Linking stomatal size and density to water use efficiency and leaf carbon isotope ratio in juvenile and mature trees
Abstract
Water-use efficiency (WUE) is affected by multiple leaf traits, including stomatal morphology. However, the impact of stomatal morphology on WUE across different ontogenetic stages of tree species is not well-documented. Here, we investigated the relationship between stomatal morphology, intrinsic water-use efficiency (iWUE) and leaf carbon isotope ratio (δ13C). We sampled 190 individuals, including juvenile and mature trees belonging to 18 temperate broadleaved tree species and 9 genera. We measured guard cell length (GCL), stomatal density (SD), specific leaf area (SLA), iWUE and bulk leaf δ13C as a proxy for long-term WUE. Leaf δ13C correlated positively with iWUE across species in both juvenile and mature trees, while GCL showed a negative and SD a positive effect on iWUE and leaf δ13C. Within species, however, only GCL was significantly associated with iWUE and leaf δ13C. SLA had a minor negative influence on iWUE and leaf δ13C, but this effect was inconsistent between juvenile and mature trees. We conclude that GCL and SD can be considered functional morphological traits related to the iWUE and leaf δ13C of trees, highlighting their potential for rapid phenotyping approaches in ecological studies.
1 INTRODUCTION
Water-use efficiency (WUE) reflects the balance between carbon gain and water loss in plants (Leakey et al. 2019; Brendel et al. 2021; Vadez et al. 2023). Intrinsic water-use efficiency (iWUE) indicates a momentary balance of leaf carbon and water fluxes and corresponds to the ratio of net CO2 assimilation rate (An) to stomatal conductance to water vapour (gs) (Roussel et al. 2009; Petek-Petrik et al. 2023). Higher iWUE can increase the establishment and survival of plants under water-deficit conditions (Ehleringer and Driscoll 2022). Enhancing iWUE is crucial for maximizing forest carbon assimilation capacity while conserving water resources (Zhang et al. 2023; Petrík et al. 2024). For long-term WUE, leaf carbon isotope composition (δ13C) is often used as a proxy because of the preference for the lighter isotope during physical and chemical processes involved in CO2 uptake and assimilation (Farquhar et al. 1989; Ma et al. 2023). The preference for the lighter 12C isotope during CO2 uptake and assimilation results in discrimination of the heavier 13C isotope. This discrimination is more pronounced when leaf internal CO2 concentrations are higher, for instance when stomata are fully open due to more intense gas exchange via stomata, which in turn is associated with a lower iWUE (Impa et al. 2005). Thus, iWUE and δ13C are critical traits affecting tree water-use use for carbon assimilation, growth and survival across different time scales under water-deficit conditions, and they are of vital importance in the context of increasing evaporative demand due to climate change (Ehleringer and Driscoll 2022; Zhang et al. 2023; Puchi et al. 2024).
Both iWUE and δ13C of plants are affected by multiple physiological and morphological traits such as stomatal morphology, cuticular conductance, mesophyll conductance, leaf nitrogen, respiration rates (Buckley and Warren 2014; Bucher et al. 2016; Cardoso et al. 2020; Paillassa et al. 2020; Eckardt et al. 2023; Petrík et al. 2023; Kurjak et al. 2024). Ontogeny can also play a crucial role in shaping the leaf physiology, anatomy and morphology of trees across different life stages. Studies have shown that as trees develop, there are significant changes in their leaf morpho-physiological traits such as photosynthetic pigment concentration, dark respiration, δ13C (Fortunel et al. 2019) and photosynthetic efficiency (Ishida et al. 2005). Understanding particularly the phenotypic and ontogenetic constraints of iWUE would allow for better insights into the impacts of rising evaporative demand and more frequent drought periods (Grossiord et al. 2020; Vicente-Serrano et al. 2020; De Souza et al. 2023).
We chose here to specifically analyse the influence of stomatal morphological traits on iWUE and leaf δ13C as the characterization of these traits may represent an affordable and robust method for a rapid and mass phenotyping of tree water-use in field and experimental studies. So far, most studies focused on linking stomatal morphology to iWUE in crops (Andrade et al. 2022; Huang et al. 2022; Ozeki et al. 2022) or used model plant species like Arabidopsis and poplars (Guo et al. 2019; Jiao et al. 2022). There are some studies that focused on tree species, but these studies only captured intra-specific variability and did not include the effect of ontogeny (Cregg et al. 2000; Dillen et al. 2008; Roussel et al. 2009; Cao et al. 2012). Liu et al. (2018) found quadratic relationships between GCL, SD and WUE among tree species in global meta-analysis, but the WUE was derived from empirical relationships based on temperature and precipitation data rather than direct measurements. A comprehensive experimental analysis of inter-specific variability and coordination between the stomatal morphology and iWUE in forest tree species is lacking thus far. It, therefore, remains inconclusive whether stomatal morphological traits such as guard cell length (GCL) and stomatal density (SD) affect the water-use efficiency in tree species across ontogenetical stages, and whether they can be considered as robust functional traits associated with the drought tolerance in trees.
Photosynthetic activity of plants can adjust to changes in irradiance in seconds, but the time lag in stomatal responses limits the CO2 uptake and therefore constrains photosynthesis and limits iWUE (Lawson et al. 2012; Nguyen et al. 2023). Several studies have reported that smaller stomata respond faster to changes in environmental conditions than larger stomata (Lawson et al. 2014; Kardiman and Raebild 2018; Durand et al. 2019), which can lead to higher long-term WUE (Drake et al. 2013; McAusland et al. 2016; Haworth et al. 2021). Stomatal size is also positively correlated with operational gs; therefore, plants with smaller stomata can limit their maximal transpiration, potentially positively affecting iWUE (Fanourakis et al. 2015). Multiple gene-manipulation studies have shown that a reduction of stomatal size leads to enhanced iWUE in plants (Lawson et al. 2014; Mohammed et al. 2019; Jiao et al. 2023). This is particularly true in crops, and as a result, stomatal morphology is already used in crop breeding programmes that aim to create varieties with greater resistance to drought (Robertson et al. 2021; Xiong et al. 2022). If the negative relationship between stomatal size (guard cell length, GCL) and iWUE also holds true in trees across ontogenetical stages, this could further support phenotyping efforts for the evaluation of the water-use ability of tree populations.
SD is also a common functional trait related to plants WUE. Multiple studies have found a significant impact of SD on the WUE of plants under well-watered but also drought stress conditions. Several gene-manipulation studies have found negative relationships between SD and iWUE or δ13C in Arabidopsis (Franks et al. 2015), various crop species (Liu et al. 2015; Guo et al. 2019; Li et al. 2020; Pitaloka et al. 2022), in poplars (Liu et al. 2021; Jiao et al. 2022) and among tropical trees (Pan et al. 2024). Nevertheless, there is some evidence that higher SD can also be associated with a greater iWUE in plants (Xu et al. 2008; Naz et al. 2010; Zhao et al. 2015; Stojnić et al. 2019; Bhaskara et al. 2022; Al-Salman et al. 2023; Caine et al. 2023), or correlate positively with assimilation without offsetting iWUE (Tanaka et al. 2013). SD is usually negatively correlated with GCL as there is a trade-off between stomatal size and frequency (Franks and Beerling 2009; Doheny-Adams et al. 2012; Driesen et al. 2023). The increase of SD and reduction of stomatal size is a common acclimation response to drought stress in trees (Dunlap and Stetter 2001; Pearce et al. 2006; Boughalleb et al. 2014; Stojnić et al. 2015). Typically, SD and GCL show a negative correlation across genera due to spatial constraints on the leaf (Liu et al. 2023). In contrast, gene manipulation in crops can reduce SD disproportionally (−80%) compared to the increase of GCL (+20%), which might not be realistic for natural populations (Franks et al. 2015). The relationship between SD and iWUE of trees is therefore probably connected to changes in stomatal size.
The leaf morphology can also constrain the iWUE via changes in CO2 and H2O pathways throughout leaf tissues (Carriquí et al. 2015; Trueba et al. 2022). Leaf morphology can be apprehended by the specific leaf area (SLA), i.e., the inverse of leaf mass per area, a widely used functional trait in plant ecology (Tian et al. 2016). SLA decreases in plants with thicker leaves (Vile et al. 2005; Homeier et al. 2021). Multiple studies have found that SLA is negatively related to iWUE and δ13C in crops (Reddy et al. 2020a; Reddy et al. 2020b), shrubs (Horike et al. 2021) and tree species (Ge et al. 2022). The correlation between SLA and iWUE might be caused by anatomical differences that affect the mesophyll tissue surface area and mesophyll conductance (Baird et al. 2017). SLA can also influence photosynthetic capacity (Liu et al. 2010) and quantum yield (Petek-Petrik et al. 2024), which may be constraining factors for iWUE. This raises the question of whether the reported association between SLA and iWUE is a general pattern, which would allow using SLA, a widely available functional trait, as a proxy of the iWUE of plants in ecological studies.
In this study, we took advantage of two experiments on temperate mature and juvenile trees (18 tree species in total) to quantify how stomatal and leaf morphology affect the short-term iWUE and long-term δ13C estimates of tree water-use. We hypothesized that i) higher values of GCL are associated with a lower iWUE and δ13C, ii) the degree of stomatal density affects iWUE and δ13C, iii) SLA is negatively related to iWUE and δ13C, iv) the relationships between morphological (GCL, SD, SLA) and physiological (iWUE, δ13C) traits do not differ between juvenile and mature tree species.
2 MATERIALS AND METHODS
2.1 Juvenile trees experimental set-up
The juvenile tree measurements were conducted in the Botanical Garden of the University of Würzburg, Germany (49°45′53.542″N, 9°55′52.92″E) in June 2022. The site has a temperate climate with an average temperature of 11.7°C and an annual precipitation of 561 mm in 2022 (Deutscher Wetterdienst, 2022). Ten individuals each from ten broadleaved tree species were used for the measurements: Acer pseudoplatanus (ACPS), Aesculus hippocastanum (AEHI), Betula maximowicziana (BEMA), Betula pendula (BEPE), Fagus sylvatica (FASY), Quercus petraea (QUPE), Quercus rubra (QURU), Sorbus aucuparia (SOAC), Tilia cordata (TICO) and Tilia tomentosa (TITO). The trees of all species except ACPS and SOAC had heights ranging from 50 cm to 120 cm and were between 3–5 years old. The height of ACPS and SOAC individuals ranged from 150 cm to 180 cm, ACPS individuals were 8 years old and SOAC individuals were 6 years old. The juvenile trees were planted in an inorganic sand/loam mixture in 10 L pots and 20 L pots (ACPS, SORB) during the spring of 2022. The trees were regularly irrigated to maintain optimal water status. The leaves for all measurements were sampled from the upper part of the crown and represented sun leaves.
2.2 Mature trees experimental set-up
The mature tree measurements were conducted on the Campus of the Université du Québec à Trois-Rivières, Canada (46°20′49.041″N, 72°34′40.932″W) in August/September 2022. The site is located on sandy soils representative of the retreat of the Champlain Sea, and in a temperate climate zone with average annual temperature and annual precipitation around 5.2°C and 872 mm (as rainfall), respectively (MDDELCC, 2015). Six individuals each from nine broadleaved tree species were tagged within the Campus area and used for the measurements. The tree species included: Acer platanoides (ACPL), Acer rubrum (ACRU), Acer saccharinum (ACSA), Betula populifolia (BEPO), Populus grandidentata (POGR), Populus tremuloides (POTR), Prunus pensylvanica (PRPE), Quercus rubra (QURU) and Tilia americana (TIAM). All mature trees had heights ranging between 8–15 m with the exception of TIAM, which measured around 4 m. The vegetation season and measurement period of 2022 received evenly distributed precipitation and, therefore, the trees were not drought-stressed, which is also visible in our leaf water potential measurements (Table 1). The leaves for all measurements were sampled with telescopic scissors from the sun-exposed Southern side in the lower third of the crown.
2.3 Gas-exchange measurements
Gas-exchange measurements were conducted between 1st and 9th of June 2022 of juvenile trees and between 25th of August and 8th of September 2022 on mature trees. The time periods for the measurements were 9:30–12:00 and 13:30–16:00 to avoid a mid-day depression. Gas-exchange measurements were done with a Li-6800 (LI-COR) equipped with a standard leaf chamber with 3 cm2 for juvenile trees and with 6 cm2 cuvette for mature trees. The chamber conditions were set to 1000 μmol m−2 s−1 PAR intensity, 420 ppm reference CO2 and fan speed of 10000 rpm in both experiments. The air temperature in the cuvette was 22 ± 0.95 °C (mean ± SE) for mature trees and 26 ± 1.05°C for juvenile trees, the relative humidity in the cuvette was averaging at 60 ± 1.6% for mature trees and 60 ± 3% for juvenile trees. Leaf gas-exchange on mature trees was measured at sun-exposed leaves from the lower third of the tree crown, immediately after excision. Leaf gas-exchange of juvenile trees was typically measured at intact, sun-exposed leaves from the upper third of their crown. For juvenile ACPS and SORB, we used excised branches originating from the upper third of the tree crown, which were immediately measured. In total, we measured gas exchange on 154 trees belonging to 18 tree species (mature: n = 54; juvenile: n = 100). Leaf gas-exchange was measured 4–5 times per individual in mature trees and 3–4 times in juvenile trees during the experimental period. The measurements were averaged per individual for further analyses. The intrinsic water-use efficiency (iWUE) was calculated as a ratio between assimilation rate (A) and stomatal conductance (gs); iWUE = A/gs.
2.4 Water potential measurements
The leaf water potential (WP) of both juvenile and mature trees was measured periodically to make sure that the trees were not drought-stressed. All juvenile trees were watered regularly (1–2× per week) and their leaf WP was measured each morning before gas-exchange measurements to test their water status (Table 1). The leaf WP of juvenile trees was measured with a Scholander pressure chamber (Model 1505D, PMS Instruments) on petioles of leaves excised from the upper part of the tree crowns, within 3 minutes after the excision.
The leaves of mature trees adjacent to the leaves used for the gas-exchange measurements were stored in plastic bags with a wet tissue and placed in a mobile cooler. In the afternoon of the same day (ca. 4–5 pm), their leaf WP was measured with a Scholander pressure chamber (Model 1505D, PMS Instruments) on petioles of the excised leaves (Table 1).
The water potential measurements confirmed that none of the species were drought-stressed during the measurement periods neither in juvenile nor mature trees.
2.5 Stomatal morphology
The stomatal imprints were done with the ‘collodion method’, where transparent nail polish is applied to the abaxial side of the leaves, as all sampled species have minimal stomatal occurrence on upper side of their leaves. After 2–3 minutes, the nail polish layer was transferred to a microscope slide using transparent tape (Petrík et al. 2022). The stomatal imprints from mature trees were collected after each gas-exchange measurement, while for juvenile trees they were taken only after the first round of measurements. The imprints were collected from the same area where leaf gas-exchange was measured. Therefore, the spatial variability of the stomatal morphology within the leaf should match the spatial variability of gas-exchange. The stomatal imprints were taken for 10 individuals per species, 4–5 imprints per individual for mature trees and one imprint per individual for juvenile trees. From these imprints, the digital photographs using Levenhuk MED 30 T equipped with Delta Optical DLT-Cam Pro 12MPx were taken at 40×10 resolution. The guard cell length (GCL) and stomatal density (SD) were measured from these digital photos with ImageJ software (Schneider et al. 2012). The GCL was measured for 3 random stomata per photo and these values were averaged per individual (three stomata per individual for juvenile and 12–15 stomata per individual for mature trees). The number of stomata for the entire area of the photo (0.416 mm2) was measured and was further recalculated to SD per 1 mm2 (one SD value per individual for juvenile and three SD values averaged per individual for mature trees).
2.6 Specific leaf area
The leaves used for the first round of gas-exchange measurements for mature trees were taken for specific leaf area (SLA) estimation, while SLA was deduced from additional leaves sampled at the end of the experiment for juvenile trees. Three leaves from the upper third of the crown were sampled from each mature tree individual. The mature tree leaves were scanned with a Perfection V800 scanner (Epson) and juvenile tree leaves with an A3 scanner (Perfection 12000XL, Seiko Epson) and their leaf area was measured with ImageJ software. Afterwards, the leaves were oven-dried at 70 °C for 48 h. Subsequently, SLA of each leaf was calculated as SLA = leaf area/dry mass. The values were averaged for each individual.
2.7 Carbon isotope analysis
The same leaf samples used for SLA measurement were further ground to fine powder and stored in a freezer for the carbon isotope (δ13C) analysis. Leaf δ13C isotopic ratios of the mature tree's samples were determined using an elemental analyser coupled with an isotope ratio mass spectrometer (EA-IRMS, Agilent technology). The juvenile tree samples were analysed at the Centre for Stable Isotope Research and Analysis (KOSI), University of Göttingen. The leaf δ13C of juvenile trees was measured with a Delta Plus Isotope mass ratio spectrometer (Finnigan MAT), a Conflo III interface (Thermo Electron Corporation) and a NA2500 elemental analyser (CE-Instruments). The replicate analyses of isotopic standard reference materials USGS 40 (δ13C = −26.39‰) and USGS 41 (δ13C = 37.63‰) were used to normalize the isotopic values of working standards to the Vienna Pee Dee Belemnite (δ13C) scales. Isotope values are expressed in δ notation following the formula δX (‰) = [(Rsample/Rstandard) – 1] × 103, where X represents 13C and R is 13C/12C isotopic ratio. Working standards were analysed after every ten samples to monitor instrument performance and ensure data normalization. The precision of the laboratory standards was ±0.3‰ for C and N.
2.8 Statistical analysis
All statistical analyses were conducted in R 4.2.1 software (R Core Team), using trait data at the individual (tree) level (n = 10 individuals × 10 species for the juvenile tree stage, and n = 6 individuals × 9 species for the mature tree stage). Prior to analyses, the normal distribution of all traits within species was tested with the Shapiro–Wilk test and the homoscedasticity between species was tested with the Bartlett's test. Variation in stomatal and leaf morphology among species was conducted using simple ANOVAs with species as fixed factor and Tukey's HSD post-hoc tests to test variation in the measured traits among species (Table 1).
Models were fitted with restricted maximum likelihood. Inference was based on Wald t-tests with Satterthwaite's approximation to the degrees of freedom based on R package lmerTest v3.1–3 (Kuznetsova et al., 2017). Model assumptions were tested by inspection of residual diagnostic plots. As there were indications of increasing variance with the mean and a non-normality of the residuals, the model for WUE was re-fitted after log-transformation of the response. Estimates of the explained variance of the marginal and conditional predictions were computed according to Nakagawa et al. (2017) using R package MuMIn v1.47.5 (Bartoń, 2023). Parametric confidence bounds on partial predictions were computed with R package ciTools v0.6.1 (Haman & Avery, 2017).
Supplementary analyses Pearson correlations between traits were assessed and visualized with R package corrmorant (Link 2020).
3 RESULTS
3.1 Species level relationships between leaf and stomatal morphology, water-use efficiency and leaf carbon isotope ratio
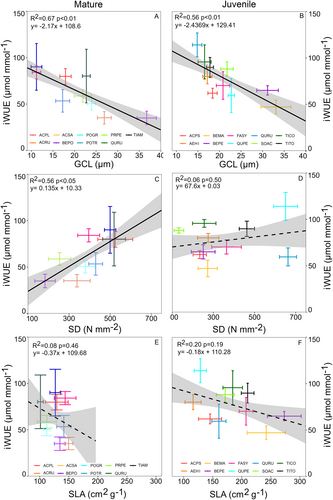
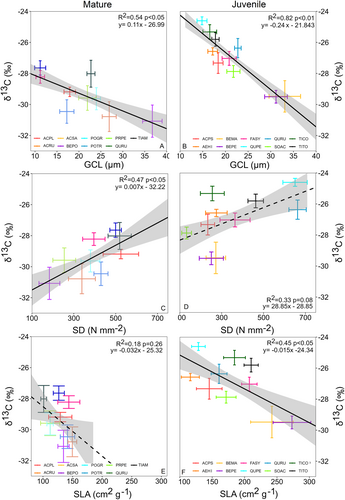
Across species, GCL was a significant predictor of iWUE and leaf δ13C. The increase of GCL corresponded to a reduction of iWUE (Figure 1A,B) and leaf δ13C (Figure 2A,B) in both juvenile and mature trees. All significant correlations for iWUE exhibited an R2 > 0.5, while those for δ13C demonstrated R2 > 0.4. The GCL and SD were negatively correlated for both juvenile and mature tree species (Figure S1). Therefore, SD showed a positive trend with iWUE and δ13C, but this trend was significant only for mature trees (Figures 1C and 2C). SLA showed a negative impact on both iWUE and leaf δ13C, but the relationship was significant only for the leaf δ13C of juvenile trees (Figure 2F).
| Species | ID | WP | SLA | GCL | SD | A | gs | iWUE | δ13C |
|---|---|---|---|---|---|---|---|---|---|
| (MPa) | (cm2 g−1) | (μm) | (N mm−1) | (μmol m−2 s−1) | (mmol m−2 s−1) | (μmol mmol−1) | (‰) | ||
| Juvenile trees | |||||||||
| Acer pseudoplatanus | ACPS | −0.66 ± 0.09 | 145.45 ± 19.7 cd | 18.4 ± 1.1cde | 233.7 ± 32.6d | 12.01 ± 2a | 207.00 ± 39.0a | 62.03 ± 5.3 cd | −27.32 ± 0.7de |
| Aesculus hippocastanum | AEHI | −0.78 ± 0.16 | 116.28 ± 13.6d | 17.19 ± 1.1ef | 274.7 ± 51.5 cd | 9.41 ± 1.1ab | 120.0 ± 19.2b | 80.1 ± 8bc | −26.58 ± 0.2 cd |
| Betula maximowicziana | BEMA | −0.71 ± 0.09 | 242.17 ± 32.1ab | 33.09 ± 3.5a | 272.3 ± 46.7 cd | 8.69 ± 1.2b | 193.6 ± 51.2a | 47.12 ± 7.4d | −29.47 ± 1f |
| Betula pendula | BEPE | −0.47 ± 0.10 | 273.17 ± 29.5a | 31.15 ± 2.5a | 250.6 ± 57.6d | 13.34 ± 2.3a | 209.8 ± 46.0a | 65.02 ± 5bcd | −29.50 ± 0.4f |
| Fagus sylvatica | FASY | −0.72 ± 0.09 | 207.33 ± 11.5b | 20.82 ± 1.6bcd | 363.9 ± 72bc | 8.08 ± 1.6b | 109.9 ± 11.8bc | 70.25 ± 14.9bcd | −27.03 ± 0.5de |
| Quercus petraea | QUPE | −0.68 ± 0.09 | 160.65 ± 12.9c | 14.84 ± 1.1b | 650.6 ± 58.2a | 10.48 ± 1.8ab | 97.6 ± 29.0bc | 114 ± 12.9a | −24.60 ± 0.3a |
| Quercus rubra | QURU | −0.69 ± 0.05 | 148.74 ± 10.2 cd | 22.74 ± 0.7f | 662.7 ± 41.4a | 7.98 ± 1.2b | 136.9 ± 39.8bc | 59.54 ± 18.4 cd | −26.36 ± 0.6bcd |
| Sorbus aucuparia | SOAU | −0.63 ± 0.09 | 171.48 ± 15.0c | 21.76 ± 1.4bc | 132.5 ± 23.3e | 7.4 ± 1.1b | 85.6 ± 15.3c | 88.04 ± 7.7bc | −27.87 ± 0.4e |
| Tilia cordata | TICO | −0.57 ± 0.06 | 184.42 ± 16.9bc | 16.55 ± 1.5ef | 255.4 ± 57d | 8.76 ± 1b | 98.1 ± 11.7bc | 95.68 ± 18.3a | −25.32 ± 0.5ab |
| Tilia tomentosa | TITO | −0.68 ± 0.10 | 210.92 ± 10.6b | 17.8 ± 0.8def | 462.7 ± 37.1b | 10.88 ± 0.8a | 124.4 ± 18.4b | 89.82 ± 10.7ab | −25.80 ± 0.4bc |
| Mature trees | |||||||||
| Acer platanoides | ACPL | −0.66 ± 0.14 | 129.22 ± 19.62b | 17.9 ± 1.31c | 524.9 ± 84.8ab | 9.64 ± 1.37a | 126.2 ± 21.1a | 79.82 ± 8.42c | −29.18 ± 0.3b |
| Acer rubrum | ACSA | −0.90 ± 0.07 | 126.74 ± 9.32a | 11.17 ± 1.25d | 699.8 ± 27.5ab | 10.13 ± 1.3ab | 125.7 ± 25.7a | 90.09 ± 25.14c | −27.64 ± 0.46a |
| Acer saccharinum | ACSX | −0.83 ± 0.16 | 143.67 ± 18.64a | 11.19 ± 0.98d | 695.8 ± 53.4b | 13.54 ± 1.48b | 181.2 ± 38.5b | 84.18 ± 7.09c | −28.22 ± 0.4a |
| Betula populifolia | BEPO | −1.00 ± 0.11 | 134.66 ± 9.51e | 36.72 ± 2.22a | 183.4 ± 49.8b | 13.53 ± 2.03b | 393.1 ± 37.2d | 35.04 ± 7.28a | −31.07 ± 1.03c |
| Populus grandidentata | POGR | −1.57 ± 0.16 | 141.43 ± 12.18b | 17.24 ± 1.62c | 429.7 ± 33.6b | 13.77 ± 1.22b | 276.0 ± 34.8c | 53.41 ± 12.08b | −30.45 ± 0.75bc |
| Populus tremuloides | POTR | −1.65 ± 0.26 | 112.2 ± 16.23c | 21.93 ± 1.98b | 354.4 ± 54.4a | 15.93 ± 1.24b | 283.4 ± 35.3c | 58.74 ± 6.79b | −29.58 ± 0.79b |
| Prunus pensylvanica | PRPE | −0.86 ± 0.17 | 149.58 ± 7.43d | 26.9 ± 1.59b | 338.7 ± 64.3b | 8.91 ± 1.9a | 254.7 ± 34.2bc | 35.26 ± 6.65a | −30.78 ± 0.96bc |
| Quercus rubra | QURU | −1.07 ± 0.26 | 101.19 ± 4.88c | 22.74 ± 0.91c | 519.1 ± 55.5a | 15.43 ± 2.44b | 236.7 ± 57.0bc | 80.18 ± 28.88c | −28.01 ± 0.86ab |
| Tilia americana | TIAM | −0.87 ± 0.11 | 112.02 ± 5.37 cd | 23.97 ± 0.93bc | 379.5 ± 45.2a | 14.72 ± 0.68b | 305.6 ± 31.6c | 50.52 ± 6.86ab | −29.63 ± 0.71bc |
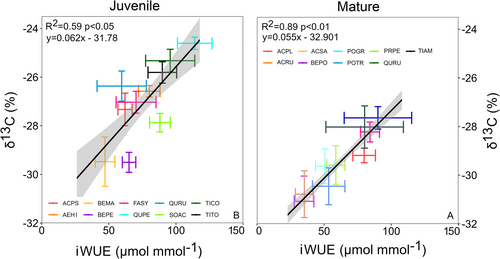
The iWUE derived from gas-exchange measurements corresponds to leaf δ13C among tree species. The iWUE and leaf δ13C showed significant positive linear relationships for both juvenile and mature trees (Figure 3A,B). The relationship between iWUE and δ13C showed greater explanatory power for mature (R2 = 0.89) than for juvenile (R2 = 0.59) trees.
3.2 Individual level relationships between leaf and stomatal morphology, water-use efficiency and leaf carbon isotope ratio
The mixed effects model of iWUE as a function of GCL, SD and SLA at the individual level explained 63.1% of the variance in iWUE, of which 45.0% were explained by the fixed effects alone (Table 2). On average, iWUE was higher for juvenile than for mature trees. iWUE was moreover significantly lower for leaves with a higher average guard cell length both for mature and juvenile trees (Figure 5A). In addition, the iWUE of the leaves of mature trees was lower for leaves with higher SLA (Figures 4C and 5A). Notably, SD did not have a significant effect on iWUE after accounting for the effect of GCL and SD (Figures 4C and 5A).
| Parameter | Ontogeny | Estimate | Std.error | Lwr 95% CI | Upr 95% CI | t-statistic | df | p-.value |
|---|---|---|---|---|---|---|---|---|
| Model for iWUE | ||||||||
| αs (Intercept) | Juvenile | 4.286 | 0.054 | 4.168 | 4.403 | 78.818 | 12.784 | <0.001 |
| Mature | 3.881 | 0.085 | 3.706 | 4.056 | 45.246 | 31.215 | <0.001 | |
| β1 (SD) | Juvenile | 0.007 | 0.039 | −0.071 | 0.085 | 0.188 | 52.876 | 0.851 |
| Mature | 0.124 | 0.090 | −0.054 | 0.303 | 1.377 | 132.133 | 0.171 | |
| β2 (GCL) | Juvenile | −0.212 | 0.053 | −0.321 | −0.104 | −3.959 | 49.194 | <0.001 |
| Mature | −0.144 | 0.067 | −0.278 | −0.009 | −2.130 | 69.997 | 0.0376 | |
| β3 (SLA) | Juvenile | 0.053 | 0.037 | −0.021 | 0.127 | 1.412 | 116.924 | 0.161 |
| Mature | −0.176 | 0.085 | −0.346 | −0.006 | −2.065 | 100.837 | 0.041 | |
| τsp | 0.149 | NA | NA | NA | NA | NA | NA | |
| σ | 0.213 | NA | NA | NA | NA | NA | NA | |
| Model for δ13C | ||||||||
| αs (Intercept) | Juvenile | −26.843 | 0.307 | −27.566 | −26.120 | −87.310 | 7.194 | <0.001 |
| Mature | −29.576 | 0.411 | −30.451 | −28.701 | −71.804 | 15.656 | <0.001 | |
| β1 (SD) | Juvenile | 0.204 | 0.161 | −0.115 | 0.524 | 1.264 | 105.781 | 0.208 |
| Mature | −0.136 | 0.340 | −0.808 | 0.536 | −0.399 | 145.903 | 0.698 | |
| β2 (GCL) | Juvenile | −0.785 | 0.226 | −1.235 | −0.335 | −3.469 | 85.023 | <0.001 |
| Mature | −0.396 | 0.287 | −0.975 | 0.182 | −1.380 | 44.570 | 0.174 | |
| β3 (SLA) | Juvenile | −0.160 | 0.142 | −0.441 | 0.121 | −1.123 | 145.995 | 0.263 |
| Mature | −0.261 | 0.332 | −0.918 | 0.396 | −0.785 | 139.41 | 0.434 | |
| τsp | 0.920 | NA | NA | NA | NA | NA | NA | |
| σ | 0.748 | NA | NA | NA | NA | NA | NA |
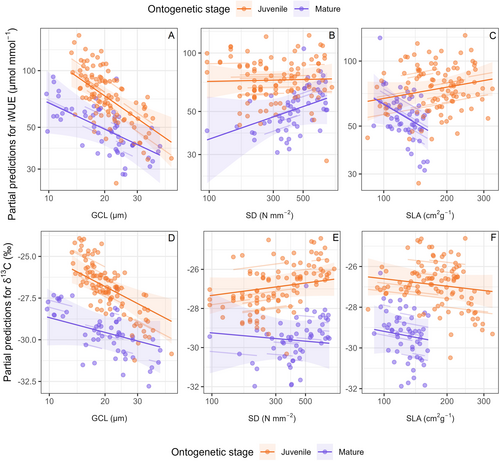
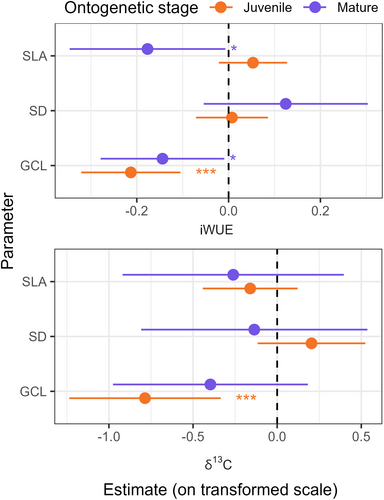
An analogous model of leaf δ13C at the individual level explained 82.8% of the variance in leaf δ13C, of which 56.8% were explained by GCL, SD an SLA. Here, only the GCL for juvenile trees had a significant impact, while the other variables did not (Figures 4D and 5B). The partial effects of the model showed similar patterns as for iWUE, where increasing GCL had a negative impact on leaf δ13C.
3.3 Variation in stomatal and leaf morphology among species and ontogenetic stages
All tested morpho-physiological traits differed significantly among species for both juvenile and mature stages (Table S1). The averages with 95% confidence intervals and results of Tukey's HSD post-hoc analysis for each measured trait are presented in Table 1. The lowest iWUE was observed for Betula sp. at both juvenile and mature stages (35–65 μmol mmol−1 average range) and for mature Prunus pensylvanica (35 μmol mmol−1). The highest iWUE was observed among the mature trees of Acer sp. and Quercus rubra (averages between 80 and 90 μmol mmol−1) and among juvenile Quercus petraea and two Tilia sp. (averages between 95 and 114 μmol mmol−1). The aforementioned species had an approximately 2.5× higher iWUE than species with a low iWUE in both juvenile and mature stages. The data from both experiments showed a dependency between GCL and SD at the species level (Figure S1). Accordingly, species with high GCL, like Betula sp., had a lower SD and species with low GCL, like Acer sp. and Quercus sp., had a higher SD in our study (Table 1). The differences in specific leaf area (SLA) between species were greater among the juvenile trees than between mature trees (Table 1). The Betula sp. had the greatest SLA among juvenile trees and Aesculus hippocastanum the lowest. The overall mean SLA of mature trees was lower than that of juvenile trees. The variability of SLA between species was much higher among juvenile trees than among mature trees (Table 1).
4 DISCUSSION
4.1 Guard cell length affects water-use efficiency and δ13C of trees
We observed significant negative relationships between GCL and both iWUE and δ13C for juvenile and mature trees both inter-specifically and intra-specifically (with the exception of mature δ13C within species). A higher guard cell length corresponded to a lower iWUE, as well as a longer-term WUE proxy of δ13C, thus confirming hypothesis 1. A negative relationship between stomatal size or GCL and iWUE has been previously reported mostly in crops and was explained by the faster response time of smaller stomata to changing environmental conditions compared to larger stomata (Drake et al. 2013; Lawson and Blatt 2014; Kardiman and Raebild 2018; Durand et al. 2019). Lei et al. (2023) showed that larger stomata exhibited a decelerated response time to fluctuations in light intensity and demonstrated an overall diminished water-use efficiency (inferred from δ13C). Larger stomata can also have higher maximal gs, which increases transpiration and, therefore, can have a negative impact on iWUE (Fanourakis et al. 2015). A genetic manipulation experiment showed that rice mutants with reduced stomatal size exhibited increased iWUE compared to mutants with larger stomatal size (Pitaloka et al. 2022). Similarly, the iWUE in wheat cultivars was correlated negatively with stomatal size and transpiration rates (Li et al. 2017). Amitrano et al. (2021) found that lettuce exhibited a substantial 49% increase in iWUE that was associated with a reduction of stomatal size under different VPD treatments. Furthermore, exposure to drought stress led to the inhibition of stomatal development, resulting in smaller stomata and an increase of iWUE in cotton (Dubey et al. 2023). On the other hand, two studies focusing on the intra-specific variability of iWUE did not find a significant relationship between GCL and iWUE, most likely due to low GCL variability across poplar genotypes (Durand et al. 2019; Durand et al. 2020). The GCL is probably under strong intra-specific genetic control, as previously observed for different European beech provenances (Petrík et al. 2020). Our results showed that GCL had a significant impact on iWUE also within species and tends to impact the leaf δ13C of mature trees. The use of stomatal imprints is a cost-effective method to characterize trees' water-use efficiency variability compared with labour-intensive gas-exchange measurements or costly carbon isotope analysis. Our results support findings from crops that species with smaller stomatal cells with lower GCL have a higher immediate leaf iWUE derived from gas-exchange and higher leaf δ13C as proxy for long-term WUE. The relationship is slightly weaker for intra-specific comparison at the individual level but is quite robust at the species level. The creation of stomatal imprints is significantly cheaper and faster than gas-exchange or δ13C measurements. Therefore, stomatal morphology traits can be measured more extensively in the field (more sites, higher sample size) compared to the other two methods, which highlights their potential value for large-scale phenotyping studies. The overall time efficiency is limited by the measurement of stomatal morphological parameters and stomatal density, which can be significantly improved by the implementation of machine learning or AI tools that automate the measurements (Casado-Garcia et al. 2020; Wu et al. 2024). Additionally, it is important to note that stomatal imprints primarily capture the water side of iWUE, and that photosynthetic efficiency must be characterised by other methods to fully understand iWUE constraints (Al-Salman et al. 2024).
4.2 Stomatal density and water-use efficiency
We observed that SD had a significant positive effect on iWUE and leaf δ13C of mature trees (p < 0.05) and a marginally significant impact on leaf δ13C (p = 0.08) of juvenile trees at the species level (hypothesis 2 confirmed). On the other hand, the mixed model showed no impact of SD on individual-level iWUE or leaf δ13C. The discrepancies between the inter- and intra-specific comparison may in part result from the different aggregation levels (Pollet et al. 2015; Isasa et al. 2023). However, the disappearing SD effect when accounting for GCL is likely also driven by the relatively high correlation between the two variables. Leaf stomatal density can have a distinct effect on overall plant water loss. For instance, genetical manipulation studies in crops show overwhelming evidence that a reduction of SD leads to increased WUE due to lower transpiration rates (Liu et al. 2015; Guo et al. 2019; Li et al. 2020; Pitaloka et al. 2022). In stark contrast, there are multiple studies that reported a positive relationship between SD and WUE in plants (Xu et al. 2008; Naz et al. 2010; Zhao et al. 2015; Stojnić et al. 2019; Bhaskara et al. 2022; Al-Salman et al. 2023; Caine et al. 2023). As there is a general trade-off between GCL and SD in plants due to space constraints of leaves (Lawson et al. 2016), increasing GCL typically leads to lower SD in natural populations (Haworth et al. 2023). The increase of SD and reduction of stomatal size is a common acclimation response to water-deficit or drought stress in trees (Dunlap and Stetter 2001; Pearce et al. 2006; Boughalleb et al. 2014; Stojnić et al. 2015). Gene manipulation techniques on crops can disproportionally reduce SD compared to an increase in GCL, which might not be realistic for natural populations, or it may influence other factors that affect iWUE (Franks et al. 2015). In a study by Hughes et al. (2017), the reduction of SD in barley via gene manipulation also led to a reduction of GCL and an improved iWUE. Therefore, the reduction of SD can have a strong positive impact on iWUE in gene manipulation studies in crops, but the applicability of lowering SD under field conditions, particularly in tree species, is still not well understood. Our results show that GCL and SD are good predictors of iWUE and leaf δ13C across species, but GCL is more reliable for capturing individual-level relationships and intra-specific variability of trees.
4.3 Specific leaf area and water-use efficiency
Specific leaf area (SLA) had a minor role in explaining water-use efficiency in our study. We found that SLA had a significant negative impact on the individual level iWUE of mature trees and a negative impact on leaf δ13C of juvenile trees at the species level. SLA is widely used in functional ecology as a proxy for plant life strategies (e.g., Wright et al. 2010), where high SLA is typically associated with an ‘acquisitive’ growth strategy and high relative growth rate (Wright et al. 2004; Baird et al. 2017). SLA can reflect the differences in leaf anatomical structure that can influence the variability of iWUE between species via changes in mesophyll conductance (Mediavilla et al. 2001; Tomás et al. 2013; Carriquí et al. 2015; Trueba et al. 2022). Previous studies reported that SLA was negatively correlated with iWUE and δ13C in crops (Craufurd et al. 1999; Reddy et al. 2020a,b), shrubs (Horike et al. 2023), trees (Wang et al. 2013; Ge et al. 2022; Zhong et al. 2022) and forests (Guerrieri et al. 2021). Our results also show the tendency of a negative correlation between SLA and both iWUE and δ13C, though this was significant only for the relationship with δ13C of juvenile species inter-specifically and with the iWUE of mature trees intra-specifically. Our measurements showed different coverage of variable ranges for juvenile and mature species that could affect these results. The lower variability in SLA for mature trees may indicate that even though we sampled sun-exposed leaves from the crown edges, these might have been still more shaded than the sun-exposed leaves of the seedlings (Baird et al. 2017). Moreover, leaf size increases and thickness declines vertically (Oldham et al. 2010; Schuldt et al. 2011; Coble et al. 2014). In comparison, GCL and SD are more influential and more robust traits capturing the iWUE and leaf δ13C variability than SLA.
4.4 Relationship between intrinsic water-use efficiency and leaf carbon isotope ratio
The significant positive relationship between iWUE and leaf δ13C observed for both juvenile and mature tree species serves as a vital indicator of the capacity of trees in regard to carbon-water utilization. The positive relationship between gas-exchange derived iWUE and leaf δ13C (or negative relationship between iWUE and δ13C) has also been observed inter-specifically (Grossnickle et al. 2005; Ducrey et al. 2008; Roussel et al. 2009; Marguerit et al. 2014; Kaluthota et al. 2015). The leaf δ13C reliably reflects seasonal iWUE and therefore can capture long-term trends as in our study, where the trees were not exposed to water-deficit stress. Exposure of plants to short-term drought or heat stress can create a discrepancy between momentary iWUE and leaf δ13C as the sampled leaves contain carbohydrates from pre-stress period not affected by current A/gs balance (Camarero et al. 2023; Pernicová et al. 2023). The leaf δ13C is thus a good proxy for a long-term iWUE, especially under relatively homogenous environmental conditions. Also, as it can be easily sampled in a large number of individuals in the field while representing plant long-term trends in water-use efficiency, the leaf δ13C offers good insights to analyse the adaptation of tree species to environmental aridity (Rabarijaona et al. 2022).
4.5 General comparison of species and ontogenetical stages
The inter-specific differences between stomatal and leaf traits, iWUE and δ13C reflect their functional adaptation to the environment. We can see a clear differentiation between pioneering species such as Betula populifolia (low iWUE), fast-growing species such as Populus grandidentata (low iWUE) and climax forest species such as Quercus petraea or Tilia cordata (high iWUE). Nevertheless, our results show that there are also intermediaries between these two edge cases, where high iWUE species can also have relatively high assimilation rates (Acer saccharinum), or low iWUE species can have relatively low assimilation rates (Prunus pensylvanica). It should be noted that forest tree species typically have a high intra-specific variability, and sample size of 7–10 trees may be inefficient in capturing the functional differences between species. We observed a very high variability of GCL and SD between the species as well. The species-level GCL correlated negatively with SD, consistent with the assumed trade-off between the size and frequency to optimize the overall conductive surface to water vapour and CO2 that was described in numerous other studies (Doheny-Adams et al. 2012; Boer et al. 2016; Rahman et al. 2022). The differences between the species we report here represent the carbon-water balance under well-watered conditions and should be without the impact of reduced stomatal conductance. Hence, we excluded any drought stress impacts that can strongly alter the iWUE/δ13C of plants (Roman et al. 2015; Hajíčková et al. 2021; Hartmann et al. 2021; Gebauer et al. 2022). Therefore, the relationship between GCL and iWUE/δ13C could be even more (or less) pronounced under drought stress conditions.
The tree age class can also affect the iWUE, which is usually species-specific and tied to the stand structure (Tanaka-Oda et al. 2010; Matoušková et al. 2022). Neither juvenile nor mature trees in our study were light-limited; therefore, we can eliminate the impact of light competition. The only species sampled in both ontogenetical stages was Quercus rubra (QURU). The juvenile QURU had significantly higher SLA and SD than mature QURU, and significantly lower A and gs, but there were no significant differences in GCL, iWUE or δ13C. Similarly, a study by Cavender-Bares & Bazzaz (2000) found no changes in iWUE between three ontogenetical stages of Q. rubra under well-watered conditions. Ontogeny had a significant impact on leaf anatomical and morphological traits, but no impact on assimilation or stomatal conductance among tropical tree species (Ishida et al. 2005; Fortunel et al. 2019). These results suggest that leaf morphological traits might change during ontogenetical stages, but the water-use efficiency remains stable. Nevertheless, our sampling did not cover very old trees in which the iWUE could be limited by soil nutrients (N, P) or aging (Munné-Bosch 2007; Brueck et al. 2008; Huang et al. 2016).
The strength and significance of relationships between SD, SLA and iWUE, δ13C differed between ontogenetical stages (juvenile, mature). Irrespective of tree age and species, GCL showed over all a consistent negative relationship with iWUE and was significant for both ontogenetical stages inter-specifically and intra-specifically. This indicates the potential of GCL as a highly effective predictor of iWUE regardless of the ontogenetical stage. The relationship between GCL and δ13C was significant for both ontogenetic stages across species, but only for juvenile trees intra-specifically. The study by Fortunel et al. (2019) found a significant impact of the ontogenetical stage on leaf δ13C and dark respiration but not on assimilation or stomatal conductance and, therefore, probably no impact on iWUE. The lower explanatory power of GCL in regards to δ13C (compared to iWUE) in mature trees could be explained by differences in the respiratory substrate used throughout the season (Salomón et al. 2023). The iWUE signal recorded in the δ13C of plant organic material is photosynthetic rate weighted, meaning that more carbon is assimilated during periods of high photosynthetic rate. Consequently, the iWUE signal from these periods is more strongly represented in the bulk leaf material (Bing et al. 2022). This also implies that δ13C is more biased toward variations in A as well as tissue growth, while GCL primarily reflects the gs component of iWUE. This difference could explain why we observed stronger correlations between GCL and iWUE compared to δ13C. Therefore, it is critical to explore if the connections between morphological and physiological traits are consistent throughout ontogenetical development (life stages) if we want to transfer inferences obtained at seedling or juvenile level to mature trees. Finally, our study used two sets of trees grown under different conditions and experienced varying environments during the sampling period. It is thus possible that the differences observed between juvenile and adult stages may also be driven by variations in soil type, nutrients, climate, watering status, as well as within-species genetic variation. Controlled condition experiments limiting environmental variations will now be needed if we are to characterize more precisely the sole effects of ontogeny on the relationships among SD, SLA, iWUE and δ13C.
5 CONCLUSION
Our study confirmed our assumption that stomatal guard cell length (GCL) and stomatal density (SD) are important determinants of both short-term intrinsic water-use efficiency (iWUE) from gas exchange and long-term WUE derived from leaf carbon isotopes (δ13C). Both iWUE and δ13C correlated positively with SD and negatively with GCL for juvenile and mature trees across species. The GCL was a stronger predictor of both iWUE and δ13C compared to SD within species. In addition, the short-term iWUE showed a strong positive correlation with leaf δ13C in both ontogenetical stages. We conclude that GCL is a valuable addition to the functional trait toolkit that permits rapid phenotyping of the WUE strategy of broadleaved tree species regardless of their age class.
AUTHOR CONTRIBUTIONS
PP, RML conceived the paper idea; PP, APP, LJL, PAW conducted the measurements; PP, RML, VM conducted the statistical analysis; PP, RML prepared the visualizations; PP, APP, LJL, RML, NKR, BS, VM wrote the first draft of the paper; BS and VM supervised all processes; all authors contributed to the final version of the paper.
ACKNOWLEDGEMENTS
We thank Romane Hubert and Vincent Paul Riedel for their technical assistance.
FUNDING INFORMATION
NKR acknowledges funding by the Helmholtz Initiative and Networking fund (grant no. W2/W3-156). The authors further gratefully acknowledge the financial support granted by the Bundesministerium für Ernährung und Landwirtschaft (Germany), Bundesministerium für Umwelt, Naturschutz und nukleare Sicherheit (Germany) and the Fachagentur Nachwachsende Rohstoffe eV (Germany) within the frame of the ‘Waldklimafond’ (project NONNATIVE) that partly funded this study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




