Effect of histone modifications on fruit ripening
Abstract
Histone modifications are canonical epigenetic modifications mediating plant growth and development. Specially, histone modifications play important regulatory roles in plant fruit ripening, directly affecting fruit color changes, soluble sugar accumulation, and fruit softening. In this review, we focus on the effects of histone acetylation and methylation during fruit ripening. In particular, histone acetylation at H3 and H4 accelerates fruit ripening, whereas removal of histone acetylation via histone deacetylases (HDACs) inhibits or delays ripening by regulating the expression of carotenoid and anthocyanin production, glycometabolism, cell wall degradation, ethylene synthesis and signalling, and cell expansin-related genes. In addition, histone methylation is also involved in fruit ripening, in which the emergence of H3K27me3 modifications represses fruit ripening and H3K4me3 modifications promote fruit ripening by affecting multiple ripening-related pathways. However, the relationship between other histone modifications and fruit ripening is currently unclear. Here, we point out that accurate and comprehensive studies concerning the regulatory mechanism of histone modifications in fruit ripening are needed to facilitate the design of high-quality and high-yield fruit.
1 INTRODUCTION
Histone modifications act as gene expression regulators by altering chromatin structure and recruiting gene expression regulatory factors (Chen et al., 2020). Generally, the dynamic changes in chromatin structure regulate DNA accessibility and DNA templating processes, affecting gene transcript abundances in eukaryotes. Nucleosomes, the basic unit of chromatin, consist each of 147 base pairs (bp) of DNA wrapping around a protein octamer, which is assembled from equal parts of the core histone H2A, H2B, H3, and H4 (Lai & Chan, 2024). Modifications are carried out in histone tails by dynamic post-translational modifications (PTMs), including acetylation, methylation, ubiquitination, phosphorylation, etc. (Huang et al., 2020). Currently, it has been found that histone modifications are extensively involved in various aspects of plant growth and development, including seed germination, flower development, female gametophyte development, and embryogenesis. For example, the histone deacetylase (HDAC) AtHDA7 is involved in the development of female and male gametophytes and early embryo formation (Cigliano et al., 2013). Histone modifications are also involved in plant abiotic stress responses. For example, in Arabidopsis thaliana, drought stress led to genome-wide dynamic changes in histone H3K4me1, H3K4me2, and H3K4me3 (Van Dijk et al., 2010). In maize (Zea mays L.), the acetylation levels of histone H3 lysine 9 (H3K9ac) and histone H4 lysine 5 (H4K5ac) were increased in the maize root system under salt stress (Li et al., 2014). In recent years, relevant studies have shown that fruit development and ripening are regulated by epigenetic modifications, including histone modifications (Tang et al., 2020).
Fruit, as unique organs of angiosperms, provide the human body with not only dietary fiber, vitamins and other nutrients but also minerals and antioxidants (Ming et al., 2023). Fruits experience significant physiological transformations during their development, which can generally be categorized into three phases: fruit set, fruit growth, and ripening (Li, Wang, et al., 2022b). To date, there have been many well-documented studies linking fruit ripening to physiological and biochemical processes, which, in different species, include coordinated changes in various hormones, coloring, soluble sugar accumulation, and fruit softening (Hu et al., 2022). The maturation process of fruit is mediated by many plant hormones, such as ethylene, abscisic acid, auxin and gibberellin; ethylene being the most important compound in ripening. Fruits are generally categorized into climacteric and non-climacteric fruits based on different respiration patterns (Brumos, 2021). The appearance of respiratory peak and ethylene burst play a distinct role in climacteric fruit ripening (Ji & Wang, 2023). However, non-climacteric fruits maintain low respiration and ethylene production during the entire ripening process. In addition, fruit ripening is also accompanied by color changes. For example, the dramatic increase in carotenoid and lycopene during ripening in tomato (Solanum lycopersicum) is responsible for the red color of the fruit (Guo, 2022; Guo et al., 2017a). Furthermore, in recent years, there has been significant progress in the understanding of anthocyanin metabolism during fruit ripening. Additionally, sucrose metabolism and fruit softening also influence the process of fruit ripening. Usually, the higher the sugar content, the faster the rate of fruit softening is promoted (Wang, Liu, et al., 2023a). Up to now, it has been shown that histone acetylation and methylation modifications are closely related to fruit ripening. For instance, endogenous ethylene usually adjusts the expression of transcription factors by influencing the corresponding histone acetylation during fruit ripening (Wang et al., 2020; Li, Guo, et al., 2020a). Besides, the accumulation of anthocyanin during fruit ripening is correlated with the enhanced levels of histone deacetylation and demethylation at the corresponding gene sequences (Jia et al., 2023). Meanwhile, sucrose metabolism and softening have also been found to be connected with histone acetylation and methylation. However, the relationship between plant fruit ripening and other types of histone modifications is still unclear, such as histone ubiquitination, histone phosphorylation, and histone SUMOylation.
Recently, the relationship between histone modifications and changes in gene expression during fruit ripening was gradually revealed. Therefore, this review mainly summarized and discussed the recent research progress on the role of histone modifications during ripening in different kinds of fruit, which may provide new insights for future studies concerning histone modifications during fruit ripening.
2 EFFECTS OF HISTONE ACETYLATION ON FRUIT RIPENING
2.1 Histone acetylases and deacetylases
Histone acetylation is an important histone modification commonly associated with enhanced gene activity and transcriptional activation, which is regulated through the action of histone acetyltransferases (HATs) (Guo et al., 2018). The addition of acetyl groups to histone neutralizes their positive charge, leading to relaxation of chromatin structure and thus allowing gene transcription (Wang et al., 2020). In plants, HATs have been distinctly divided into four groups, including GCN5-related N-terminal acetyltransferase (GNAT) superfamily, MOZ, Ybf2/Sas3, Sas2, and Tip60 (MYST) superfamily, CREB-binding protein (p300/CBP) family, and TATA-binding protein-associated factors (TAFII250) family (Li, Liu, et al., 2022c). Meanwhile, different kinds of HATs can catalyze the acetylation on different sites of histone. For example, in A. thaliana, the GNAT family proteins HAG1 and HAG2 catalyze the acetylation of H3K14 and H4K12, respectively. The MYST family members HAM1 and HAM2 redundantly acetylate H4K5. Alternatively, the acetylation of H3K9, H4K8, and H4K16 is generated by the cooperation of multiple HATs (Hu et al., 2019).
In addition, histone deacetylation is also an important epigenetic change that occurs during many plant biological activities. This process is mediated by HDACs (Fu et al., 2019) and involves the removal of acetyl groups from histones, resulting in a tighter chromatin structure and a lower transcriptional level of specific genes (Shen et al., 2015). In plants, HDACs can be divided into three subfamilies: reduced potassium dependency 3/histone deacetylase 1 (RPD3/HDA1), silent information regulator 2 (SIR2), and histone deacetylase 2 (HD2) (Alinsug et al., 2009). It has been found that plant HDACs have multiple functions in flowering, seed maturation, gamete development, and early embryo formation (Cigliano et al., 2013). Recent studies have shown that histone acetylation plays a crucial role in fruit ripening and, in this review, we highlighted the findings on histone acetylation in fruit ripening events.
2.2 The influence of histone acetylation on fruit ripening
Previous studies reported that histone acetylation is involved in many kinds of plants' fruit ripening, including tomato (Guo et al., 2022), pear (Pyrus pyrifolia) (Li, Guo, et al., 2020a), sweet orange (Citrus sinensis) (Xu et al., 2015), and banana (Musa acuminata) (Fan et al., 2016). However, most studies reporting the involvement of histone acetylation focus on climacteric fruits, whereas the regulation of this modification in non-climacteric fruits' ripening is unclear. For example, a gene named SlHAF1, encoding a TAFII250 family HAT, was identified and was expressed in all organs of tomato, with the strongest expression level in roots and fruits (Aiese Cigliano et al., 2013). Besides, overexpression of SlHAF1 promoted the maturation of tomato fruit. Similarly, in sweet orange, the expression of CsHAF1 and CsHAF2 showed an increasing trend during fruit ripening (Xu et al., 2015). Thus, HATs may indeed take part in the process of fruit ripening. Besides, multiple studies have also suggested the link between tomato fruit ripening and histone deacetylases (Table 1). Up to now, histone acetylation has been found to regulate fruit ripening by modulating phytohormone signalling, fruit coloration, glycometabolism, and fruit softening, as summarized and discussed below (Figure 1).
| Histone modifications | Species name | Histone modifiers | Specific histone modification | Regulated genes | Physiological processes | Promote or repress | References |
|---|---|---|---|---|---|---|---|
| Histone acetylation | Banana | —— | H3ac, H4ac | MaEXP1/3, MaPG1, MaXTH10, MaPL3 and MaPME | Fruit ripening | ↓ | (Fan et al., 2016) |
| Pear | —— | H3ac, H4ac | PuSWEET15 | Glycometabolism | ↑ | (Li, Jiang, et al., 2020a) | |
| Pepper | CaHAG3, CaHAG9 | —— | —— | Fruit coloration | ↑ | (Cai et al., 2022) | |
| Sweet orange | CsHAF1/2 | —— | —— | Fruit ripening | —— | (Xu et al., 2015) | |
| Tomato | SlHAF1 | —— | —— | Fruit ripening | —— | (Aiese Cigliano et al., 2013) | |
| Histone deacetylation | Apple | MdHDA19 | —— | MdCCD1, MdPDS | Fruit coloration, Fruit softening | ↓ | (Li, Guo, et al., 2020) |
| Apple | —— | H3K9ac | MdACS3a | Fruit ripening | ↓ | (Hu et al., 2020; Hu et al., 2022) | |
| Banana | MaHDA1 | H3ac, H4ac | MaACO1, MaEXP2, MaEXP7 and MaEXP8 | Fruit ripening | ↓ | (Han et al., 2016) | |
| Grape | VvHDAC19 | H3ac, H4ac | VvMYB5a | Fruit coloration | ↓ | (Jia et al., 2023) | |
| Papaya | CpHDA3 | —— | CpPME1, CpPME1 and CpERF9 | Fruit softening | ↓ | (Fu et al., 2019) | |
| Pear | Sirtuin | —— | PbSRT2 | Glycometabolism | ↑ | (Vall-Llaura et al., 2021) | |
| Tomato | SlHDA1/SlHDA3/SLHDT1 | H4ac | SICYC-B, SILCY-B, SILCY-E, SlPSY1, SlHEX, SlMAN, SlTBG4, SlXTH5 and SlXYL | Fruit coloration, Fruit softening | ↑ | (Guo et al., 2018; Guo et al., 2017b; Guo, 2022) | |
| Tomato | SlHDA1/SlHDA3 | H3K9ac, H3K27ac | SlACS2, SlACS4 | Fruit ripening | ↓ | (Deng et al., 2022) | |
| Tomato | SIHDT3 | —— | SlCYC-B, SlLCY-B, SlLCY-E, SlPSY1, SlHEX, SlMAN, SlTBG4, SlXTH5 and SlXYL | Fruit coloration, Fruit softening | ↓ | (Guo et al., 2017a) | |
| Tomato | SlHDA7 | H4ac | SlGGPPS2, SlZISO | Fruit coloration | ↑ | (Wang et al., 2024) | |
| Strawberry | FaSRT1-2 | —— | FaSPS1, FaSPS2 and FaSUS2 | Glycometabolism | ↑ | (Wang et al., 2024) | |
| Histone methylation | Grape | VvHKMT | H3K4me | VvH3K4-5 | Fruit development | —— | (Liu et al., 2021) |
| Tomato | SlEZ1 | H3K27me3 | SlEZ1 | Fruit development | ↑ | (Li, Wang, Zhang, et al., 2022a) | |
| Tomato | SlEZ2 | H3K27me3 | SlEZ2 | Fruit softening | ↓ | (Boureau et al., 2016) | |
| Tomato | —— | H3K27me3 | SlACS4, SlACS2, SlPG2a, SlRIN and SlNOR | Fruit ripening | ↓ | (Liang et al., 2020) | |
| Histone demethylation | Banana | MaJMJ15 | H3K27me3 | MaNAC1, MaNAC2, MaACS1, MaACO1, and MaEXP2 | Post-harvest ripening of fruit | ↑ | (Zeng et al., 2023) |
| Tomato | SlJMJ3 | H3K27me3 | SlRIN, SlNOR, SlFUL1, SlACS2, SlACS4, SlPSY1, SlDXS1, SlPL, SlPG2a and SlTBG4 | Fruit ripening | ↑ | (Li et al., 2024) | |
| Red-fleshed pummelo | CgSDG40 | —— | CgPSY1 | Fruit coloration | ↑ | (Fu et al., 2024) | |
| Tomato | SlJMJ6 | H3K27me3 | SlRIN, SlACS4 and SlACO1, SlPL,SlTBG4 and SlDML2 | Fruit ripening | ↑ | (Li, ,Jiang, et al., 2020b) | |
| Tomato | SlJMJ7 | H3K4me3 | SlACS2, SlACS4, SlACO6, SlRIN, SlNOR and SlDML2 | Fruit ripening | ↓ | (Ding et al., 2022) |
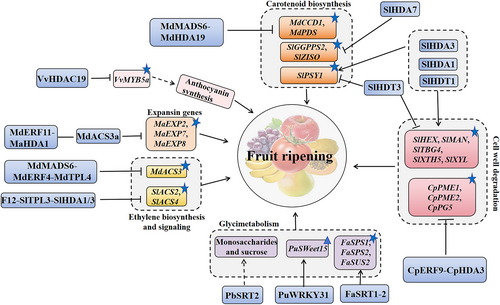
2.2.1 Phytohormone signalling
In plants, the expression of ethylene biosynthesis and signalling pahway-related genes has been shown to be regulated by histone acetylation within many biological processes, especially in fruit ripening. For instance, in banana, MaDEAR1 acts as a DREB transcription factor subfamily member of the AP2/ERF protein family, negatively participates in ethylene mediation and inhibits fruit ripening by reducing histone H3 acetylation (H3ac) level and histone H4 acetylation (H4ac) levels (Fan et al., 2016). In addition, HDAC is able to affect ethylene synthesis and the expression of ethylene signal transduction-related genes, thereby affecting fruit ripening. Ethylene response factors (ERFs) belong to the Apetala2 (AP2)/ERF superfamily, which acts downstream of the ethylene signalling pathway to regulate the expression of ethylene-responsive genes (Feng et al., 2020). ERFs have been reported to play important roles in plant development, flower abscission, and fruit ripening (Fan et al., 2016; Feng et al., 2020; Nakano et al., 2014). In addition, ERF may regulate the process of fruit ripening in correlation with HDAC proteins. In A. thaliana, overexpression of HDA19 affected the expression of AtERF1 (Wang et al., 2020). Subsequent studies showed that HDAC interacted with ERF and affected fruit ripening by regulating ethylene synthesis. For example, in banana, MaERF11 interacted with a histone deacetylase MaHDA1 during fruit ripening. By directly binding to the promoters of the ethylene biosynthesis gene 1-aminocyclopropane-1-carboxylate oxidase 1 (MaACO1) and ripening-related expansin genes MaEXP2, MaEXP7, and MaEXP8, MaERF11-MaHDA1 complex reduced the acetylation levels of histone H3 and H4 and suppressed the expression of the above genes (Han et al., 2016). Furthermore, as the fruit ripened, the accumulation of MaERF11 was inhibited, and the expression of MaACO1 and the expansins was enhanced. In early-ripening apple fruit, the appearance of the ethylene peak was accompanied by an upregulation of MdACS3a expression, which promoted fruit ripening, whereas no ethylene peak appeared in late-ripening varieties (Hu et al., 2020; Hu et al., 2022). Moreover, the histone deacetylase MdHDA19 inhibited the expression of MdACS3a in apple and suppressed fruit ripening by forming a complex with MdERF4 and the TOPLESS co-repressor (MdTPL4) and promoting the H3K9 deacetylation at the MdACS3a locus. In addition, in tomato, SlHDA1 and SlHDA3 could also form a protein complex with SlERF.F12 and repress the transcription of SlACS2 and SlACS4 by reducing the related H3K9Ac and H3K27Ac levels (Deng et al., 2022). Therefore, histone deacetylation can inhibit the expression of ethylene synthesis genes and repress fruit ripening by collaborating with ERFs (Figure 1).
2.2.2 Fruit coloration
Plants HDAC is associated with coloring during fruit ripening. During tomato fruit ripening, the fruit coloration is always accompanied by the degradation of chlorophylls and the accumulation of carotenoids (Guo et al., 2017a). PHYTONE SYNTHASE 1 (PSY1) is a critical metabolic flux regulator in the pathway of carotenoid biosynthesis during fruit ripening. Previous studies showed that in tomato pericarp, the expression level of SlPSY1 was up-regulated in the silenced SlHDA1 and SlHDA3 RNAi plants, whereas the expression of chromoplast-specific lycopene-β-cyclase (SlCYC-B), chloroplast-specific lycopene-β-cyclase (SlLCY-B), and SlLCY-E were significantly down-regulated, which led to an increase in carotenoid content (Guo et al., 2018; Guo et al., 2017b). Thus, histone deacetylase SlHDA1 and SlHDA3 contribute to the repression of fruit ripening and carotenoid accumulation. Similarly, in the SlHDT1 RNAi lines, the level of acetylation on histone H3 was increased, affecting carotenoid accumulation and ethylene biosynthesis (Guo, 2022). In contrast, the SlHDT3 RNAi lines of histone deacetylase SlHDT3 delayed fruit ripening and suppressed carotenoid accumulation in tomato (Guo et al., 2017a). Furthermore, in slhda7 mutant fruit, the expression of key genes involved in the carotenoid biosynthesis genes GERANYLGERANYL PYROPHOSPHATE SYNTHASE 2 (SlGGPPS2) and 15-cis-ZETA-CAROTENE ISOMERASE (SlZISO) were significantly increased but decreased in SlHDA7-overexpressing fruits. Meanwhile, the H4ac modifications were removed at these ripening-related gene loci by SlHDA7 (Zhou et al., 2024). Thus, HDAC may play a dual role in regulating carotenoid biosynthesis during fruit coloring, while the influence of HDAC on fruit chlorophyll content is waiting to be explored. In apple (Malus domestica), the transcription factor (MdMADS6) formed a complex by recruiting and interacting with MdHDA19 to depress the expression of the carotenoid metabolism-related genes MdCCD1 and MdPDS in the early stages of fruit development (Li, Wang, Xu, et al., 2022a). Then, this complex was dissociated with the increased expression of MdMADS6, leading to the accumulation of carotenoids at the late stages of fruit development. In addition, in pepper (Capsicum annuum L.), CaHATs regulated the metabolism and signalling of ABA to judge the biosynthesis of capsaicinoid and carotenoid during fruit development and ripening (Cai et al., 2022). However, the specific role of histone acetylation on ABA during fruit ripening is waiting to be uncovered. In addition, HDACs could also regulate the color changes during fruit ripening by interacting with ERFs. Usually, the content of carotenoid and anthocyanin increases gradually, and the chlorophyll level decreases during fruit ripening, turning the color of ripe fruit into yellow, pink, red or purple. In purple-flowered Mimulus Iuteus var. Variegatu, the knockout of MYB5a reduced the transcription of certain anthocyanin regulatory factors, certifying the involvement of MYB5a in anthocyanin formation (Zheng et al., 2021). Similarly, in grape (Vitis vinifera) (Jia et al., 2023), the histone deacetylase VvHDAC19 recruited VvERF4 to the VvMYB5a promoter region, suppressing anthocyanin biosynthesis and accumulation by down-regulating the related H3 and H4 acetylation levels, which contributed to a delay of grape fruits' color transformation. Thus, HDAC could influence fruit ripening in conjunction with ERFs through affecting anthocyanin-based fruit coloring (Figure 1).
2.2.3 Glycometabolism
Histone acetylation has also been reported to influence carbohydrate metabolism during fruit ripening. In pear fruit, the high level of histone acetylation in the promoter region of the transcription factor PuWRKY31 enhanced its expression level. Subsequently, PuWRKY31 bound to the sugar transporter (PuSWEET15) promoter sequence, thereby increasing its expression level and strengthening the accumulation of sucorse in ‘Nanguo’ (NG) and its bud sport (BNG) fruit (Li, Guo, et al., 2020a). However, the specific HAT involved in fruit sucrose accumulation is unknown. Secondly, Sirtuin (SRT), as a specific NAD+-dependent HDAC, can participate in plant energy and sugar metabolism. In A.thaliana, AtSRT1 could inhibit cellular glycolysis and enhance mitochondrial respiration in Seedlings (Liu et al., 2017). In rice (Oryza sativa L.), the nucleus-localized OsSRT1 restrained the glycolytic pathway in seedlings by inducing histone deacetylation of glyceraldehyde 3-phosphate dehydrogenase (AtGAPDH) locus (Zhang et al., 2017). Nevertheless, during pear fruit ripening, the increase of PbSRT2 gene expression was positively correlated with sugar accumulation (Vall-Llaura et al., 2021). Furthermore, in strawberry (Fragaria × ananssa Duch.), overexpression of FaSRT1-2 gradually increased the expression levels of the sucrose phosphate synthase genes (FaSPS1 and FaSPS2) and sucrose synthase (FaSUS2) involved in glucose metabolism pathway, thereby promoting fruit ripening (Wang et al., 2024). Thus, plant SRT may also reinforce sugar accumulation through depressing glycolysis and promoting sucrose production during fruit ripening, in which the specific histone-deacetylated sites need to be uncovered (Figure 1).
2.2.4 Fruit softening
Finally, it has been found that HDAC is involved in fruit softening during post-harvest ripening. With the softening process in the stored fruit, the fruit firmness gradually decreases, leading to reduced fruit quality, shelf life and commercial value. Additionally, the excessive softening led to post-harvest fruit rot, making the fruit susceptible to pathogen infection (Farinati et al., 2017; Peng et al., 2022). Previous studies found that in tomato SlHDA1 and SlHDA3 RNAi lines, the transcript abundances of cell wall metabolism genes SlHEX, SlMAN, SlTBG4, SlXTH5, and SlXYL were promoted, which in turn accelerated cell wall degradation and the subsequent fruit softening. However, down-regulation of SlHDT3 expression in RNAi fruit repressed the expression of the above cell wall metabolism-related genes, thereby delaying softening and prolonging fruit shelf life. Thus, histone deacetylation may also play a dual role in affecting fruit softening during storage. Yet, the specific kinds of histone deacetylation are unclear.
Furthermore, HDACs and ERF may be able to cooperatively regulate fruit ripening by influencing the softening process. In apple, MdHDA19 was recruited to the co-repressor MdERF4-MdTPL4 and introduced H3K9 deacetylation to impact ethylene production and suppress fruit softening (Li, Wang, Xu, et al., 2022). Previous studies showed that the softening of fruit was caused by denaturation or degradation of cell wall polysaccharides (Wang et al., 2018). For example, in tomato, the cell wall degradation-related polygalacturonase (SlPG) was able to promote fruit softening (Hu et al., 2021; Nie et al., 2022). In addition, during the ripening process of papaya (Carica papaya L.) fruits, the expression levels of PECTIN METHYLESTERASE 1 (CpPME1), CpPME2, and CpPG5 were gradually increased, thereby promoting fruit softening (Fu et al., 2016). Moreover, a RPD3/HDA1 subfamily protein CpHDA3 interacted with CpERF9 and strengthened the transcriptional repression activity of CpERF9 by promoting histone deacetylation at its downstream genes CpPME1, CpPME2, and CpPG5 within this process (Fu et al., 2019). Hence, HDAC may associate with ERF to depress fruit softening via affecting the transcriptional levels of the related genes (Figure 1).
3 EFFECT OF HISTONE METHYLATION ON FRUIT RIPENING
3.1 The involvement of histone methylation in fruit ripening
Histone methylation generally refers to the methylation of arginine or lysine in histone proteins (Jia et al., 2022). There are two main types of plant histone methyltransferases (HMTs): lysine methyltransferases (HKMTs) and arginine methyltransferases (PRMTs) (Liu et al., 2021). Plant HMTs are mainly encoded by a family of SET structural domain (SDG) group genes (Berr et al., 2011). The SDG family is categorized into different classes based on sequence similarity; it includes the suppressor of variegation 3–9 [SU(VAR)3–9], enhancer of zeste [E(z)], trithorax (TRX), and absent, small, or homeotic disks 1 (ASH1) (Xu et al., 2015). The lysine residues of histone can undergo different degrees of methylation, including monomethylation (me1), dimethylation (me2) and trimethylation (me3) (Xiao et al., 2022). H3K4me3, one of the most extensively studied histone methylation patterns, is recognized for promoting gene transcription initiation through the trimethylation of histone H3 lysine 4 (Wang, Wang, et al., 2023b). Furthermore, histone methylation has now been found to regulate fruit ripening in plants, and the corresponding studies were collected and reviewed below.
Histone lysine methylation is involved in a variety of biological processes in plants, including the ripening process (Figure 2, Table 1). For example, during grape fruit ripening, the expression of VvH3K4–5 was significantly up-regulated by the endogenous reactive oxygen species (ROS) (Liu et al., 2021). Thus, it was shown that histone H3K4 methylation might be involved in the grape fruits' development process, but the exact mechanism remains to be explored. Additionally, in red-fleshed pummelo (Citrus maxima), the histone methyltransferase (CgSDG40) positively regulates carotenoid biosynthesis. By activating the expression of CgPSY1, carotenoid accumulation is promoted and the flesh turns red (Fu et al., 2024). Hence, histone methyltransferases can promote the accumulation of pigments and thereby influence the process of fruit color change (Figure 2). In addition, a recent study revealed that H3K27 methylation was able to participate in the regulation of the fruit ripening process. In tomato, E(z), as a type of HMTs, was part of the core element of PRC2s that mediated H3K27me3 (Li, Wang, Zhang, et al., 2022c). Besides, the two genes encoding E(z), named SlEZ1 and SlEZ2, had different functions during fruit development and ripening (Boureau et al., 2016). Knockdown of SlEZ1 resulted in an increased number of fruit chambers and altered flower morphology but had no effect on fruit coloration during fruit ripening. In contrast, silencing of SlEZ2 led to accelerated fruit softening, which in turn promoted the fruit ripening process. Thus, different types of HKMT changes may bring about different effects on fruit development and ripening. In animals, another kind of PCG protein family, PRC1, is required for transcriptional repression through its chromodomain-specific binding function to genes marked with H3K27me3 (Grossniklaus & Paro, 2014). In plants, it has been found that LIKE HETEROCHROMATIN PROTEIN 1 (LHP1) is a candidate PRC1 protein (Liang et al., 2020). To be specific, in tomato fruit, knockdown of SlLHP1b promoted ripening initiation and the related earlier climacteric ethylene biosynthesis and fruit softening. However, overexpression of SlLHP1b retarded the ripe process of the fruit. In addition, SlLHP1b bound to the ripening-related genes marked by H3K27me3, including the ethylene biosynthesis genes 1-CARBOXYLATE SYNTHASE 2 (SlACS2), and SlACS4, cell wall degradation gene SIPG2a, and fruit ripening-related transcription factors SlRIN and SlNOR. Nevertheless, the H3K27me3 level of these gene sites was promoted by SILHP1b, and the related gene expression levels were depressed. Thus, histone methylation of H3K4 and H3K27 may take part in regulating plant's fruit ripening. However, the accurate mechanism of dynamic regulation of histone methylation concerning fruit ripening, especially the modifications of H3K4, is not yet discovered.
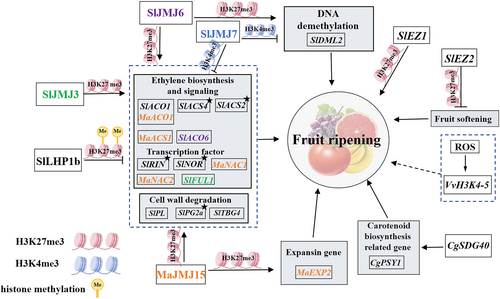
3.2 The adjustment of histone demethylation during fruit ripening
Histone methylation is dynamically regulated by HMTs and histone demethylases (HDMs). Histone lysine demethylases include two subfamilies, namely lysine-specific demethylase 1 (LSD1) and jumonji C domain-containing proteins (JMJs) (Li, Jiang, et al., 2020b). These enzymes are dependent on the cofactors ligand iron II (Fe2+) and 2-oxogluconate (α-KG) for histone methylation removal (Accari & Fisher, 2015).
It has now been found that histone lysine demethylases (JMJs) are able to participate in the process of fruit ripening in plants (Figure 2). For instance, the grape berries treated with an H3K4 methylation inhibitor (MTA) presented an early ripening phenotype. Additionally, the expression of VviJMJ gene was increased by MTA, suggesting a close relationship between JMJ and fruit ripening (Cheng et al., 2022). Furthermore, SlJMJ6 in tomato encodes a histone lysine demethylase that specifically demethylates histone methylation at H3K27 sites. In SlJMJ6 overexpression fruit, ripening-related regulators, such as ripening inhibitor (SlRIN), SlACS4, SlACO1, pectate lyase (SlPL), and SlTBG4, were directly up-regulated by the removal of H3K27me3, which accelerated the ripening process (Li, Jiang, et al., 2020b). Thus, SlJMJ6 acted as an H3K27me3 demethylase and positively regulated tomato fruit ripening. Similarly, in banana post-harvest fruit, the transcript abundance of MaJMJ15 was gradually and significantly up-regulated with the extension of storage time (Zeng et al., 2023). And MaJMJ15 was also able to promote the banana post-harvest ripening process by binding to the MaNAC1, MaNAC2, MaACS1, MaACO1 and MaEXP2 genes and reducing the corresponding H3K27me3 methylation levels. Similarly, SlJMJ3, a member of the KDM4/JHDM3 family, acted as an H3K27me3 demethylase in tomato and promoted fruit ripening through removing H3K27me3 marks at ethylene biosynthesis and response, carotenoid biosynthesis, and cell wall degradation-related gene loci (Li et al., 2024). Thus, the demethylation at H3K27me3 by JMJ proteins may promote fruit ripening. Subsequent studies revealed that SlJMJ7, as a negative regulator of fruit ripening, directly inhibited the expression of SlACS2, SlACS4, SlACO6, SlRIN, SlNOR, and a DNA demethylase DEMETER-like 2 (SLDML2) by decreasing H3K4me3 demethylation, causing a delay in fruit ripening (Ding et al., 2022). In addition, it was recently shown that SLDML2 promotes fruit ripening through inducing genome-wide DNA demethylation (Lang et al., 2017; Liu et al., 2015). Interestingly, SlJMJ6 promoted SlDML2 expression by removing H3K27me3 modifications, while SlJMJ7 inhibited SlDML2 expression by repressing H3K4me3 level during fruit ripening. Thus, the histone demethylation at H3K27me3 and H3K4me3 loci may also influence fruit ripening by regulating the DML2-mediated DNA demethylation process.
4 EFFECTS OF OTHER HISTONE MODIFICATIONS ON FRUIT RIPENING
Ubiquitin (Ub) is a class of low molecular weight proteins consisting of 76 amino acids with a molecular weight of approximately 8.451 kD. Ubiquitination is the process by which ubiquitin molecules are specifically modified by a family of enzymes, including E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes, and E3 ubiquitin-ligase enzymes (Wang, Wang, et al., 2023b). Histone ubiquitination is generally a modification of the lysine (Lys) at the C-terminus of histone and is associated with initiating gene expression. Ubiquitination modifications of histone play a role in changing the conformation of chromosomes, recruiting and activating downstream proteins, and degrading proteins as degradation signals (Wang, Wang, et al., 2023b). To date, the most well-studied histone ubiquitinations are the ubiquitination of H2A and H2B (Zhang et al., 2023). Overall, ubiquitinated H2A is found mostly in heterochromatin, whereas ubiquitinated H2B is found mostly in active euchromatin. Ubiquitinated histones can alter chromatin structure, and in eukaryotes, HISTONE 2A MONOUBIQUITINATION (H2Aub1) is associated with transcriptional repression mediated by the PRC1 pathway, while HISTONE 2B MONOUBIQUITINATION (H2Bub1) is involved in transcriptional activation (Zarreen et al., 2022). Furthermore, it can interact with various post-translational modifications, such as methylation and acetylation, to synergistically regulate various life activities, including fruit ripening.
Protein ubiquitination controls many essential aspects of plant vegetative and reproductive processes, including fruit ripening (Zarreen et al., 2022). Among them, the ubiquitination modifications of histone play an important role in plant growth, development, and environmental adaptation. For instance, previous research found that, in A. thaliana, the HISTONE MONOUBIQUITINATION 2 (HUB2) promoted the monoubiquitination of histone H2B. Overexpression of AtHUB2 enhanced the drought resistance of transgenic cotton (Gossypium hirsutum L.) (Chen et al., 2019). In rice, knockout of either OsHUB1 or OsHUB2 caused depressed levels of H2Bub1 and H3K4me2 modifications, which was correlated with abnormal anther development (Cao et al., 2015). Although histone ubiquitination has been reported to regulate stress tolerance and control growth and development in plants, the specific mechanisms in the regulation of fruit ripening have yet to be explored in depth. In contrast, the mechanism of protein ubiquitination in promoting fruit ripening has been reported. In A. thaliana, ETHYLENE INSENSITIVE 3 (EIN3) binding F-box proteins (EBFs) are ubiquitination-related proteins that may interact with EIN3 and cause ubiquitination-dependent EIN3 removal and influence ethylene response (Gagne et al., 2004). In banana, the expression of MaEBF2 was increased in ethylene-treated fruit during the ripening process (Kuang et al., 2013). The above results suggest that there may exist a relationship between ethylene-induced protein ubiquitination and fruit ripening. Moreover, in apple, BEL1-LIKE HOMEODOMAIN transcription factor 7 (MdBEL7) potentially functions as a transcriptional repressor of the chlorophyll catabolic genes (CCGs). In addition, MdPUB24, an ethylene-activated U-box-type E3 ubiquitin ligase, could degrade MdBEL7 by enhancing the ubiquitination modifications of MdBLE7, which led to chlorophyll degradation during apple fruit storage (Wei et al., 2021). Similarly, MdCOP1 E3 ubiquitin ligase interacted with MdMYB1, regulating light-induced anthocyanin biosynthesis and the development of red fruit coloration in apple (Li et al., 2012). Thus, protein ubiquitination is a multifaceted regulator of fruit ripening by directly modulating ethylene signalling and affecting pigment metabolism. However, the specific mechanism by which histone ubiquitination modifications regulate fruit ripening needs to be explored further.
5 CONCLUSIONS AND PERSPECTIVE
In summary, histone modifications play an important role in the regulation of fruit ripening. Specifically, histone acetylation may accelerate the ripening process by increasing acetylation levels of histone H3 and H4, which promote fruit color and sugar metabolism pathways. In addition, the deacetylation of H3ac, H4ac, H3K9ac, H3K14ac, and H3K27ac inhibits or delays fruit ripening through the ethylene generation and signalling, carotenoid and anthocyanin synthesis, cell wall degradation, and sugar metabolism. Besides, in tomato, histone methylation, in particular H3K4me3 and H3K27me3 modifications, has a critical impact on the regulation of fruit ripe gene expression. In detail, during fruit ripening, the expression of fruit ripening-related genes is inhibited by the increase of H3K27me3 methylation by mediating SlEZ1, SlEZ2, and SlLHP1b. In contrast, histone demethylation of H3K27me3 via MaJMJ15, SlJMJ6, and SlJMJ3 enhances the expression of ripening-related transcription factors, ethylene signalling pathway genes, and cell wall degradation concerning genes, thereby accelerating fruit ripening. In addition, SlJMJ7 reduces H3K4me3 level to depress fruit ripening. Notably, in tomato, SIJMJ6 and SIJMJ7 may also mediate DNA demethylation via modulating the levels of H3K27me3 and H3K4me3 modifications at the SlDML2 locus during fruit ripening. Although the evidence that histone ubiquitination regulates fruit ripening is scarce, the protein ubiquitination pathway has already been shown to indirectly participate in fruit ripening regulation by affecting the stability of proteins related to ethylene signalling and pigment metabolism. Overall, although the current understanding of the role of histone modifications in fruit ripening has progressed, a comprehensive understanding of their complex regulatory networks still needs to be deepened, which will provide key scientific support for optimizing plant breeding and fruit storage technologies. Future research should focus on the detection of more histone modifiers and their specific mediated mechanisms during fruit ripening. In addition, the effective reagents that can specifically adjust histone modifications in fruit ripening process should be identified.
AUTHOR CONTRIBUTIONS
All authors contributed to the conceptualization and design of the study. Conceptualization, Chunlei Wang; Fund Acquisition, Caiting An, Chunlei Wang; Original Writing, Chunlei Wang, Caiting An; Copy Editing, Caiting An, Zesheng Liu, Xuejuan Pan, Chunlei Wang; Character Creation and Adaptation, Caiting An and Chunlei Wang. All authors read and approved the manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No. 32102370); the key project of Gansu Provincial Natural Science Foundation, China (No. 23JRRA1406); and the Fuxi Young Talents Fund of Gansu Agricultural University (Gaufx-03Y07).
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were cre-ated or analyzed in this study.




