CmPYL7 positively regulates the cold tolerance via interacting with CmPP2C24-like in oriental melon
Abstract
Pyrabactin or Actin Resistance1/PYR1-Like/Regulatory Components of abscisic acid (ABA) Receptors (PYR/PYL/RCARs, referred to as PYLs) are direct receptors of ABA that function pivotally in the ABA-signaling pathway. Previously, we discovered that CmPYL7 was strongly upregulated by cold stress in oriental melon (Cucumis melo). In this study, we demonstrated that CmPYL7 was strongly induced by cold treatment (Cold), Cold+ABA, and Cold+fluridone (Flu, an ABA inhibitor) treatments, while the expression level of CmPYL7 under Cold+Flu is lower than that of cold treatment. Silencing CmPYL7 in oriental melon seedlings significantly decreased cold tolerance due to the reduced activities of antioxidant enzymes [superoxide dismutase (SOD); catalase (CAT), and ascorbate peroxidase (APX)] and the accumulation of H2O2, accompanied by higher electrolyte leakage and MDA content, but lower proline and soluble sugar content. In contrast, overexpressing CmPYL7 in Arabidopsis plants significantly increased cold tolerance owing to the enhanced activities of antioxidant enzymes (SOD, CAT, and APX) and limited H2O2, accompanied by lower electrolyte leakage and MDA content, but higher proline and soluble sugar contents. CmPYL7 was found to interact with CmPP2C24-like in vivo and in vitro, whose expression is downregulated under cold stress. Furthermore, silenced CmPP2C24-like in oriental melon plants significantly increased cold tolerance, exhibiting lower electrolyte leakage and MDA content and higher proline and soluble sugar contents. The activities of SOD, CAT, and APX were further enhanced and contents of H2O2 were significantly limited from increasing in TRV-CmPP2C24-like seedlings. These results demonstrated that CmPYL7 functions positively in the ABA-signaling pathway to regulate cold tolerance by interacting with CmPP2C24-like protein.
1 INTRODUCTION
Low temperature is an important factor that restricts the growth, productivity, and quality of many species (Zhu, 2016). Melon (Cucumis melo) is sensitive to cold stress since it is native to tropical areas (Nuñez-Palenius et al., 2008). Oriental melon is very popular in China and is mainly produced in the north of China. Currently, the off-season production of oriental melon is becoming more popular in crude facilities such as greenhouses, which makes the production highly susceptible to cold stress. When subjected to cold stress, melon seedlings may exhibit symptoms of cold injury, including stunted growth, reduced yield and quality, increased susceptibility to pathogens, necrosis of tissues, and even death of plants (Korkmaz and Dufault, 2003, 2004). The best way to limit investment and protect products during off-season production is to enhance the cold tolerance of oriental melon varieties.
Abscisic acid (ABA) is a crucial stress-signaling molecule that plays an important role in regulating cold-responsive physiological metabolism, including reactive oxygen species (ROS) scavenging by antioxidant enzymes [superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX)], to enhance cold tolerance in plants (Zhang, 2014). Inhibiting ABA synthesis significantly reduces the tolerance to cold stress, whereas the exogenous application of ABA can improve the survival rate under chilling stress (Shinkawa et al., 2013; Tian and Li, 2018). The classical ABA regulatory pathway begins with the combination of ABA with its receptor, Pyrabactin or Actin Resistance1/PYR1-like/Regulatory Components of ABA Receptors (PYR/PYL/RCARs, referred to as PYLs), to form an ABA-PYL dimer. This dimer further combines with type 2C protein phosphatases (PP2Cs), inhibits their activities, and then releases and activates SNF1-related protein kinase 2 s (SnRK2s) and subsequent downstream responses (Zhang et al., 2017).
Recent studies have highlighted the significant role of PYLs in regulating cold stress responses. Previous studies have observed that a high proportion of PYL family members respond positively to cold stress (Zhao et al., 2020; Hou et al., 2020). Overexpression of important PYL members improves cold tolerance. For example, overexpression of rice OsPYL3 in Arabidopsis enhanced sensitivity to ABA and tolerance to cold stress in transgenic plants (Lenka et al., 2018). Overexpression of AtRCAR12 or AtRCAR13 in Arabidopsis and PtPYRL1 or PtPYRL5 in poplar improved cold tolerance (Zhang et al., 2019; Yu et al., 2017). Overexpression of PYL(s) results in the accumulation of more PYL receptors, promoting a quick combination with ABA and the rapid regulation of downstream metabolism. These findings revealed the crucial role of PYLs in cold tolerance in certain species.
PP2C is a large family present in various species and can be divided into multiple subfamilies. Subfamily A PP2Cs, including ABI1, ABI2, HAB1, HAB2, etc., are major switches that regulate the activities of SnRK2s and downstream responsive metabolic pathways (Krzywinska et al., 2022). Arabidopsis AtPP2CA and ABI1 can be induced by low temperature and ABA treatment. In contrast, silencing AtPP2CA in Arabidopsis significantly enhanced freezing tolerance after cold exposure or ABA treatment and significantly upregulated the characteristic genes that can be induced by cold stress and ABA (Tähtiharju and Palva, 2001). Rice OsPP2C27 negatively regulates the activities of OsMAPK3 and OsbHLH002 through dephosphorylation, thereby negatively regulating cold tolerance in rice plants (Xia et al., 2021). However, a previous study found that overexpression of ZmPP2C2 could significantly enhance antioxidant enzymes (SOD, POD, and CAT) and reduce cold damage, thereby improving the cold tolerance of transgenic plants (Hu et al., 2010). AtPP2CA, OsPP2C27, and ZmPP2C2 may originate from different subfamilies, which may explain the differences in cold response regulation.
However, reports on PYL in melon are scarce. We identified 12 CmPYL genes in the melon genome and found that CmPYL7 expression was strongly induced by cold stress (Liu et al., 2024). This study revealed that CmPYL7 positively regulates cold tolerance by interacting with downstream CmPP2C24-like, identifying it as a new gene associated with cold tolerance in oriental melon.
2 METHODS
2.1 Materials and treatment
Two oriental melon genotypes (Cucumis melo), cold-tolerant No.330 and cold-sensitive No.410, were used for expression analysis. No.330 was used for CmPYL7 functional analysis. Seeds were sterilized before sowing, and seedlings were grown in pots (peat:perlite:roseite = 2:1:1) in Panasonic plant growth chambers (MLR-352H-PC, Panasonic). Virus-induced gene silencing (VIGS) seedlings of TRV-CmPYL7 or TRV-CmPP2C24-like were cultivated as described by Liu et al. (2020). After 25 d, seedlings of No.330 and No.410, TRV (No.330 injected with Agrobacterium tumefaciens containing TRV vector), TRV-CmPYL7 or TRV-CmPP2C24-like were either cultivated under normal conditions as control or treated with 4°C as cold treatment. Normal cultivated condition was set as 28°C /20°C, 16 h/8 h, and 250 μE m−2 s−1/0 μE m−2 s−1 day/night cycles at 70% humidity. Cold treatment condition was set as: 4°C, 16 h/8 h, and 50 μE m−2 s−1/0 μE m−2 s−1 day/night cycles at 70% humidity. Leaves were collected for analysis 0, 3, 6, 9, 12, and 24 h after treatment. Phenotypes were photographed 12 or 24 h after treatment.
Transgenic Arabidopsis plants overexpressing CmPYL7 were also cultivated in pots (peat:perlite:roseite = 2:1:1) in Panasonic plant growth chambers under normal conditions of 24°C/20°C, 16 h/8 h, and 200 μE m−2 s−1/0 μE m−2 s−1 day/night cycles at 70% humidity. Col-0 plants were used as the controls. Plants were subjected to cold in the dark when they began to bolt, and leaves were collected at 0, 0.5, 1, 3, 5, and 7 d after treatment for analysis. The phenotypes were photographed 7 d after cold treatment.
Tobacco seedlings were cultivated in pots (peat:perlite:roseite = 2:1:1) in Panasonic plant growth chambers under conditions of 24°C/20°C, 16 h/8 h, and 200 μE m−2 s−1/0 μE m−2 s−1 day/night cycles and 70% humidity. Plants were cultivated at 24°C in the dark for 24 h after Agrobacterium injection and then reverted to the normal condition.
2.2 Electrolyte leakage
Electrolyte leakage was determined using 0.2 g fresh leaf samples. Fresh samples were immersed in distilled water for 2 h and then measured as Value 1 after mixing. Thereafter, the samples were boiled for 20 min and Value 2 was measured after cooling to 25°C. Electrolyte leakage was calculated as Value 1/Value 2 × 100.
2.3 Proline content
Proline content was measured using sulfosalicylic acid colorimetry (Bates et al. 1973). Briefly, leaf samples (0.5 g) were immersed in 5 mL 3% sulfosalicylic acid solution and boiled for 10 min. After cooling to room temperature and filtration, 2 mL solution was mixed with 2 mL glacial acetic acid and 2 mL acid ninhydrin, and the mixture was boiled for 30 min. After cooling, 4 mL methylbenzene was added, and the mixture was centrifuged for 5 min at 1 000 g after shaking for 30 s. The red supernatant was measured spectrophotometrically at a wavelength of 520 nm, and the proline content (mg/g FW) was calculated according to the standard curve.
2.4 Soluble sugar content
Soluble sugar content of the leaf samples (0.2 g) was extracted and quantified according to the method described by Du et al. (2020). Samples were ground and extracted with 80% (v/v) aqueous ethanol at 80°C for 30 min, followed by centrifugation at 10,000 × g for 10 min. The supernatant was transferred to a new tube, and the residue was extracted twice. The three supernatants were mixed, and 80% ethanol was added to a total volume of 10 mL. The extract was analyzed spectrophotometrically at 620 nm, and the soluble sugar content (mg/g FW) was calculated according to the standard curve.
2.5 Malondialdehyde (MDA) content
MDA content was determined using the trichloroacetic acid reaction method described by Li et al. (2011), with slight modifications. Briefly, 0.5 g fresh leaf samples were homogenized in 5 mL 10% trichloroacetic acid solution and then centrifuged at 1 800 g for 10 min. Subsequently, 2 mL was mixed with 2 mL 0.6% thiobarbituric acid and the mixture was boiled for 15 min. After quick cooling, the mixture was centrifuged at 1 800 g for 5 min, and then 2 mL supernatant was collected for detection at 532, 600, and 450 nm wavelengths. MDA content was calculated according to the following formula: MDA content (μmol g−1) = [6.45 × (OD532 − OD600) − 0.56 × OD450] × V / W, where V is the volume of the extracted trichloroacetic acid solution, and W is the weight of the fresh sample.
2.6 Antioxidant enzymes activities
The enzyme solution was extracted as previously described (Liu et al., 2020). Superoxide dismutase (SOD) activity was determined and calculated following the methods of Giannopolitis and Ries (1977). One unit of SOD activity was defined as the amount of enzyme required for a 50% reduction in nitro blue tetrazolium (NBT) as monitored at 560 nm. Catalase (CAT) and ascorbate peroxidase (APX) activities were measured as described by Noctor et al. (2016). CAT activity was determined with a mixed solution containing 0.1 M phosphate buffer (pH 7.5), 40 mM H2O2 and 50 μL enzyme extract, and measured by monitoring the decomposition rate of H2O2 within 2 min at 240 nm. APX activity was determined with a reacted solution comprising 10 mM ascorbate, 0.1 M phosphate buffer (pH 7.5), 20 mM H2O2, and 50 μL of enzyme extract and measured by detecting the decreasing rate of ascorbate within 2 min at 290 nm.
2.7 H2O2 content
H2O2 content was assayed using fresh leaves (0.1 g) and detected according to the instructions of the H2O2 Content Assay Kit (AKAO009M, Boxbio Science & Technology Company). The H2O2 concentration was determined at a wavelength of 415 nm.
2.8 RNA extraction, cDNA synthesis, RT-qPCR analysis
Total RNA was extracted from the leaf samples of melon seedlings using an Ultrapure RNA Kit (Cat#CW0581M, Kangwei Biotech) following the manufacturer's recommendations and then treated with DNase I (Cat#M6101, Promega), as described by Jin et al. (2014). cDNA was synthesized according to the instruction of GoScript Reverse Transcriptase (Cat#A2790, Promega) and the following RT-qPCR was executed according to Jin et al. (2014). RT-qPCR analysis was performed on an ABI7500 (Applied Biosystems) using SYBR Green PCR Real Master Mix (Cat#FP217, Tiangen). ACTIN (LOC103499652) was used as the reference gene. The Ct values for all CmPYLs were normalized to the Ct value for actin and calculated using the formula 2-ΔΔCt to determine relative fold changes for each sample in each experiment. Primers used are listed in Table S1.
2.9 VIGS vector construction and virus injection
cDNA extracted from untreated melon leaves was used for VIGS and overexpression vector constructions; it was prepared using the same process as for RT-qPCR. PrimeSTAR HS (Premix) (Code No. R040A, TaKaRa Bio) was used for all clonings in the following processes to ensure correct sequences. Plasmids of TRV1 and TRV2 have been described in detail by Liu et al. (2002), and TRV2 was used to carry specific fragments of the target genes CmPYL7 and CmPP2C24-like. Specific 350-bp fragments of CmPYL7 and CmPP2C24-like coding sequences (CDSs) were first T-cloned using primers without restriction sites, and then poly-A tails (Cat#RT124, Tiangen) were ligated into the T-vector (pMD19 (simple), Code No. 3271, TaKaRa Bio) using a T4 DNA ligase kit (Cat#RT406, Tiangen) for sequencing. The correct sequences were then cloned using primers containing restriction sites, and the purified fragments were fused to TRV2 using a T4 DNA ligase kit to form TRV2-CmPYL7 and TRV2-CmPP2C24-like vectors. Primers used are listed in Table S2.
Virus injection was performed as described by Liu et al. (2014) with slight modifications. Briefly, TRV1, TRV2, TRV2-CmPYL7, and TRV2-CmPP2C24-like vectors were introduced into Agrobacterium tumefaciens (strain GV3101) competent cells using the freeze–thaw protocol, then screened and inoculated with YEB medium containing 25 mg L−1 rifampicin and 50 mg L−1 kanamycin. The bacterial solutions were inoculated at a density of 0.8–1.0 at 600 nm and centrifuged to collect cells as pellets. The harvested Agrobacterium pellets were resuspended to a density of 1.0 at 600 nm in infiltration buffer (10 mM MgCl2, 10 mM MES, and 0.1 mM acetosyringone). After incubation and mixing (Liu et al., 2014), infiltration cultures were pressure-injected into newly expanded cotyledons using a 1 mL syringe minus needle, as described by Liu et al. (2002).
2.10 Overexpression vector construction and generation of transgenic Arabidopsis lines
These processes were performed as previously described by Liu et al. (2020). The full-length coding sequence of CmPYL7 was cloned into T-vector (pMD19 (simple), Code No.3271, TaKaRa Bio) for sequencing, and the correct sequence was amplified and ligated into linearized pCAMBIA1300 by using BamHI (5′) and SalI (3′) restriction sites. The constructed vector was introduced into Agrobacterium tumefaciens (strain GV3101) using the freeze–thaw protocol, and thereafter, the gene was transformed into Arabidopsis Col-0 (wild type, WT) using the floral dip method (Zhang et al., 2006). Transgenic plants were screened using hygromycin staining, GUS staining, and RT-qPCR. The T3 homozygous generations were used for further analysis. Primers used are listed in Table S2.
2.11 Yeast two-hybrid (Y2H) assay
The Y2H assay was performed as described by Xia et al. (2021). A cold-treated melon cDNA library was constructed according to the MatchMaker cDNA synthesis protocol (Clontech Laboratories, Inc.). The full-length CDS of CmPYL7 was ligated into the pGBKT7 (Clontech Laboratories, Inc.) binding domain (BD) vector using the EcoRI (5′) and BamHI (3′) restriction sites. For library screening, dsDNA, SmaI-linearized pGADT7-Rec vector, and pGBKT7-CmPYL7 were co-transformed into the competent yeast strain AH109. Full-length CDS of CmPP2C24-like was ligated into the pGADT7 (Clontech Laboratories, Inc.) activation domain (AD) vector using the EcoRI (5′) and BamHI (3′) restriction sites. The BD and AD vectors were co-transformed into the Y2H Gold yeast strain. The transformants were cultured on synthetic medium plates (SD medium) lacking Leu and Trp (SD/−Trp/−Leu) at 30°C for 2 d, then transferred onto SD/−Leu/−Trp/-His/−Ade medium firstly for cultivation and SD/−Leu/−Trp/-His/−Ade containing X-α-gal (5-bromo-4-chloro-3-indolyl-α-D-galactopyranoside) and AbA for blue color development. Primers used are listed in Table S3.
2.12 Coimmunoprecipitation (co-IP) assay
Co-IP assays were performed as described previously (Feng et al., 2014). The full-length CDS of CmPYL7 was cloned into the pCAMBIA1300-MYC vector, using BamHI (5′) and SalI (3′) restriction sites and yielding MYC-CmPYL7. The full-length CDS of CmPP2C24-like was cloned into the pCAMBIA1300-3xflag vector using BamHI (5′) and SalI (3′) restriction sites to yield Flag-CmPP2C24-like. The reconstructed vectors were co-transformed into Agrobacterium GV3101 competent cells using the freeze–thaw protocol, and then Agrobacterium cells were infiltrated into tobacco leaves. Tobacco plants were cultivated at 24°C for 72 h for protein expression. For protein extraction, the leaves were collected and homogenized in extraction buffer (50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM DTT, 1 mM PMSF, 2 mM EDTA, 0.1% Triton X-100, and 1 x protease inhibitor cocktail) and centrifuged at 12 000 × g for 15 min at 4°C. The supernatant was incubated with 50 μL anti-Flag agarose (Sigma-Aldrich) for 2 h at 4°C. The agarose samples were then washed five times with extraction buffer, and immunoprecipitation products were detected via immunoblot analysis. Anti-Myc (Sigma-Aldrich) or anti-FLAG (Sigma-Aldrich) was used at 1:5,000 dilutions, and the chemiluminescence signal was detected by autoradiography. Primers used are listed in Table S3.
2.13 Bimolecular fluorescence complementation (BiFC) assay
The BiFC assays were performed as described previously (Walter et al., 2004). The full-length CDS of CmPYL7 was cloned into the pSPYNE vector, and the full-length coding sequence of CmPP2C24-like was cloned into the pSPYCE vector. The reconstructed vectors were co-transformed into Agrobacterium GV3101, and the Agrobacterium cells were infiltrated into tobacco leaves. Tobacco plants were cultivated at 24°C for 72 h to measure protein expression. Fluorescent signals were observed and imaged using a confocal laser scanning microscope (TCS SP5II, Leica). Primers used are listed in Table S3.
2.14 Statistical analysis
Data were collected and analyzed using Excel 2021 and SPSS 18.0, and graphs were generated using Origin 2021, Photoshop 2021, and Illustrator 2020. Significance was tested using Duncan's multiple range test with p < 0.05 or p < 0.01.
3 RESULTS
3.1 CmPYL7 positively participates in the ABA-mediated cold response
We previously discovered that CmPYL7 is significantly upregulated in oriental melon seedlings under cold stress. In this study, two melon cultivars, cold-tolerant No.330 and cold-sensitive No.410, were subjected to cold stress, and the expression patterns of CmPYL7 in their leaves were analyzed. As shown in Figure 1A, the expression level of CmPYL7 initially increased and then decreased in both No.330 and No.410. However, the expression levels of CmPYL7 were approximately 10 times higher in No.330 than in No.410. CmPYL7 expression increased approximately 300-, 550-, 400, and 200 times at the various time points (3, 6, 12, and 24 h) after cold treatment in No.330. However, it increased approximately 30-, 60-, 20, and 20 times at the corresponding time points in No.410. The effect of ABA on CmPYL7 was also investigated (Figure 1B). The exogenous application of ABA further promoted the expression of CmPYL7 and the application of Flu, an ABA inhibitor, restricted the expression of CmPYL7 from increasing to the level of that in the cold treatment in both cultivars.

3.2 Silenced CmPYL7 reduces the cold tolerance of No.330
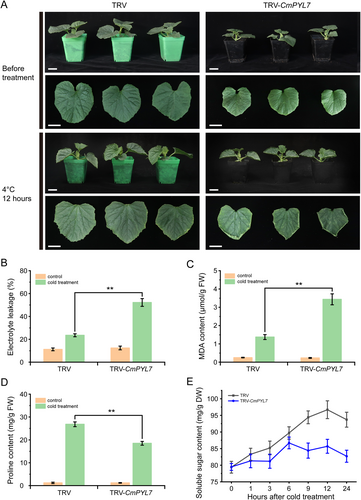
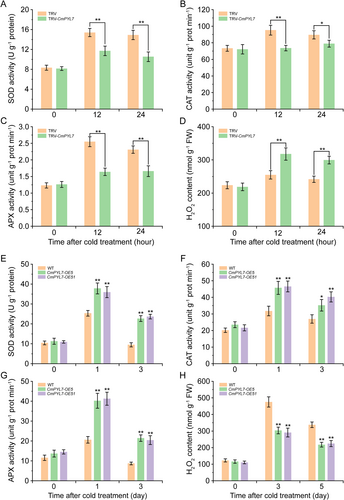
Next, we transiently silenced CmPYL7 in cold-tolerant No.330 seedlings and evaluated the cold tolerance of the silenced plants. The silencing efficiency is shown in Figure S1A. After 12 h, the leaves of TRV-CmPYL7 seedlings exhibited severe damage and wilting, whereas there was no noticeable change in the control seedlings. Stress-related physiological indices were measured to evaluate damage levels. Under normal conditions, electrolyte leakage and MDA, proline, and soluble sugar content in TRV-CmPYL7 seedlings were not different from those in the control. However, electrolyte leakage and MDA content in TRV-CmPYL7 seedlings were significantly higher than in the control after 12 h. Conversely, the proline and soluble sugar contents in TRV-CmPYL7 seedlings were significantly lower than those in the control. The activities of three enzymes involved in the antioxidant enzymes (SOD, CAT, and APX) and H2O2 content were measured in TRV-CmPYL7 seedlings. The results were consistent with the phenotypes exhibited. When exposed to cold stress, the activities of SOD, CAT, and APX in TRV-CmPYL7 seedlings were significantly lower than those in the control, resulting in excessive accumulation of H2O2 (Figure 4A-D). These findings indicated that silencing CmPYL7 can significantly reduce the cold tolerance of seedling No.330.
3.3 Overexpressed CmPYL7 enhances the cold tolerance of Arabidopsis transgenic plants
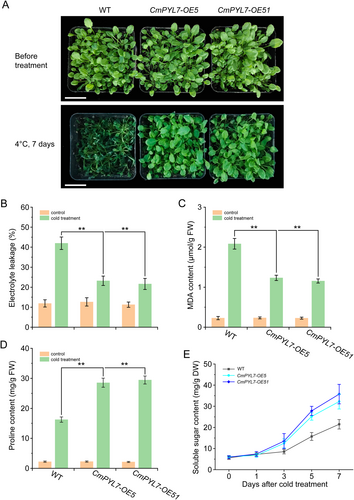
CmPYL7 was overexpressed in Arabidopsis Col-0 (WT) and two transgenic lines were obtained, CmPYL7-OE5 and -OE51. In those lines, CmPYL7 could be detected whereas it was not detected in WT (Figure S1B). The transgenic lines were subsequently exposed to cold stress for 7 d. After 7 d of cold stress, the WT plants exhibited cold damage to the leaves and wilting, whereas the CmPYL7-OE lines showed only mild damage (Figure 3A). Stress-related physiological indices were measured. Under normal cultivation conditions, no differences were observed between the CmPYL7-OE lines and WT plants; however, electrolyte leakage and MDA content of CmPYL7-OE5 and -OE51 plants were significantly lower than those of WT plants after 7 d of cold treatment (Figure 3B, C), whereas proline and soluble sugar content of CmPYL7-OE5 and -OE51 were significantly higher than those of WT plants (Figure 3D, E). In addition, the activities of SOD, CAT, APX, and H2O2 were measured in CmPYL7-OE transgenic lines. CmPYL7-OE transgenic lines significantly enhanced the activities of SOD, CAT, and APX and reduced the accumulation of H2O2 (Figure 4E-H). These results suggest that CmPYL7 effectively enhances cold tolerance in Arabidopsis transgenic lines.
3.4 CmPYL7 interacts with CmPP2C24-like which negatively responds to cold stress
A yeast library was constructed and Y2H library screening was performed to investigate the downstream regulatory function of CmPYL7. Two PP2C members, CmPP2C24-like (MELO3C020601.2) and CmPP2C77-like (MELO3C011469.2), were found to interact with CmPYL7 (Table S4). Phylogenetic analysis of the two CmPP2Cs and the AtPP2C family members of Arabidopsis thaliana revealed that CmPP2C24-like and CmPP2C77-like belong to subfamily A PP2Cs (Figure S2). The expression levels of CmPP2C24-like and CmPP2C77-like genes were investigated in No.330 leaves at various time points after cold treatment. CmPP2C24-like expression decreased by half at 12 and 24 h after cold treatment compared to at 0 h (Figure 5A), while CmPP2C77-like exhibited little change (Figure 5B). Therefore, CmPP2C24-like was selected for further investigation.
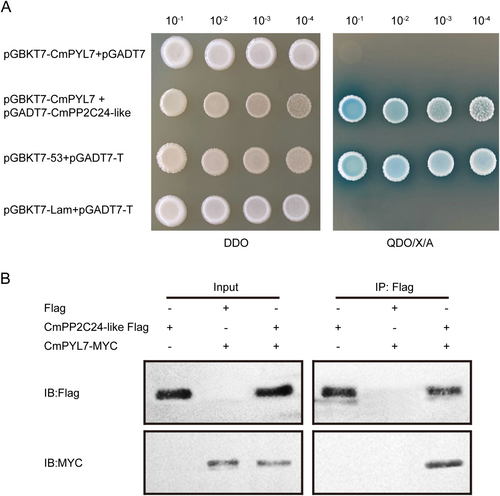
This interaction was first verified using the Y2H experiment. The pGBKT7-CmPYL7 and pGADT7-CmPP2C24-like co-transformed bacteria could grow on a QDO/X/A plate (Figure 6A), demonstrating the interaction between CmPYL7 and CmPP2C24-like in vitro. A co-IP experiment was conducted and MYC-CmPYL7 and FLAG-CmPP2C24-like co-transformed in tobacco leaves could be co-precipitated with the FLAG antibody (Figure 6B), further confirming the interaction between CmPYL7 and CmPP2C24-like in vivo.
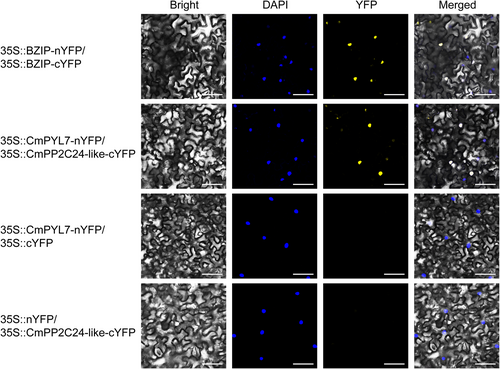
The interaction between the CmPYL7 and CmPP2C24-like proteins was further examined using BiFC. CmPYL7-nYFP and CmPP2C24-like-cYFP were co-expressed or individually expressed in tobacco leaves for 72 h and observed using a Leica confocal laser scanning microscope. As shown in Figure 7, no YFP fluorescence signal was detected when CmPYL7-nYFP or CmPP2C24-like-cYFP were introduced individually; however, YFP fluorescence signals were observed when CmPYL7-nYFP was co-expressed with CmPP2C24-like-cYFp and localized in the nucleus. Therefore, an interaction between CmPYL7 and CmPP2C24-like was confirmed in vivo.
3.5 CmPP2C24-like negatively regulates the cold tolerance of No.330
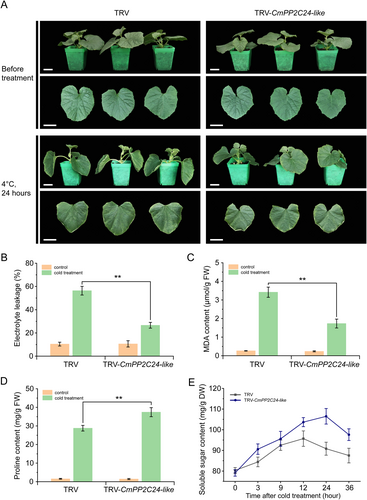
CmPP2C24-like was transiently silenced using the VIGS method to investigate the function of CmPP2C24-like in cold tolerance. More than 45% downregulation was observed in TRV-CmPP2C24-like seedlings compared to that in TRV (Figure S3), confirming the silencing efficiency. There was no significant difference in the phenotype between TRV-CmPP2C24-like and control seedlings. However, after 24 h cold treatment, the leaves of the control seedlings suffered severe damage, whereas the TRV-CmPP2C24-like seedlings exhibited no obvious phenotypic changes (Figure 8A). The four stress-related indices were re-measured. There was no difference between TRV-CmPP2C24-like and control seedlings under normal conditions, whereas the electrolyte leakage and MDA content of TRV-CmPP2C24-like seedlings were significantly lower than those of the control after 24 h of cold treatement (Figure 8B, C), and the proline and soluble sugar contents were significantly higher in TRV-CmPP2C24-like seedlings than in control seedlings (Figure 8D, E). These results indicated that silencing CmPP2C24-like gene can improve the cold tolerance of oriental melons.
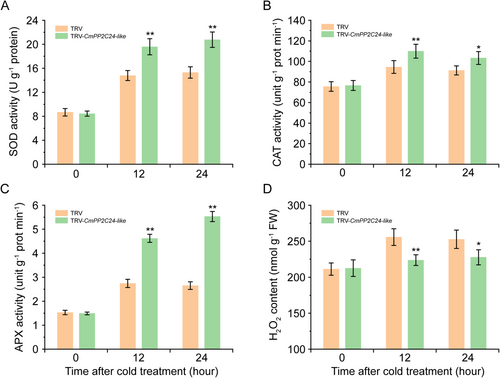
The activities of SOD, CAT, APX, and H2O2 were also detected in TRV and TRV-CmPP2C24-like seedlings after cold treatment. The results revealed that the activities of antioxidant enzymes were further promoted in TRV-CmPP2C24-like seedlings and were significantly higher than those in TRV seedlings under cold stress (Figure 9A-C). H2O2 contents in TRV-CmPP2C24-like seedlings were significantly lower than those in TRV seedlings at 12 h and 24 h after cold treatment (Figure 9D).
4 DISCUSSION
4.1 CmPYL7 positively regulates the cold tolerance of oriental melon
Cold stress becomes much more prominent during melon production at high latitudes and may lead to reduced production and quality (Korkmaz and Dufault, 2003, 2004). ABA is an important stress-regulatory signaling molecule in plants, and their receptors (PYLs) have recently been studied for their function in regulating cold tolerance. Certain members of the PYL family exhibit positive responses to cold stress. For instance, in grape and apple PYL families, 6 PYL members responded positively to cold stress (Hou et al., 2020; Zhao et al., 2020), whereas 5 out of 12 CmPYL family genes in melon responded positively to cold stress, with CmPYL7 showing the strongest response (in press). This suggests that certain PYL members within a family actively participate in the response to cold injuries. Expression of rice OsPYL3 and grape VaPYL4 and VaPYL9 can be significantly upregulated by cold injury (Lenka et al., 2018; Nai et al., 2022; Ren et al., 2022). As research progresses, there are increasing reports on the role of PYLs in the regulation of cold tolerance.
Recent studies on PYLs have shown that they play crucial roles in ABA-mediated cold tolerance, as verified using transgenic methods (Lenka et al., 2018; Nai et al., 2022; Zhang et al., 2019). CmPYL7-silenced oriental melon plants (TRV-CmPYL7) and CmPYL7-overexpressed Arabidopsis lines (CmPYL7-OE5 and -OE51) were generated to evaluate the cold tolerance and preliminarily reveal the function of CmPYL7 in cold tolerance. After cold treatment, several characteristic cold injury indexes, H2O2 content, and antioxidant enzyme activities were determined. The results showed that CmPYL7-silenced seedlings had higher electrolyte leakage and MDA content and lower proline and soluble sugar content (Figure 2), whereas the CmPYL7-OE lines had lower electrolyte leakage and MDA content and higher proline and soluble sugar content (Figure 3) compared to WT, demonstrating CmPYL7 was positively correlated with cold tolerance. ABA triggers the synthesis of downstream transcription factors (e.g., WRKY and MYC2) for soluble sugar synthesis (such as galactinol and raffinose oligosaccharides), which helps alleviate oxidative stress by inhibiting ROS (Saddhe et al., 2020). Overexpression of PtPYRL1 or PtPYRL5 in poplar significantly increased the growth potential, promoted proline accumulation, and improved tolerance to drought and cold stress in transgenic plants (Yu et al., 2017). Similar results were observed where VaPYL9 could significantly decrease cold damage in transgenic lines (Nai et al., 2022). Cold stress can cause excessive accumulation of ROS and oxidant damage, and cold-tolerant cultivars usually have a strong antioxidant system (Distelbarth et al., 2013). The overexpression of genes encoding antioxidant enzymes confers cold tolerance in transgenic lines (Shafi et al., 2014). We observed that silencing CmPYL7 significantly withheld the increase in antioxidant enzymes (SOD, CAT, and APX) and resulted in higher H2O2 accumulation in TRV-CmPYL7 seedlings than in the control, consequently leading to decreased cold tolerance (Figures 2 and 4). Overexpression of CmPYL7 significantly increased the levels of antioxidant enzymes (SOD, CAT, and APX) and resulted in lower H2O2 accumulation in CmPYL7-OE lines than in the control, consequently leading to improved cold tolerance (Figures 3 and 4). Similarly, there is an enhanced antioxidant system and ROS scavenging ability in heterologously overexpressed VaPYLs transgenic plants and improved cold tolerance of AtRCARs, OsPYLs, and VaPYLs transgenic plants (Lenka et al., 2018; Nai et al., 2022; Ren et al., 2022; Verma et al., 2019; Zhang et al., 2019).
In addition, we found that the expression pattern of CmPYL7 positively correlated with that of ABA (Figure 1), indicating CmPYL7 may act as an important ABA receptor. Overexpression of VaPYL9 improves ABA content and the ABA/IAA ratio in tomato transgenic lines (Nai et al., 2022), and overexpression of OsPYL3 or AtRCAR12 significantly improves hypersensitivity to ABA and enhances cold tolerance in transgenic plants (Lenka et al., 2018; Zhang et al., 2019). Additionally, transcription factors (CBFs), antioxidant enzyme genes (SOD), and cold-responsive genes (COR15A) are expressed in PYL-overexpressing transgenic lines (Nai et al., 2022; Ren et al., 2022; Zhang et al., 2019). Therefore, our results suggested that CmPYL7 plays a crucial role in regulating the cold response via the ABA-signaling pathway.
4.2 CmPYL7 regulates cold tolerance by interacting with CmPP2C24-like
The ABA-PYL-binding dimer inactivates the negative signaling regulator PP2Cs in the classical ABA-signaling pathway, leading to the activation of SnRK2s through phosphorylation (Wang and Zhang, 2014). Subfamily A PP2Cs are commonly used as negative regulators of cold tolerance in plants. Two PP2C members, CmPP2C24-like and CmPP2C77-like, were identified using Y2H library screening (Table S4) when investigating the downstream interaction factors of CmPYL7. Phylogenetic analysis revealed that they belong to subfamily A PP2Cs (Figure S2), suggesting that they may function downstream of CmPYL7 in the ABA-signaling pathway. RT-qPCR analysis revealed that CmPP2C24-like was downregulated after cold treatment, whereas little change was observed in CmPP2C77-like (Figure 5). Thus, CmPP2C24-like was selected for further analysis. The interaction between CmPYL7 and CmPP2C24-like was confirmed using Y2H, co-IP, and BiFC assays, revealing that the interaction was localized to the nucleus (Figures 6 and 7). Previous studies demonstrated that PYL primarily functions in the nucleus and cytoplasm (Fidler et al., 2022). PYL8 is generally distributed in the cell membrane and nucleus; however, it mainly accumulates in the nucleus under ABA stimulation (Lee et al., 2015). Belda-Palazon et al. (2018) further validated that PYL8 recognizes ABA and reduces its ubiquitination levels, thereby promoting its accumulation in the nucleus. In addition, PYL8/RCAR3 in Arabidopsis was found to interact with the FsPP2C1 of Fagus sylvatica in the nucleus, positively regulating ABA signal-mediated seed germination and stress responses (Saavedra et al. 2010). Grape VaPYL4 is primarily localized in the nucleus (Ren et al., 2022). These pieces of evidence further suggest that CmPYL7 may serve as a crucial ABA receptor and play a significant role in positively regulating ABA stress signals.
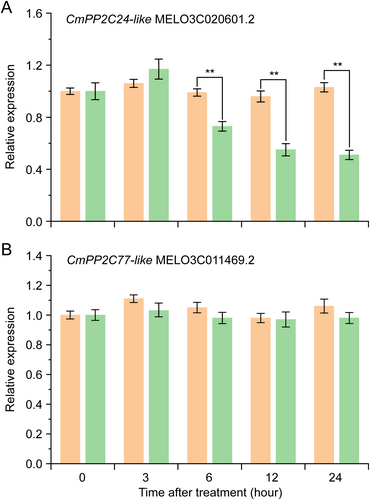
Subsequent transient silencing of CmPP2C24-like in No.330 seedlings significantly enhanced cold tolerance, indicating its negative regulatory function in cold tolerance in oriental melon. Similarly, antisense inhibition of AtPP2CA or PdPP2C can accelerate cold adaptation in transgenic plants, thereby improving their tolerance to cold or freezing stress (Ajab et al., 2021; Tähtiharju and Palva, 2001). OsPP2C27 negatively regulates the OsMAPK3-OsbHLH002-OsTPP1 signaling pathway in rice, leading to increased expression levels of OsTPP1 and OsDREBs and a significantly higher survival rate after chilling treatment in OsPP2C27-silenced plants than in control plants. Conversely, OsPP2C27-overexpressing plants exhibited the opposite results (Xia et al., 2021). A study on maize revealed that the overexpression of ZmPP2C reduced the expression of stress-related genes (RD29A, P5CS1, etc.) and ABA-related genes (ABI1 and ABI2) in Arabidopsis transgenic plants, resulting in decreased sensitivity to ABA and reduced tolerance to salt and drought stress (Liu et al., 2009). However, the overexpression of ZmPP2C2 and ZmPP2C55 enhances the antioxidant system and consequently improves cold resistance (Hu et al., 2010; Zhang et al., 2022). These contrasting results may be due to the different ZmPP2Cs subfamilies. We found that CmPP2C24-like was classified into the subfamily A PP2Cs, and silencing CmPP2C24-like gene enhanced cold tolerance by enhancing the antioxidant system, suggesting an important role of CmPP2C24-like in the ABA-signaling pathway (Figure 9). Previous studies have indicated that subfamily A PP2Cs are key negative regulators of SnRKs in the ABA-signaling pathway (Krzywinska et al., 2022), whereas other subfamily members may cooperate with ABA signaling for positive regulatory effects. For instance, SlPP2C, a subfamily F member in tomato, can be upregulated by ABA signaling but does not interact with any SlPYLs. Silencing SlPP2C significantly delays the aging process in RNAi plants (Jiang et al., 2023).
5 CONCLUSION
CmPYL7 was strongly induced by cold stress in oriental melons, and its expression profile positively correlated with ABA treatment. CmPYL7 positively regulated the activities of antioxidant enzymes in oriental melon and Arabidopsis, thereby influencing cold tolerance. CmPP2C24-like interacted with CmPYL7 in the nucleus and positively regulated cold tolerance by improving the antioxidant system of TRV-CmPP2C24-like seedlings. These results suggested that CmPYL7 positively regulates cold tolerance by interacting with CmPP2C24-like proteins in oriental melon.
AUTHOR CONTRIBUTIONS
Wei Liu and Jiawang Zhang: experiments design. Lili Zhang and Shilei Liu: cold treatment, ABA, and Flu treatments. Wei Liu and Yanling Lv: gene silencing and overexpressing analysis. Wei Liu and Yun Jiang: Y2H, co-IP and BiFC assays, data analysis, photographs generation, manuscript preparation. Ming He and Jiawang Zhang: revision.
FUNDING INFORMATION
This work was supported by the PhD Start-up Fund of Liaoning Province (2022-BS-045), the President's Fund of the Liaoning Academy of Agricultural Sciences (2022BS0702), the Seed Industry Innovation Project of Shenyang (22–318–2-14), and the China Agriculture Research System (CARS-25).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




