Multifaceted growth promotion and biocontrol of Agroathelia rolfsii and induction of defense mechanism by Bacillus amyloliquefaciens SS-CR10 on chilli
Abstract
Plant-growth-promoting (PGP) endophytic bacteria are beneficial microorganisms that can help plants withstand biotic stress caused by fungal phytopathogens. In the present study, 78 endophytic bacterial isolates were isolated from chilli (Capsicum annuum L.). A potent isolate with several PGP attributes and better inhibition against Agroathelia rolfsii was selected and identified as Bacillus amyloliquefaciens using 16S rDNA homologies. Phosphate solubilization (65.9 μg ml−1), nitrogen fixation, production of IAA (9.77 to 24.45 μg ml−1), ammonia, ACC deaminase, siderophore, and hydrolytic enzymes were among the PGP traits shown by the strain SS_CR10. Furthermore, the strain demonstrated the ability to colonize roots. It significantly improved the plant's developmental traits, such as fresh and dry weight, photosynthetic pigments, and root and shoot length. LC–MS analysis and PCR amplification of lipopeptide genes also showed that surfactin, iturin, and bacilysin lipopeptides were present. Following treatments with lipopeptide extract and bacterial suspension, A. rolfsii mycelia showed severe deformation and cell death, as seen by live dead staining by fluorescent microscopy and scanning electron microscopy (SEM). Finally, the upregulation of defense-related genes CaPR1, CaPR2, and CaPR4 after bacterial treatment confirmed the induction of systemic resistance. In conclusion, this study shows how strain SS_CR10 might be useful for promoting plant growth in chilli and controlling A. rolfsii in an eco-friendly way, which would protect the health of the soil.
1 INTRODUCTION
Chilli (Capsicum annuum L.) is one of the most widely grown and consumed vegetable cum spice crops, with high nutritional and medicinal value. It is a good source of vitamins such as A, B, C, E, and K, as well as dietary fibers, phenolics, and carotenoids (Bal et al., 2022). Chilli, with its ethnopharmacological properties, contains capsaicinoid, a compound with anti-cancer, anti-oxidant, and anti-obesity properties as well as body temperature regulatory effects (Meghvansi et al., 2010). However, several pathogens affect the growth and development of chilli, leading to an overall reduction in yield. The damping-off disease caused by Pythium spp., Fusarium spp., wilting caused by Verticillium dahliae, Fusarium solani, root rot caused by Rhizoctonia solani, fruit rot caused by Colletotrichum spp., Phytophthora blight caused by Phytophthora capsici, and leaf spot caused by Cercospora capsici are among the major fungal diseases of chilli (Saxena et al., 2016). In addition, southern blight and stem rot caused by the soil-borne pathogen A. rolfsii has also been a major concern in recent years (Sharf et al., 2021). Agroathelia rolfsii (aka Sclerotium rolfsii, Athelia rolfsii) is known to cause southern blight, foot rot, leaf blight, and rot on several commercially important crop plants (tomato, brinjal, cabbage, China aster, chrysanthemum, common beans, maize, and many more) and adversely affect the overall productivity (Mahadevakumar et al., 2016, 2018, 2023; Tejaswini et al., 2022, 2023). Currently, this soil-borne pathogen has been found to infect approximately 500 different host plants worldwide (Mullen, 2001; Punja, 1985; Mahadevakumar et al., 2023). The fungi penetrate the collar region of the plant and cause lesions on the stem, typically close to the soil line. According to Abeysinghe (2009), the lesions tend to rapidly spread along the stem, causing the stem to become girdled and the plant to permanently wilt.
Plant growth-promoting (PGP) endophytic bacteria are symbiotically colonized bacteria that do not cause any harm to their hosts. The endophytic bacterium can actively enter the host plant by producing hydrolytic enzymes. Colonization of the endophytic microbial population stimulates plant growth and stress tolerance. Plants become more prone to biotic and abiotic stress conditions without the colonization of beneficial microorganisms. PGP endophytic bacteria promote plant growth by different mechanisms, which include phosphate solubilization, production of indole acetic acid (IAA), atmospheric nitrogen fixation, 1-aminocyclopropane-1-carboxylate (ACC) deaminase production, and siderophore production (Liu et al., 2017). PGP endophytic bacteria employ various mechanisms, such as antagonism, antibiotic production, nutrient check through competition, siderophore production, hydrolytic enzyme production, and induction of systemic resistance, to suppress plant pathogens and maintain the health of host plants (Elnahal et al., 2022).
Bacillus species have been reported to improve plant growth and inhibit various phytopathogens in different crop plants. Owing to their great potential for inhibitory activity against various phytopathogenic fungi and growth-promoting activities, they have been widely used in agricultural field applications (Fira et al., 2018). More recently, studies have revealed the growth-promoting and biocontrol efficacy of Bacillus cereus YN917 in rice (Zhou et al., 2021). B. subtilis ZD01 limits the development of potato early blight, causing mycelial and conidial deformations in Alternaria solani (Zhang et al., 2022). The antifungal lipopeptides produced by Bacillus spp. are the major inhibitory compounds that affect cell membrane permeability and induce systemic resistance in plants. Bacillus spp. produce antifungal lipopeptides, including surfactin, iturin, fengycin, and bacillomycin (Gond et al., 2015). Among them, fengycin shows strong antifungal properties against phytopathogens, specifically filamentous fungi. Surfactins have low antifungal properties but are high in antibacterial activities. Iturins have strong antifungal activities against some fungi, such as Botrytis cinerea, Fusarium graminearum, Sclerotinia sclerotiorum, Phytophthora infestans, and Candia albicans (Calvo et al., 2019; Gong et al., 2015; Lei et al., 2019; Wang et al., 2022; Kaushal et al., 2017). Studies have found that lipopeptides produced by Bacillus amyloliquefaciens YN201732 are the main active ingredients against the fungal phytopathogen Erysiphe cichoracearum, which causes powdery mildew in tobacco (Jiao et al., 2021). Lipopeptides from Bacillus amyloliquefaciens FZB42 are necessary for root colonization, biofilm formation, inducing resistance, and successful control of phytopathogens (Chowdhury et al., 2015).
Induction of systemic resistance (ISR) is a novel approach to controlling phytopathogens. Plants have evolved with a variety of defense mechanisms that enable them to avoid tissue damage by the pathogen and survive adverse environmental conditions (Kamle et al., 2020). Several Bacillus spp. have been investigated for their role in ISR by triggering cellular defense responses, such as increased production of defense-related enzymes, cell wall thickening, and callose deposition. ISR, triggered by beneficial microorganisms such as plant growth-promoting endophytic bacteria, improves plant's immunity by regulating jasmonate (JA) and ethylene-dependent signaling pathways. The increased expression of genes related to defense enzymes such as superoxide dismutase (SOD), peroxidase (POD), polyphenol oxidase (PPO), and phenylalanine ammonia-lyase (PAL) has been investigated in chickpea, tomato, and sugarcane (Mageshwaran et al., 2022; Shu et al., 2021; Singh et al., 2021). This study aims to isolate endophytic bacteria from chilli plant tissues and investigate their plant growth promotion and biocontrol characteristics against A. rolfsii in vitro and in vivo, as well as the mechanism of induced systemic resistance against the blight disease of chilli.
2 MATERIALS AND METHODS
2.1 Isolation of endophytic bacteria
Endophytic bacteria were isolated from healthy chilli plants collected from several agricultural fields in the Mandya districts of Karnataka, India, across the Kaveri River basin. Samples collected from several sites were combined, and the cleaned leaf, root, and stem were randomly chosen and chopped into smaller pieces of 0.5 cm2. Samples were surface sterilized by rinsing in 70% (v/v) ethyl alcohol for 2 min, 2% NaOCl for 3 min, 95% (v/v) ethyl alcohol for 1 min, followed by sterile distilled water (SDW) for the final three rounds. Aliquots of water from the final rinsing were spread on nutrient agar (NA) plates to confirm the efficacy of the surface sterilization process. After a 24 h incubation period at 27 ± 2°C, plates were observed for the existence of colonies along the tissue boundaries. Selected cultures were purified by streaking on nutrient agar plates. Cultures were stored and preserved at −20°C as glycerol stocks (Liotti et al., 2018).
2.2 Isolation of pathogen and pathogenicity test
The chilli plants infected with southern blight were collected from the farmer's fields and used for the isolation of the pathogen (Figure S1). Infected stem and root sections (4–5 cm in length) of plants were rinsed thoroughly with tap water and then cut into smaller pieces of about 1 cm in length with the help of a sterile scalpel. After surface sterilization with 2% sodium hypochlorite (NaOCl) for 5 min, stem and root samples were rinsed thoroughly in SDW to remove the traces of NaOCl, dried on sterilized filter paper, and placed onto potato dextrose agar (PDA) medium amended with streptomycin sulphate (200 mg l−1) (w/v). Plates were incubated for 4–5 days at room temperature. The pathogenicity of the isolates was confirmed on chilli plants, and the pathogen was re-isolated from the artificially inoculated plant tissues, fulfilling the Koch postulates. The most virulent isolate was selected for the in vitro and in vivo experiments (Tejaswini et al., 2022).
2.3 Molecular identification of endophytic bacteria and phytopathogen
Genomic DNA was isolated using the CTAB method from fungal phytopathogens and endophytic bacteria for molecular identification. Internal transcribed regions (ITS) of fungi were amplified using ITS1/ITS4 primers (White et al., 1990), while the 16S rDNA sequence of endophytic bacteria was amplified using 27F/1429R forward and reverse primers (Kumar et al., 2021a). A 30 μL reaction mixture was used for the PCR reaction, which included 11 μL of sterile deionized water, 2.0 μL of each primer (100 μmol), 2.0 μL of 100 ng gDNA as a template, and 15 μL of Taq DNA polymerase Master mix RED (Amplicon®). The PCR products were analyzed by 1% (w/v) agarose gel electrophoresis, and the gel image was documented using a gel documentation system (Bio-Rad). The amplified products were forwarded to Barcode Bioscience (Bangalore) for sequencing. The obtained sequences were aligned and identified using BLASTn NCBI (http://blast.ncbi.nlm.nih.gov). The sequences that have close matches were identified as the same species and aligned using Clustal W with outgroups. The phylogenetic tree was constructed to reveal the phylogeny of the endophytic strain and fungal phytopathogen using MEGA11 software (Adhikari and Pandey, 2020).
2.4 In-vitro evaluation of PGP and antifungal activities
The isolated bacterial endophytes were tested for plant growth-promoting traits such as nitrogen fixation, inorganic phosphate solubilization, IAA production, siderophore production, ACC deaminase activity, ammonia production, and antifungal activity against the southern blight pathogen A. rolfsii. An isolate with superior plant growth-promoting traits and antifungal activity against the fungal phytopathogen was chosen for further investigation of its potential plant growth-promoting and protective capabilities.
2.4.1 Phosphate solubilization
The ability of the selected endophytic strain SS_CR10 to solubilize inorganic phosphorus tricalcium phosphate (TCP) has been evaluated in triplicate. Overnight-grown culture (OD620nm - 0.5) was spot inoculated on Pikovskaya (PKV) agar media with TCP (5 g l−1) as the sole source of phosphate. The plates were incubated at 28°C for 4 days. The typical transparent halo zones surrounding the colonies were monitored for phosphate solubilization activity, and the solubilization index (SI) was calculated. SI = (Halo zone + diameter of the colony)/diameter of the colony. For quantitative evaluation of the day-wise kinetics of phosphate solubilization by SS_CR10, the bacterial isolate was grown in PKV liquid broth. A triplicate Erlenmeyer flask containing 100 mL of the freshly prepared PKV broth media was inoculated with 100 μL of overnight grown culture (OD620nm – 0.5) and incubated in a shaking incubator at 180 rpm for 7 days at 28°C. The soluble phosphate in the culture supernatant (CS) was determined using the method described by Fiske and Subbarow (1925). In a test tube, 1 mL of CS was mixed with 0.5 mL of 10% (w/v) trichloroacetic acid (TCA). To the mixture, 4 mL of colour reagent (3 M H2SO4, 10% (w/v) ascorbic acid, 2.5% (w/v) ammonium molybdate, and distilled water) was added in a ratio of 1:1:1:2. The test tubes were kept for incubation at room temperature for 15 min. The measure of absorbance was recorded after the development of a blue colour using a spectrophotometer at 820 nm. The standard curve constructed using KH2PO4 was used to quantify the amount of soluble phosphate produced by the isolate per ml of PKV medium (μg ml−1) over time intervals (Adeleke et al., 2021).
2.4.2 Indole-3-acetic acid (IAA) production
The production of IAA by the strain SS_CR10 was determined by culturing the bacterial strains in nutrient broth supplemented with L-tryptophan, a precursor of IAA. Briefly, the SS_CR10 strain was inoculated in nutrient broth media amended with 1% L-tryptophan and incubated for 3 days in a shaking incubator at 150 rpm. The CS obtained by centrifugation at 4°C in 8000 g for 20 min was subsequently treated with Salkowski reagent (0.5 M FeCl3 1 mL, 95% sulphuric acid 30 mL, distilled water 50 mL) (Tarroum et al., 2022). The change in colour of the supernatant to pink and its intensity were visually measured. For kinetics of IAA production, the isolate was inoculated in nutrient broth media amended with different concentrations of L-tryptophan (0, 1, 3, 5 mg ml−1) and incubated until it reached an OD600 of 0.6–0.8 at 28°C in a shaking incubator at 160 rpm. The CS and Salkowski reagents were mixed in a 1:2 ratio and incubated in the dark for 30 min at room temperature. The absorbance of the pink colour developed in a reaction mixture was estimated at 530 nm. The estimation of IAA from the reaction mixture [micrograms (μg) of IAA produced per mL of growth media by the bacterium] was done using a standard graph obtained by diluting pure IAA (Hassan, 2017; Glickmann and Dessaux, 1995).
2.4.3 Nitrogen fixation and ammonia production
The qualitative estimation of nitrogen fixation ability by the bacterial strain SS_CR10 was conducted by streak inoculation onto the nitrogen-free Ashbey media (Zhang et al., 2012). The plate was then incubated at 28°C for 48 h, and bacterial growth was monitored. The appearance of bacterial growth on media was considered positive for nitrogen fixation. Further PCR amplification of the target fragment within the nitrogenase reductase protein gene (nifH, ~ 760 bp) was carried out for the confirmation of the nitrogen fixation ability of the bacterial strain. The results were visualized using gel electrophoresis and documented using the gel documentation system.
Additionally, the bacterial production of ammonia was confirmed by inoculating 100 μL of bacterial suspension into triplicate test tubes containing 10 mL of peptone water. The tubes were incubated at 28°C with shaking at 150 rpm for 72 h. The development of a brown to pale yellow colour after the addition of Nessler's reagent to CS was indicated as a positive test for the production of ammonia (Anwar et al., 2016).
2.4.4 ACC deaminase activity
The bacterial isolate was assessed for ACC deaminase production by the methodology of Dworkin and Foster (1958). The isolate was streak inoculated on DF (Dworkin and Foster) salt minimal media amended with 3 mmol ACC as the sole source of nitrogen. The appearance of bacterial growth after 48 h of incubation on an ACC-containing plate was considered positive for ACC deaminase production. Further confirmation of ACC deaminase activity was carried out by the PCR amplification of the ACC deaminase gene (acds ~ 860 bp). Gel electrophoresis was carried out to identify and analyze the amplified product, which was visualized and documented using the Gel documentation system (Kumar et al., 2021a).
2.4.5 Siderophore activity
Siderophore production by the strain SS_CR10 was confirmed by a blue agar chrome azurol S (CAS) assay (Louden et al., 2011). Bacterial culture was spot-inoculated on triplicate plates containing chrome azurol and hexadecyl tri-methyl ammonium bromide (HDTMA) indicators. The change in colour of CAS agar from blue to colourless halo indicated the production of siderophore. The halo, or area of colour conversion around the bacterial colony, was measured after 5 days of incubation at 28°C (Passari et al., 2016).
2.4.6 Hydrolytic enzyme production
For qualitative evaluation of cellulase activity, bacterial strain SS_CR10 was spot-inoculated on carboxymethyl cellulose (CMC) agar plates supplemented with 1% CMC and 0.5% tryptone. After the plates were incubated at 28°C for 48 h, each plate was flooded with 0.5% (w/v) congo red solution for 20 min (Yang et al., 2017). The appearance of a clear zone around the colony confirms the bacterial ability to hydrolyze cellulose. The non-inoculated plates were used as a control.
Protease activity was determined by spot inoculation on skim milk Luria Bertani (LB) agar medium supplemented with 10% (w/v) sterile skim milk powder. The appearance of a clear zone around the colony after 2–5 days of incubation at 28°C indicates a positive result for protease production.
Amylase activity was determined by spot inoculation on soluble starch agar media supplemented with 1% (w/v) soluble starch. Plates were incubated at 28°C for 4 days (Padda et al., 2017). The appearance of a clear zone around the colony after incubation indicates a positive result for enzyme activity. The addition of Lugol's iodine solution and 70% ethanol for 10 min confirms the activity. The non-inoculated plate served as a control.
2.5 Re-inoculation experiment for seedling growth measurements
Re-inoculation experiments were carried out using the methodology described by Kumar et al. (2021b). Sterilized mixed substrate (cocopeat, perlite, and sand) growth media in two different ratios (2:2:1 and 2:1:1) were prepared in magenta boxes. Further, various treatments were applied to chilli seeds: 1) completely disinfected seeds as a negative control (surface sterilized with 4% NaOCl and treated with streptomycin sulphate 100 μg ml−1 for 8 h); 2) only surface sterilized seeds with 4% NaOCl as a positive control; and 3) completely disinfected seeds re-inoculated with the endophytic bacterial strain SS_CR10 were transferred to magenta boxes containing growth media. For re-inoculation, approximately 100–150 chilli seeds were treated with 5 mL bacterial cell suspension (106–107 cfu) for 2 hours. Further, around 150 seeds were transferred to magenta boxes containing two different growth media. Treatments were done in triplicate, and each magenta box was transferred with 25 seeds. All of them have been transferred to the growth chamber for the maintenance of controlled conditions (12-h photoperiod, 28 ± 2°C temp, and 80–85% humidity). Seedling growth parameters such as root length, shoot length, dry weight, fresh weight, and photosynthetic pigments in the leaves were measured after 10 and 30 days, respectively (Kumar et al., 2021b).
2.6 Quantification of photosynthetic pigments
2.7 Visualization of root colonization by SS_CR10 using fluorescent microscopy
Root colonization of the potent endophytic bacterial strain SS_CR10 was analyzed with the help of SYTO-9 (Thermo Fisher Scientific) fluorescent dye using a fluorescent microscope. SYTO-9 stain, which stains both the live and dead bacteria present inside the root tissues of the seedlings, was mixed with the sterilized deionized water to prepare 50 μM final concentrations and incubated for 20–30 min in a dark condition at 28°C. Roots from both the negative control and treatment seedlings were collected from the magenta boxes containing different growth media and cleaned thoroughly to remove associated dirt and soil particles. By using the sterile scalpel, root samples were cut into 0.5 cm lengths, sectioned, and then transferred to slides. 20 μL of SYTO-9 solution with a 50 μmol final concentration was poured on the root surfaces and incubated for 5–10 min in dark conditions. Roots were then examined for the presence of bacteria inside the root tissues (Pal et al., 2022; Kumar et al., 2021b).
2.8 Antifungal activity against A. rolfsii
Where RC represents the radius of fungal growth (control plate without bacteria), and R represents the radius of fungal growth in the presence of bacteria.
2.9 Extraction, well diffusion, and UPLC-ESI-QTOF-MS analysis of antifungal lipopeptides
Lipopeptides were extracted using the acid precipitation method. The culture broth of SS_CR10 was centrifuged to remove bacterial cells at 6000 g for 20 min. With the concentrated HCl, the CS was acidified to pH 2 using 6 N HCl and incubated overnight at 4°C. The precipitate was collected by centrifugation at 6000 g for 20 min and extracted three times with methanol, followed by filtration with 0.45 μm PTFE membrane filters to remove larger particles and cell debris (Gond et al., 2015). The methanol was evaporated to dryness using a vacuum evaporator, and the final concentrated methanolic crude extract was stored at 4°C. Crude methanolic extracts of lipopeptide were then tested against the fungal phytopathogen A. rolfsii. For that, a mycelial block from the 7-day-old culture of the pathogen was then placed in the center of culture dishes containing freshly prepared PDA medium. Further, wells were formed around the pathogen inoculated center at a distance of 2 cm, and 30 μL of crude lipopeptide extract was loaded onto a well. The negative control was set with the solvent methanol loaded. The inhibitory activity of the crude extract was observed after 4 days of incubation at 28°C. To characterize the antifungal lipopeptide produced by the bacterial strain SS_CR10, crude lipopeptide extracts were subjected to UPLC-MS analysis. Lipopeptide extract was dissolved in 20% methanol and analyzed by Ultra-performance liquid chromatography (UPLC) (Acquity UPLC® BEH, Waters) connected with a high-definition mass spectrometer (SYNPTG2 HDMS Q-TOF, Waters). Mass spectroscopy was carried out in both positive and negative modes of electron spray ionization. The obtained data was analyzed, and molecular mass was determined using MassLynx4.1 SCN 9.16 software (Waters) (Gorai et al., 2021).
2.10 Amplification of lipopeptide genes
Bacillus amyloliquefaciens strain SS_CR10 was screened for the presence of lipopeptide genes, including surfactin (sfp), iturin A (ItuD), fengycin (FenD), bacillomycin D (BamC), iturin C (ItuC), bacilycin A (BacA), and surfactin C (srfC) using the specific primer sequences (Table S1). The PCR condition for amplification was set up as initial denaturation (5 min at 95°C), followed by 30 cycles of denaturation (1 min at 94°C), annealing (1 min at 55°C), extension (1 min at 72°C), and final extension for 10 min at 72°C (Gond et al., 2015).
2.11 Microscopic analysis of the effects of Bacillus amyloliquefaciens SS_CR10 and its lipopeptide extract on A. rolfsii
Bacillus amyloliquefaciens SS_CR10 and its lipopeptide treatment on A. rolfsii hyphae were analyzed using bright field, fluorescent, light, and scanning electron microscopy (SEM). For the light microscopic analysis (Olympus), fungal mycelia were treated with lactophenol cotton blue. For the fluorescent microscopic analysis, fluorescent stains such as DAPI (4′,6-diamidino-2-phenylindole) (Sigma-Aldrich) and Propidium Iodide (PI) (Sigma-Aldrich) were used. From the stock solutions, 25 μM concentrations of DAPI and PI in the ratio 1:1 was prepared, and 20 μL of this combination was used to stain fungal mycelia. Samples were incubated in dark conditions for about 10 min at room temperature and then examined under a fluorescent microscope (Zeiss Axio Imager A2 M, DAPI-FITC-Bright field combination).
For SEM analysis, samples were prepared following the methodology described by Wu et al. Briefly, a portion of fungal mycelia from the control and treated plates was placed on slides and fixed with 2.5% glutaraldehyde. After 24 h of incubation, it was rinsed with a 10 mM solution of phosphate buffer and then dehydrated using increasing concentrations of ethanol. After dehydration, samples were examined under SEM (EVO LS 15, Zeiss).
2.12 Seedling protection assay
Seedling protection assays were carried out using the methodology described in Kumar et al. (2021b). Magenta boxes containing the growth media cocopeat, perlite, and sand in a ratio of 2:2:1 were used. Surface sterilized chilli seeds (4% NaOCl for 5 min) were treated separately with Bacillus amyloliquefaciens (10−4 to 10−6 mL−1) and its lipopeptide extract (300 μg ml−1). After the treatment, 25 seeds from each treatment were transferred to magenta boxes containing the sterile growth medium. Seeds that were only surface sterilized were transferred to control sets and incubated in the plant growth chamber for about six days at 27 ± 2°C. On the sixth day, two infective mycelial discs of A. rolfsii were transferred to potting mixtures in magenta boxes, and the analysis was carried out after 24 h. Each experimental treatment was done in triplicate.
2.13 Plant protection assay
2.13.1 Defense-related gene expression
The effect of chilli inoculation with strain SS_CR10 was studied in the greenhouse at the University of Mysore, Karnataka, India. 20-day-old seedlings were transferred to flowerpots (10 cm in depth and 10 cm in diameter) containing a sterile soil and sand mixture (3:1) and placed in a growth chamber (25–28°C, 12/12 h photoperiod, and 75–80% relative humidity). Two plants per pot were used as experimental units (replicates), with each treatment containing five replicates. 30-day-old seedlings were treated with four treatments: CT (control treated with water), SS_CR10 (inoculated with SS_CR10), SR (inoculated with the pathogen), and SS_CR10 & SR (infected with the pathogen and inoculated with bacteria). The method for microbial treatment is as follows: 6 mL of bacterial cells at 10−8 CFU ml−1 was pipetted into 2 cm depth soil around the roots of seedlings using a sterile syringe; a mycelial mat of pathogen cultured in potato dextrose liquid medium for 7 days was selected and cut into 1 cm2 and buried at a depth of 2 cm around the roots of seedlings; and chilli seedlings treated with mycelial mat of pathogen were inoculated with 6 mL of bacterial cells at 10−8 CFU ml−1 was pipetted into 2 cm depth soil around the roots of seedlings using a sterile syringe (Singh et al., 2021; Gorai et al., 2023).
Leaf tissue from all treatments and controls was sampled after 7 DAI, and total RNA was isolated with Trizol reagent (TaKaRa) using the manufacturer's instructions. First-strand cDNA was synthesized using the cDNA synthesis kit PrimeScript™ RT Reagent Kit (TaKaRa) following the manufacturer's protocol. The oligo (dT) primers were synthesized at Eurofins (Bangalore, India). The primers used in qRT-PCR are described in Table S2. qRT-PCR reactions were performed with TB Green® Premix Ex Taq™ II (Tli RNaseH Plus;TakaRa) using manufacturer instructions in the Applied Biosystem RT-PCR machine. Expression of C. annuum's all defense gene PROTEINASE INHIBITOR II (CaPIN II), PATHOGENESIS-RELATED 1 (CaPR1), PATHOGENESIS-RELATED PROTEIN 4 (CaPR4), and β-1,3-GLUCANASE (CaPR2) previously reported to have defensive roles was calibrated using CaACT1 (GenBank Acc. No. AY572427) housekeeping gene. The relative expression of all genes was calculated with a 2¯ΔΔCt procedure (Livak and Schmittgen, 2001).
2.14 Statistical analysis
Results are the means of independent replicates expressed as the mean ± standard deviation (SD). The data was subjected to statistical analysis by the statistical tool GraphPad Prism 8.0.2 software (GraphPad). The mean differences in the growth promotion and defense gene expression of treatments and controls were analyzed by analysis of variance (ANOVA) and Tukey's HSD (honestly significant difference) test at p < 0.05.
3 RESULTS
3.1 Isolation, selection, and identification of endophytic bacteria
A total of seventy-eight endophytic bacteria were isolated from the chilli plant endosphere. The surface sterilization process was found effective by the absence of bacterial growth in the last rinse solution of the treatment. All isolates were tested for qualitative PGP activities using a plate assay, including inhibitory activity against the fungal phytopathogen A. rolfsii. Among the 78 isolates tested, SS_CR10 isolated from the root tissue showed positive results for all the PGP activity tested, with maximum inhibitory activity against A. rolfsii. Therefore, the isolated strain was selected for further evaluation of PGP activity. Morphological and biochemical characterization of SS_CR10 revealed the isolate as a Gram-positive and rod-shaped bacterium with positive results for catalase, motility test, and Voges-Proskauer test but negative results for indole, methyl red, and urease test.
3.2 Molecular identification and phylogenetic tree construction
The strain SS_CR10 was subjected to molecular identification using the NCBI database. The analysis revealed that it shared a sequence similarity of 99.93% with Bacillus amyloliquefaciens strain GIS-SCA2 (PP355697) and Bacillus amyloliquefaciens strain KDP3 (MZ396973). The fungal isolate exhibited a sequence similarity of 100% with A. rolfsii strain SrSh7 (OM021880) and A. rolfsii (AB075298). The sequences generated in the present investigations have been deposited in GenBank. Accession numbers OR563700 and OR659546 have been obtained. A phylogenetic tree was generated for each isolate by utilising reference sequences retrieved from GenBank (Figures S2 & S3).
3.2 Plant growth-promoting properties.
3.2.1 Phosphate solubilizing potential
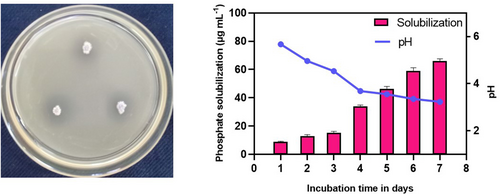
The endophytic strain Bacillus amyloliquefaciens SS_CR10 was observed to exhibit the ability to solubilize inorganic phosphate TCP. This was evidenced by the formation of a clear halo zone surrounding the colonies when grown on the Pikovskaya agar medium. After incubating the sample at a temperature of 28°C for a duration of 72 h, a solubilization index of 1.94 was recorded. The solubilization kinetics of inorganic tricalcium phosphate (TCP) by strain SS_CR10 was studied at various time intervals (days). The results showed a notable increase in solubilization as the pH of the medium decreased from 5.6 to 3.2 (Figure 1). After a 7-day incubation period, an observed solubilization of TCP was recorded at a concentration of 65.9 ± 1.5 μg ml−1.
3.2.2 Indole acetic acid (IAA) production
The production of indole-3-acetic acid (IAA) by the endophytic strain SS_CR10 was confirmed through the observation of a pink chromogenic reaction upon the addition of Salkowski reagent to the culture medium (CS). The experiment included carrying out a quantitative test to investigate the impact of precursor concentration on the production of IAA. The test measured the production of IAA at various concentrations of L-tryptophan (1, 3, and 5 mg ml−1), as well as a control group without any tryptophan precursor. The production of indole-3-acetic acid (IAA) was observed to increase as the concentration of tryptophan increased. The IAA production ranged from 9.77 ± 0.41 μg ml−1 to 24.45 ± 0.68 μg ml−1 at concentrations of 0 and 5 mg ml−1 of tryptophan, respectively (Figure S4).
3.2.3 Nitrogen fixation and ammonia production
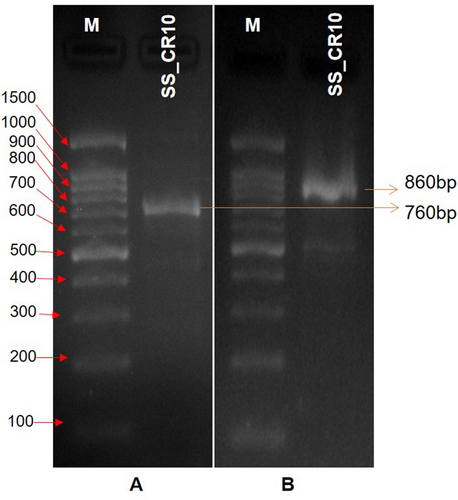
The strain's nitrogen-fixing capability was evaluated by observing its growth on nitrogen-free media and confirming the presence of the nifH gene fragment through PCR amplification. The ability of strain SS_CR10 to fix nitrogen was detected through its growth on a nitrogen-free Ashbey medium. The nitrogen fixation ability was further confirmed by performing PCR amplification of a ~ 760 bp nifH gene fragment (Figure 2a). The alteration in the colour of the medium following the application of Nessler's reagent to a deeper shade of yellow or brown indicates a positive result for the production of ammonia.
3.2.4 ACC deaminase production
The activity of ACC deaminase in the selected strain was evaluated based on its capability to utilise ACC as the exclusive nitrogen source. This was confirmed by performing PCR amplification of the acds gene fragment. The production ability of the ACC deaminase enzyme in strain SS_CR10 was determined by observing visible growth on DF salt minimal media supplemented with 3 mM ACC as the sole source of nitrogen. The confirmation of ACC deaminase production ability was achieved through the PCR amplification of an approximately 860 bp acds gene fragment (Figure 2b).
3.2.5 Siderophore production and hydrolytic enzyme production
The production of siderophore was confirmed through the observation of a yellow halo forming around the colony (Figure S5). The strain SS_CR10 exhibited strong siderophoric activity, indicating its ability to produce and utilise siderophores. The cellulase production was assessed qualitatively, and it was found that the cellulase activity resulted in the formation of a clearance zone measuring 1.66 ± 0.05 cm (Figure S5). The protease activity on skim milk agar was assessed qualitatively, and the results indicated a positive stain with a clearance zone measuring 1.40 ± 0.10 cm. The amylase activity on soluble starch agar media was assessed qualitatively, indicating that the strain tested positive for amylase. The clearance zone observed ranged from 1.36 ± 0.05 cm.
3.3 Disinfection and re-inoculation of endophytic bacteria for seedling growth and improved photosynthetic pigments
The disinfection of chilli seeds was done by washing the seeds with a 4% sodium hypochlorite solution for 10 minutes. This was followed by a treatment with streptomycin at a concentration of 100 μg ml−1 for 8 h. The absence of bacterial growth around the disinfected seeds cultured on nutrient agar media confirmed the efficacy of the disinfection process. Furthermore, we considered the thoroughly disinfected seeds to be free of endophytes. The bacterial isolate with the highest level of activity that promotes plant growth was also treated. The re-inoculation of disinfected seeds was performed using B. amyloliquefaciens strain SS_CR10. The seeds that were inoculated with the bacterial strain SS_CR10 exhibited a significant increase in root and shoot length, as well as the fresh and dry weight of the seedlings. This increase was observed in comparison to both the positive and negative control groups (Figure 3). However, seedling growth parameters for seeds in both growth media (cocopeat, perlite, and sand in a ratio of 2:2:1 and 2:1:1) produced more or less similar results. Furthermore, the treatments significantly improved the synthesis of photosynthetic pigments within the seedling leaves, including chlorophyll a, chlorophyll b, and carotenoid. This improvement was observed in comparison to both the positive and negative control groups, as indicated in Table 1.

| Parameters | Treatments | GM 1 (2:2:1) | GM 2 (2:1:1) | ||
|---|---|---|---|---|---|
| 10 dpi | 30 dpi | 10 dpi | 30 dpi | ||
| Shoot length plant−1 (cm) | NC | 4.66 ± 0.51a | 8.10 ± 0.64a | 4.39 ± 0.46a | 7.89 ± 0.41a |
| PC | 4.88 ± 0.45a | 8.89 ± 0.65b | 4.77 ± 0.52b | 8.79 ± 0.65b | |
| SS_CR10 | 6.28 ± 0.34b | 10.56 ± 0.57c | 6.10 ± 0.38c | 10.28 ± 0.66c | |
| Root length plant−1 (cm) | NC | 3.99 ± 0.37a | 5.29 ± 0.46a | 3.94 ± 0.41a | 5.08 ± 0.35a |
| PC | 4.30 ± 0.47a | 5.97 ± 0.77a | 4.26 ± 0.53a | 5.84 ± 0.71b | |
| SS_CR10 | 5.16 ± 0.38b | 9.34 ± 1.17b | 5.10 ± 0.44b | 9.34 ± 1.16c | |
| Fresh weight plant−1 (mg) | NC | 57.8 ± 6.90a | 105.2 ± 11.15a | 58.1 ± 8.74a | 105.5 ± 8.78a |
| PC | 64.3 ± 10.49b | 115.4 ± 7.18a | 63.7 ± 11.33a | 113.6 ± 9.67a | |
| SS_CR10 | 77.7 ± 8.12c | 157.4 ± 12.90b | 79.4 ± 10.47b | 157.5 ± 15.72b | |
| Dry weight plant−1 (mg) | NC | 6.3 ± 0.77a | 10.7 ± 0.91a | 6.26 ± 0.87a | 10.93 ± 0.88a |
| PC | 6.3 ± 0.56a | 10.6 ± 1.75b | 6.86 ± 0.73a | 10.73 ± 1.86a | |
| SS_CR10 | 6.7 ± 0.85a | 13.7 ± 1.21c | 8.3 ± 0.76b | 14.16 ± 0.62b | |
| Chlorophyll a (mg g−1 of FW) | NC | ND | 13.31 ± 1.22a | ND | 14.00 ± 1.01a |
| PC | ND | 15.38 ± 0.31b | ND | 15.38 ± 0.65a | |
| SS_CR10 | ND | 18.77 ± 0.71c | ND | 19.01 ± 0.79b | |
| Chlorophyll b (mg g−1 of FW) | NC | ND | 0.63 ± 0.08a | ND | 0.60 ± 0.01a |
| PC | ND | 0.58 ± 0.06a | ND | 0.60 ± 0.10a | |
| SS_CR10 | ND | 0.86 ± 0.09b | ND | 0.83 ± 0.10b | |
| Carotenoid (mg g−1 of FW) | NC | ND | 0.95 ± 0.07a | ND | 0.93 ± 0.15a |
| PC | ND | 1.08 ± 0.07a | ND | 1.24 ± 0.09b | |
| SS_CR10 | ND | 1.47 ± 0.18b | ND | 1.67 ± 0.11c | |
- GM – Growth media; NC – Negative control; PC – Positive control; FW – Fresh weight; dpi – Day post inoculation; ND – not done. Each value represents the mean ± SD, values followed by different letters show significant difference between treatments at p ≤ 0.05.
3.4 Root colonization potential of endophytic isolate Bacillus amyloliquefaciens SS_CR10
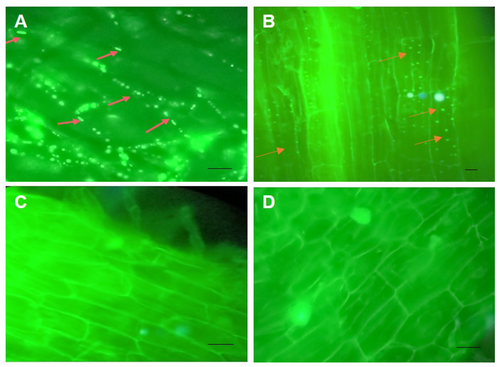
The presence of internal root colonisation was detected within the intra and inter-cellular spaces of the root parenchyma cells in the seedlings treated with bacteria. The visualisation of bacterial cells was facilitated by the emission of green fluorescence following staining with SYTO-9 stain and observation under a fluorescent microscope. The negative control seedlings that were examined for root colonisation showed no bacterial colonisation on root parenchyma cells and root hairs (Figure 4). An examination was conducted on root samples taken randomly from the seedlings of each treatment, revealing similar colonization patterns.
3.5 Antifungal activity, amplification of lipopeptide gene
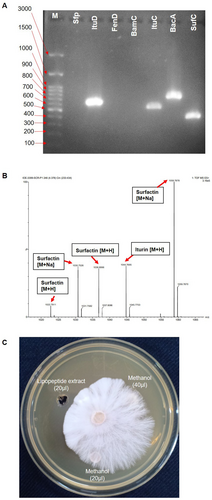
The bacterial strain SS_CR10 demonstrated antifungal activity against the fungal phytopathogen A. rolfsii. The growth inhibition percentage of 82.2% was observed in the dual culture plate (Figure 2). The polymerase chain reaction (PCR) amplification of the lipopeptide genes was performed using specific primers. The results revealed the presence of Iturin D, Iturin C, Bacilysin A, and Surfactin C genes (Figure 5). These genes are responsible for the antifungal activity. There was no amplification detected for the genes Sfp, FenD, and BamC, suggesting that these genes are not present in the bacteria.
3.6 Microscopic observation of the effects of SS_CR10 and its lipopeptide on A. rolfsii
The study revealed that B. amyloliquefaciens strain SS_CR10 and its lipopeptide extracts inhibit the growth of A. rolfsii by damaging and deforming the mycelium. The effects were studied using bright field, light, and fluorescent microscopy. Bright-field microscopy and light microscopic studies using lactophenol cotton blue (LCB) recorded the abnormal structures, swelling, cell wall deconstruction, and degradation of mycelia treated with the lipopeptide extracts and bacteria. Furthermore, fluorescent microscopic studies using propidium iodide (PI) that stains only the dead cells revealed a significant number of dead A. rolfsii mycelia in Bacillus and lipopeptide-treated samples. In addition, studies using DAPI that stain both live and dead mycelia helped identify the dead portion of the mycelia with an intense colour compared to the living cells. Only PI stained the deformed and deconstructed portions of the mycelia, while DAPI stained all parts of the fungal mycelia (Figure 6). However, a light and bright field microscope observed the samples from the untreated control as normal, smooth mycelial structures, free of any deformations. After staining the untreated control samples with PI and DAPI, a fluorescent microscope revealed no dead mycelia. Further, mycelial deformation of A. rolfsii growing in the presence of strain SS_CR10 was seen by scanning electron microscopy (SEM), demonstrating the ability of the strain to impede mycelial development (Figure S6).
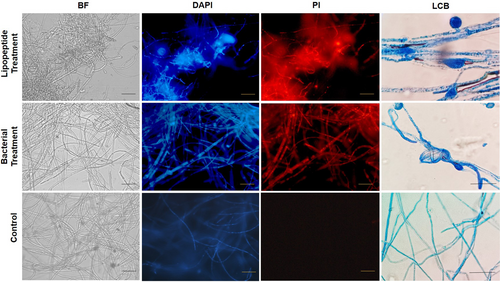
3.7 Detection of the lipopeptide produced by SS_CR10
The crude extract of lipopeptide was analyzed by electrospray quadrupole time-of-flight mass spectrometry (Q-TOF MS) to identify the lipopeptides produced by strain SS_CR10. The analysis revealed that the strain SS_CR10 isolate produces different forms of lipopeptides, with the m/z corresponding to cyclic lipopeptides previously reported in the Bacillus genera. Two MS signals with a [M + H] peak at m/z 1022.79 and 1036.80 were hypothesized to be surfactin with C14 to C15 fatty acid chain length, peaks at m/z 1030.75 and 1058.78 were speculated to be Na + adduct ions of surfactin [M + Na]. The peak at 1044.76 m/z is considered the [M + H] of iturin (Table 2). However, there were no signals for the fengycin and bacillomycin lipopeptides (Figure 5).
| Lipopeptide | Fatty acid chain | Molecular formula | Calculated (m/z) | |
|---|---|---|---|---|
| [M + H]+ | [M + Na]+ | |||
| Surfactin | C13 | C51H89N7O13 | 1008.77 | 1030.75 |
| C14 | C52H91N7O13 | 1022.79 | ||
| C15 | C53H93N7O13 | 1036.80 | 1058.78 | |
| Iturin | C14 | C48H73N12O14 | 1044.76 | |
3.8 Seedling protection from A. rolfsii infection
The use of B. amyloliquefaciens strain SS_CR10 and its lipopeptide-treated seeds effectively prevented infection and promoted healthy growth in seedlings challenged with A. rolfsii. The untreated control group experienced significant wilting and death, with extensive colonization of the fungus in the collar regions of the seedlings. In contrast, the seedlings treated with the bacterium and its lipopeptide treatments showed no signs of colonization (Figure S7).
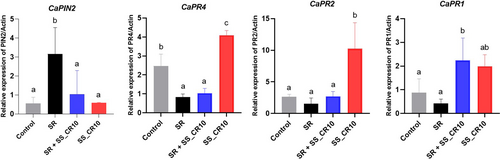
3.9 Relative expression of induced systemic resistance and systemic acquired resistance defense-related genes
The quantification of the induction of the defense gene in pepper by strain SS_CR10 was performed using RT-PCR. The expression levels of CaPR1, CaPR2, and CaPR4 genes in pepper leaves treated with strain SS_CR10 were markedly elevated compared to those treated with water and SR (pathogen). Pepper leaves treated with SR showed a notable up-regulation of the CaPIN2 gene expression relative to other treatments (Figure 7). Hence, the potential mechanism via which strain induces systemic resistance is by activating the salicylic acid and ethylene response pathways.
4 DISCUSSION
Beneficial bacteria can colonize a plant's endosphere by entering the internal tissues within the plant, and some of them have been shown to promote plant growth and induce systemic resistance. Bacillus spp. are very common and reported to harbour many crop plants, including rice, maize, sugarcane, and tomato (Shahid et al., 2021). The ability to promote plant growth and biocontrol, including phosphate solubilization, IAA production, nitrogen fixation, siderophore production, ACC deaminase activity and antifungal effects on several phytopathogens, have been studied in Bacillus spp. The present study evaluates the potential of Bacillus amyloliquefaciens SS_CR10 for plant growth promotion and biocontrol. This is the first time the biocontrol mechanisms of SS_CR10 against A. rolfsii on chilli was comprehensively illustrated.
The solubilization of complex inorganic phosphate is one of the key characteristics of nutrient acquisition. The mechanism by which the endophytic bacteria solubilize inorganic phosphate may involve the production of organic acids such as citric, acetic, lactic, gluconic, fumaric, melonic, glyconic, oxalic, propionic and succinic acids. Organic acids chelate mineral ions, such as Ca, and liberate the complexed phosphate, or they reduce the pH to bring phosphorus into the soil. Our results indicated that SS_CR10 inoculation released significant amount of phosphorus from the inorganic tri-calcium phosphate in pikovskaya broth (Figure 1). Several bacillus species have already been reported to solubilize phosphate in varieties of crop plants, including chickpeas and cotton (Gorai et al., 2021; Ahmad et al., 2021); results of the present study are in accordance with those published reports.
IAA is one of the crucial plant growth regulators that improve plant growth and anatomical structures by increasing the cell length, helping root development, maintaining apical dominance, and preventing cell senescence (Gorai et al., 2021). Three strains (GSW-E-5, GSW-E-6, and GSW-E-7) related to Bacillus spp. were found to promote the growth of wheat plant under high saline condition (Albdaiwi et al., 2019). Zhou et al. (2021) documented significant IAA production by the Bacillus cereus YN917. In accordance with those studies, SS_CR10 produced a significant amount of IAA and this might contribute to the direct growth promotion of chilli. Nitrogen is a vital macronutrient and constituent of proteins, enzymes, nucleic acid, and chlorophyll. Though nitrogen is abundant in the environment and present in the inert form of dinitrogen (N2), plants can only access nitrogen in the form of nitrate (NO3-) and ammonia (NH4+). Some of the free-living and symbiotic organisms can fix atmospheric nitrogen into ammonia and enhance the availability of nitrogen to plants with the help of the enzyme nitrogenase (Raina and Mazahar, 2022). Several studies have proven the efficacy of nitrogen-fixing endophytes over rhizospheric organisms. Endophytic Bacillus sp. having nitrogen fixation capability that enhances the growth and development of plants has been reported from different crops, such as chickpeas and sugarcane (Gorai et al., 2021; Singh et al., 2021). In this investigation, B. amyloliquefaciens strain SS_CR10 produced amplified fragments of nifH and acds genes using specific primers (Figure 2). These genes play a role in PGP traits such as nitrogen fixation and ACC deaminase production, which enhance plant growth and nutrition in both normal and stressful conditions.
Plants produce high levels of ethylene under biotic and abiotic stress conditions. The endophytic bacteria produce ACC deaminase, which boosts plant growth by mitigating the stress ethylene causes. ACC deaminase reduces the levels of ethylene through the degradation of its precursor (Kumar et al., 2021a). Siderophores are iron-chelating compounds produced by various organisms that help in the development of soluble Fe2+ complexes from insoluble ferric iron for easy iron acquisition by plants. Siderophore production by endophytic bacteria can also protect plants against phytopathogens by limiting the iron availability of pathogens. Strain SS_CR10 produced a substantial amount of siderophore in the present investigation, an ecofriendly and sustainable approach over agrochemicals for the bio-availability of iron, contributing to plant growth and mitigation of biotic stress. In addition, strain SS_CR10 also produced hydrolytic enzymes such as protease, amylase and cellulase. Hydrolytic enzymes help bacteria colonise the internal plant tissue and establish themselves inside. Our microscopic study of seedling roots revealed the colonized SS_CR10 in inter and intracellular spaces of root parenchyma (Figure 4). Hydrolytic enzymes might also be helpful in the introduction of systemic resistance in plants. Upon successful colonisation of the root tissue, endophytic bacteria would multiply on the root surface and establish themselves as rhizospheric microbiomes. Endophytic bacteria on the rhizosphere help to develop seedlings through the production of plant hormones, nutrient acquisition, and mineralization. This might be the cause of the improved shoot and root length and enhanced fresh and dry weight of seedlings (Figure 3) in treatments compared to positive and negative control in our study.
Bacillus species, such as Bacillus velezensis, B. subtilis, and B. pumilus, are well-known inhibitors of different fungal phytopathogens such as Rhizoctonia solani, Fusarium oxysporum, Alternaria sp., and Curvularia sp. (Shahid et al., 2021). Many researchers report that several bacteria exhibit antifungal activity against phytopathogens. Biocontrol agents that inhibit the mycelial growth of pathogens are considered significant in disease suppression. Bacteria produce antifungal metabolites, lipopeptides, cell wall degrading enzymes, and volatiles for phytopathogen control. The lipopeptides, such as surfactin, iturin, fengycin and bacillomycin, are considered to be the main antifungal agents against phytopathogens. Lipopeptide inhibits the growth of fungal mycelia. Bacillus amyloliquefaciens YN201732 showed antagonistic properties against Fusarium solani in plate antagonism test. Furthermore, LCMS analysis identified the n-butanol extraction component as bacillomycin D (Jiao et al., 2021). Gond et al. (2015) reported the strong antifungal activity of lipopeptides obtained from Bacillus spp. such as B. subtilis and B. amyloliquefaciens against F. moniliforme. MALDI-TOF-MS analysis of lipopeptide extracts detected the presence of antifungal lipopeptides (iturin, fengycin and bacillomycin). These substances cause mycelial deformations, such as swelling, cytoplasmic leakage, pore formation, and the death of fungal hyphae and spores (Figure 6). Lipopeptides have fungitoxic effects because they compete with fungal cell membrane compounds such as phospholipids, sterols, and oleic acids. Lipopeptides inhibit chitin and glucan synthase enzymes and cause apoptosis by changing how mitochondria work in fungal cells (Kumar et al., 2021b).
Plants resist tissue damage and survive harsh environmental conditions through different defense mechanisms, which include the production of defense-related enzymes, cell wall thickening, and callose deposition. Researchers have investigated several Bacillus spp. for inducing systemic resistance by regulating jasmonate (JA), ethylene (ET), and salicylic acid-dependent signalling pathways. The JA/ET-dependent pathway is the main signaling pathway and the SA-dependent pathway is the minor signaling pathway. Activation of the SA-dependent signaling pathway leads to an increase in the levels of pathogenesis related (PR) proteins, including β-1,3-glucanase and chitinase, in the plant (Kamle et al., 2020). The increased level of transcription of pathogenesis-related protein genes CaPR1, CaPR2, and CaPR4 in our present investigation indicates the ability of SS_CR10 to combat pathogenesis by inducing the host ISR (Figure 7). Similarly, Gond et al. (2015) reported the upregulation of pathogenesis-related genes, PR-1 and PR-4, in maize after root treatment with endophytic B. subtilis SG_JW.03. In this study, CaPR1 expression is related to the SA-responsive signalling pathway. The ET-responsive signalling pathway primarily drives the expression of CaPR2 and CaPR4 (Wu and Huang, 2019). Hence, SS_CR10 can stimulate chilli systemic resistance through the activation of both SA- and ET-dependent signaling pathways.
The present research indicates that the B. amyloliquefaciens SS_10 isolate possesses antifungal properties. Furthermore, the findings demonstrate siderophore production, enhanced hydrolytic enzymes, lipopeptide gene production, seedling protection, growth promotion, and induced defense signaling molecules via activation of CaPR1, CaPR2, and CaPR4 genes during host-pathogen interaction. All these traits substantiate the biocontrol potential of B. amyloliquefaciens as an antifungal agent against A. rolfsii; thus, it could be used as a biocontrol agent against the southern blight pathogen to protect agricultural crops from the menace.
AUTHOR'S CONTRIBUTION
SCR, NBR, and SS conceptualized, and designed; SCR, BM and MM performed experiments; SCR, PJ and MKS data analysis, molecular identification and phylogeny, In-vivo studies, drafting manuscript; and SCN data analysis and interpretation, SS supervised the work and corrected the draft. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank the Institution of Excellence (IOE), Vijnana Bhavana, University of Mysore, for providing the necessary facilities.
Open Research
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the GenBank Nucleotide Database at https://www.ncbi.nlm.nih.gov/genbank/, and can be accessed with accession numbers OR563700 and OR659546.




