, which was statistically similar to that of the positive control (Trolox) and leaves (Figure 4), except for the MC extract, which was two times lower than that of the control and the leaves for the DPPH radical (Figure 4a). Compared with those of the control (Trolox), leaves, and green galls, the methanol extracts of the IC and OC of the red galls had a lower antioxidant capacity to eliminate DPPH and ABTS radicals; however, the ABTS radical activity of the IC extract did not significantly differ from that of the other treatments (Figure 4b). The MC extract of red galls had a high antioxidant capacity, which was not significantly different from that of the control and leaves for the DPPH and ABTS radicals (Figure 4a-b).
4 DISCUSSION
The red color in the outermost surface of the bud galls induced on N. obliqua does not relate directly to high anthocyanin concentrations. Nevertheless, entire red galls had higher concentrations of total chlorophyll and lower carotenoid concentrations than green galls and leaves. Although leaves had higher photosynthetic pigment concentrations than galls, unexpectedly, the gall tissues showed higher photochemical yield and red galls had a higher capacity to dissipate heat. The photosynthetic activity of galls plays an important role in regulating oxygen and carbon dioxide metabolism (Pincebourde and Casas, 2016). The red color in galls coincides with the higher accumulation of aluminum in the chlorenchyma of the OC and with the lowest capacity to reduce the Fe3+ to Fe2+, as indicated by the higher Fe2+/Fe3+ ratio if compared with the OC of green galls. Accordingly, the OC of red galls presented a lower antioxidant capacity for both DPPH and ABTS radicals than the green galls. On the other hand, the MC of the red galls presented higher antioxidant capacity and higher anthocyanin concentrations than the MC of the green galls. Despite that, the MC of the red galls does not correspond to the tissues where the red coloration of vacuole concentrations is observed. These results indicate that the red color of galls may be influenced by an intricate combination of differences in metal accumulation and tissue acidity, as reported for flowers (Chenery, 1948; Houghton et al., 2021; Kalisz et al., 2023; Schreiber et al., 2011), and an imbalance in the ratio of photosynthetic pigments induced by E. nothofagi.
4.1 The anthocyanin concentration does not determine the red color in galls but impacts some photosynthetic parameters
The conspicuous red coloration in galls is commonly related to anthocyanin accumulation (Lev-Yadun, 2016), which in green tissues can be masked by chlorophyll pigments, as observed in the pericarp of litchi fruits (Wang et al., 2002, 2005), and corroborated by the results for the OC of green galls. Overall, there is a decrease in pigment concentrations in galls compared with non-galled tissues (Dias et al., 2013; Carneiro et al., 2014). However, anthocyanin accumulation can occur at high concentrations in galls in different ways, such as through interactions with cytokinin and sugar metabolism, light exposure, the accumulation of defensive substances, or genetic interactions (Connor et al., 2012; Bomfim et al., 2019; Korgaonkar et al., 2021; Lev-Yadun and Inbar, 2023; Guedes et al., 2023, 2024). Both chlorophyll pigments and anthocyanins were lower in entire bud galls induced on N. obliqua compared with leaves. Also, there is no difference between the concentrations of carotenoids and anthocyanins of red and green galls. However, the anthocyanin concentrations observed for the MC of red galls were higher than those observed in the MC of green galls and for the leaves, but they were similar to the OC of green galls. Therefore, given that our field observations do not indicate differences in light exposure in galls, the red coloration as a mechanism of photoprotection seems to be questionable in our case, which is corroborated by the discrepancies in anthocyanin concentrations in the OC of the galls studied herein, reinforcing the idiosyncratic pathway proposed by Bomfim et al. (2019) for the formation of red colors in galls.
Despite the idiosyncratic pathway, the red coloration associated with anthocyanins seems to impact some photosynthetic parameters. In this study, the photosynthetic performance significantly differed between leaves and green and red galls. The Fv/Fm and (Fm′ – F′)/Fm′, for example, were higher in green galls than in red galls and leaves. Although lower values of both parameters are usually reported as indicators of PSII decreased operating efficiency in galls (Oliveira et al., 2017; Rezende et al., 2018; Martini et al., 2020), the bud galls on N. obliqua showed higher values for these parameters than leaves. The comparison of the photosynthetic performance parameters between the tissue compartments of both galls revealed similar results. The OC and IC of the green galls had significantly higher values of (Fm′ – F′)/Fm′ than the red galls; however, there were no differences for the Fv/Fm. Concomitantly, in the red galls, the values of NPQ and Rfd increased, indicating a mechanism of energy dissipation, which is lost as heat, guaranteeing greater vitality of the tissues, as indicated by higher Rfd values.
The lower photosynthetic performance of red galls compared to green galls induced by Cecidomyiidae (Diptera) on Qualea parviflora Mart. (Vochysiaceae) was related to the presence of anthocyanins as a result of greater exposure to sunlight (Bomfim et al., 2019). Herein, the differences in photosynthesis performance and color observed in the OC compartment between red and green galls may be related to the higher Fe-use efficiency in green galls and, consequently, higher Fe2+ availability for the photosynthetic apparatus and chloroplast synthesis (Vigani et al., 2019). In red galls, the Fe ions are not efficiently used by the photosynthetic tissues and the surplus Fe3+ may react with the available anthocyanin molecules, forming the red coloration, as observed in some flowers and fruits (Ratanapoompinyo et al., 2017; Houghton et al., 2021; Bahreini et al., 2024). From a general perspective, the elevated antioxidant capacity of N. obliqua green galls (Guedes et al., 2022) favors the maintenance of the balance of ROS in the higher presence of Fe ions. The differences in the antioxidant activity among the gall tissue compartments, as demonstrated herein, explain the differences in the Fe abundance patterns among the gall tissues and promote high Fe2+ concentrations in nutritive tissues, as predicted by Arriola et al. (2020, 2024). Additionally, the high antioxidant activity of the MC from red galls is associated with higher anthocyanin concentrations, corroborating the significant role of this pigment as an antioxidant (Sharma et al., 2024).
4.2 The bridge between photosynthesis and Fe-use efficiency in galls
The photosynthetic pigment concentrations of galls induced on N. obliqua follow the patterns found for other gall systems, with lower concentrations of chlorophylls and carotenoids than non-galled organs (Gailite et al., 2005; Kmieć et al., 2018; Martini et al., 2020; Guedes et al., 2023). Although photosynthesis deficiency is common in many galls induced on leaves in the Tropical Region (Rezende et al., 2018; Bomfim et al., 2019; Kuster et al., 2022), it is not a rule, as demonstrated by the variable effects on photosynthesis for leaf galls from the Temperate Region (Samsone et al., 2012). On the other hand, galls induced on buds have attracted attention as structures with a high capacity to maintain or increase photosynthetic activity (Fay et al., 1993; Dorchin et al., 2006; Haiden et al., 2012), which is herein corroborated. This improvement in the photosynthesis of bud galls is usually related to the avoidance of hypercarbia and hypoxia in closed gall tissues (Pincebourde and Casas, 2016) and is also an advantage in relation to the competition for photosynthates with multiple sinks (Larson and Whitham, 1997; Dorchin et al., 2006).
The increase in photosynthesis of N. obliqua galls is guaranteed by leaf-like emergences on the gall surface. The tissues of these structures, mainly chlorenchyma, concentrate higher amounts of Fe, as well as the nutritive tissues, which also had elevated photochemical yields. Both tissues are characterized by the presence of chloroplasts, which constitute 90% of the total Fe amount in the plant body (Vigani et al., 2019). Chloroplasts are usually less abundant in nutritive tissues than in the OC (Castro et al., 2012; Huang et al., 2015); however, the nutritive tissues of N. obliqua galls are rich in chloroplasts, which explains the high values of F0, Fm, and Fv in the IC. The high presence of total Fe and the capacity to control the Fe oxidation state indicate the capacity of the nutritive tissues to maintain the high Fe requirements of the PSI, mainly for the maintenance of the integrity of thylakoid membranes and chlorophyll synthesis (Cakmak et al., 2022). Moreover, the total concentration of chlorophyll in overall gall tissues is lower than that in non-galled leaves, which may be explained by the dilution of the total chlorophyll in the water-storage parenchyma (Martini et al., 2020; Kuster et al., 2022). The MC of N. obliqua galls represents the main tissue compartment in terms of overall volume (Aguilera et al., 2022), and it is the tissue compartment with the lowest number of chloroplasts and, consequently, low Fe concentrations and decreased photosynthetic efficiency. This compartmentalization of the efficiency of photosynthesis activity corroborates the observation of tissue-specific expression of photosynthesis-related genes in galls (Martinson et al., 2022), which are directly controlled by the constant supply of Fe and cross-talk with other minerals (Therby-Vale et al., 2022). The iron supply in plant tissues and its mobilization inside cell organelles are controlled by many metal transporters, usually those from the IRT, ZIP, and NRAMP families (Castaings et al., 2016; Rodrigues et al., 2023). The transport of vacuolar Fe in seeds and vegetative organs is controlled by NRAMP transporters, which have a divalent affinity (Vigani et al., 2019), requiring the reduction of Fe3+ to Fe2+, whose mobilization between cell compartments is mediated by secondary metabolites (Grillet et al., 2014; Arriola et al., 2020). In addition, some transporters, such as OsNRAMP4 or NRAT1 (NRAMP ALUMINUM TRANSPORTER 1), also recognize aluminum (Al3+), a trivalent metal (Ishida and Corcino, 2022); consequently, the high Fe requirements and mobilization in the gall tissues of N. obliqua could result in incidental aluminum accumulation.
Aluminum accumulation was demonstrated in the most photosynthetically active tissues of the galls, the nutritive tissues in the IC, and the chlorenchyma of the OC. Aluminum is a toxic metal for most plants (Ma et al., 2022), but many species adapted to Al-rich soils can tolerate moderate contents of Al ions in their tissues (Bojórquez-Quintal et al., 2017). The low levels of Al-staining in non-galled organs indicate that N. obliqua does not normally accumulate aluminum, but it is adapted to P-poor and aluminum-iron-rich soils from southern Chile (Ramírez et al., 1997; De Brouwere et al., 2003). Besides, Al stress can influence gall color by affecting pigment concentration and stability, antioxidant activity, and the bridge between Fe homeostasis and photosynthesis (Jiang et al., 2008; Mukhopadyay et al., 2012; Ribeiro et al., 2013; Herburger et al., 2016; Guo et al., 2018; Houghton et al., 2021).
4.3 Gall color and functional metal compartmentalization
As discussed, anthocyanins are plant-derived flavonoids responsible for the huge colors in plant organs, ranging from red to purple and deep blue (Davies et al., 2022). The up-regulation of anthocyanin biosynthesis can occur due to different environmental constraints, such as the elevated UVB radiation in southern Chile (Huovinen et al., 2006), as well as the accumulation of metals. Iron, for example, has been shown to significantly affect anthocyanin production (Sharma et al., 2024). In fact, anthocyanins can attenuate the stress induced on cellular processes (especially photosynthesis) under conditions of mineral imbalance (Landi et al., 2015). The lowest reduction rate of Fe3+ to Fe2+ in the red galls indicates a lower antioxidant capacity and higher use of FeIII-chelates as precursors of chlorophyll synthesis (Vigani et al., 2019), which is corroborated by the high chlorophyll concentrations and low antioxidant capacity of the OC in the red galls. In this scenario, we expect a low pH environment in the tissues of red galls, explaining the higher presence of Al3+ and Fe3+ in the OC, which interact with anthocyanin concentrations to form a red gall color, a process called metallic co-pigmentation (Ratanapoompinyo et al., 2017; Houghton et al., 2021). This is corroborated by the higher concentrations of anthocyanins in the MC of red galls, where the red contents of cell vacuoles are not visible and metallic ions are less frequently detected.
Herein, we did not find any relationship among high anthocyanin concentrations, light exposure, and red coloration in galls, which led us to associate red pigmentation with the formation of metallo-anthocyanins. The ability to form metallo-anthocyanin seems to be the first line of defense against excess UV radiation, especially when the effects of metal toxicity impair chloroplasts (Landi et al., 2015; Herburger et al., 2016), leading to changes in photosynthetic performance in galls. The formation of metallo-anthocyanins can occur with Al3+, Fe2+, and Fe3+, leading, in general, to blue coloration in plant reproductive organs (Takeda, 2006; Momonoi et al., 2009; Tanaka et al., 2010). However, the pH dependence of anthocyanin coloration has long been known and is associated with an acid solution for red hues and an alkaline solution for blue and green hues (Bayer et al., 1966; Tang & Giusti, 2020; Câmara et al., 2022). Succulent cynipid galls, such as those induced by E. nothofagi, can accumulate different organic acids, which turn their tissues into an acidic solution with a pH between 2–3 (Guiguet et al., 2023). Differential tissue acidity has a direct effect on Fe and Al dynamics and pigment stabilization in cell symplast (Herburger et al., 2016; Houghton et al., 2021), so the low pH, low anthocyanins and Al contents combined with high tissue hydration can confer the red coloration to galls. This finding is similar to the observations of reddening of metallo-anthocyanins from Hydrangea petals at pH values between 3 and 5 (Chenery, 1948; Houghton et al., 2021; Schreiber et al., 2011) and herein indicated as a potential explanation for the color shifts in the bud galls induced on N. obliqua. Additionally, beyond the genetic similarities between reproductive organs and plant galls (Schultz et al., 2019), the biochemical phenomena involved in flower color modulation also help understand the nature of conspicuous gall colors.
Besides the effect on gall color and photosynthesis, our results corroborate the high presence of Fe in the tissues of hymenopteran galls, as previously reported for those induced by Diplolepis spinosa Ashmead, 1887 on Rosa rugosa Thunb. (Rosaceae) (Bagatto et al., 1991) and Hemadas nubilipennis Ashmead, 1887 on Vaccinium angustifolium Steud. (Ericaceae) (Bagatto and Shorthouse, 1994). Furthermore, the combination of high antioxidant capacity, photosynthesis efficiency, and potential acidity contributes to high Fe homeostasis and use-efficiency in gall tissues, consequently benefiting the gall inducer by guaranteeing the phosphorous supply, as phospholipids, for the gall inducers (Aguilera et al., 2022; Therby-Vale et al., 2022). The Fe-use efficiency is characterized by the high presence of Fe2+ in the cell walls and vacuoles, which act as storage pools of Fe for both gall colors (Vigani et al., 2019; Liu et al., 2023), as well as by the functional compartmentalization of Fe2+ and Fe3+ between gall compartments, highlighting the mechanisms of Fe delivery for the gall inducer diet (Arriola et al., 2020, 2024). As evidenced by studies in Drosophila melanogaster (Diptera), the enrichment of the insect diet with Fe is necessary for the proper development of the reproductive and immune systems (Cardoso-Jaime et al., 2022). On the other hand, Al toxicity in insects is dose-dependent, and moderate intake of Al increases the male life span and level of locomotor activity in adult stages (Kijak et al., 2014).
5 CONCLUSIONS
The combination of tissue-specific compartmentalization of high antioxidant capacity and Fe2+ and Fe3+ distribution is related to the high photosynthesis efficiency in the bud galls induced by E. nothofagi on N. obliqua. The high photosynthesis efficiency benefits the inducer through the avoidance of hypercarbia inside the galls, as well as by guaranteeing phospholipid supply and improving Fe-use efficiency in gall tissues toward its availability as a food resource in nutritive tissues. Additionally, we corroborate the hypothesis that red gall color is a product of an idiosyncratic pathway, herein explained by the imbalance between pigments and metal contents in the outer compartments of gall tissues, which promotes the formation of metallo-anthocyanins in a similar way as that reported for Hydrangea flowers. Accordingly, our results highlight the complex connection between soils and gall chemistry, which affects its physiology and phenotype.
AUTHOR CONTRIBUTIONS
Í.A.A., L.M.G, A.S.F.P.M, and D.C.O. planned the research, conducted the experiments and statistics, and drew the figures and tables. N.A., R.M.S.I, and D.C.O. obtained the funding and are the project leaders in the respective institutions. All authors participated in the formal analysis and manuscript edition.
ACKNOWLEDGMENTS
We thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support (403159/2023-7), scholarship for Í.A. Arriola (141510/2020-0), and Research Fellowship for D.C. Oliveira (303691/2022-0), A.S.F.P. Moreira (309044/2021-9 and 404855/2021-0) and R.M.S. Isaias (309713/2023-4). We also thank Agencia Nacional de Investigacion y Desarrollo de Chile (ANID) for the support of Project FONDECYT/Postdoctorado for L.M. Guedes (3220169) and a research fellowship for N. Aguilera (11200360). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES/Print) - Finance Code 001 with travel support for the corresponding author.
FUNDING INFORMATION
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Grant numbers: 403159/2023–7, 141510/2020–0, 303691/2022–0, 309044/2021–9, 404855/2021–0, 309713/2023–4. Agencia Nacional de Investigación y Desarrollo (ANID), Grant numbers: 3220169, 11200360.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




