Identification of novel inhibitors of plant GH3 IAA-amido synthetases through molecular docking studies
Abstract
Auxins play a critical role in several plant developmental processes and their endogenous levels are regulated at multiple levels. The enzymes of the GRETCHEN HAGEN 3 (GH3) protein family catalyze the conjugation of amino acids to indoleacetic acid (IAA), the major endogenous auxin. The GH3 proteins are encoded by multiple redundant genes in plant genomes, making it difficult to perform functional genetic studies to understand their role in auxin homeostasis. To address these challenges, we used a chemical approach that exploits the reaction mechanism of GH3 proteins to identify small molecule inhibitors of their activity from a defined chemical library. The study evaluated receptor-ligand complexes based on their binding energy and classified them accordingly. Docking algorithms were used to correct any deviations, resulting in a list of the most important inhibitory compounds for selected GH3 enzymes based on a normalized sum of energy. The study presents atomic details of protein-ligand interactions and quantifies the effect of several of the identified small molecule inhibitors on auxin-mediated root growth processes in Arabidopsis thaliana. The direct effect of these compounds on endogenous auxin levels was measured using appropriate auxin sensors and endogenous hormone measurements. Our study has identified novel compounds of the flavonoid biosynthetic pathway that are effective inhibitors of GH3 enzyme-mediated IAA conjugation. These compounds play a versatile role in hormone-regulated plant development and have potential applications in both basic research and agriculture.
1 INTRODUCTION
The phytohormone auxin plays a pivotal role in regulating the growth and development of plants throughout their life cycle and in response to various environmental cues (Benjamins and Scheres 2008). The regulation of auxin metabolism (biosynthesis, conjugation, and degradation) and polar auxin transport are mutually dependent processes that function by establishing a tightly controlled spatiotemporal distribution of cellular auxin levels, which, in turn, could lead to concentration- and cell-type-dependent cellular responses (Ruiz Rosquete et al. 2012).
Group II members of the GRETCHEN HAGEN3 (GH3) family of acyl amido synthetases mainly catalyze the conjugation of aspartate (Asp) and glutamate (Glu) to indole-3-acetic acid (IAA), resulting in the formation of inactive IAA-Asp and IAA-Glu conjugates (Staswick et al. 2005). Some group II GH3 enzymes have the capacity to add amino acid residues to other hormones, including jasmonic acid and salicylic acid, which enables them to regulate the levels of these hormones as well (Gutierrez et al. 2012; Westfall et al. 2016). Genes encoding group II GH3 enzymes have been identified in multiple plant species, including Arabidopsis thaliana, rice, grape, and tomato (Jez 2022). In A. thaliana, eight genes are known to encode group II GH3 enzymes, which presents a challenge for their functional dissection due to genetic redundancy. This is demonstrated by the observation that the single loss-of-function gh3 mutants display subtle IAA-related phenotypes (Zheng et al. 2016). The early availability of the crystal structure of the grapevine GH3-1 protein (Peat et al. 2012) facilitated the rational design of a competitive inhibitor, adenosine-5′-[2-(1H-indol-3-yl)ethyl]phosphate (AIEP), with broad activity across all members of this enzyme family (Böttcher et al. 2012). Molecular docking assays of AIEP into the active site of the rice OsGH3-8 corroborated the effectiveness of this GH3 inhibitor also in monocots (Xu et al. 2021). AIEP has been employed to diminish endogenous IAA-Asp levels in grape berry tissues (Böttcher et al. 2012) and in carnation stem cuttings, where it has been demonstrated to markedly enhance the rooting performance of a rooting-deficient commercial cultivar (Cano et al. 2018).
Two phenotype-based screens using synthetic chemical libraries have recently been conducted with the objective of identifying additional inhibitors of group II GH3 amido synthetases (Hayashi et al. 2021; Fukui et al. 2022; Xie et al. 2022). The application of the GH3 inhibitors kakeimide and nalacin resulted in the manifestation of high-auxin phenotypes in A. thaliana and other plant species, including the development of shorter primary roots (PRs) and an increase in the number of lateral roots (LRs) and adventitious roots. This phenocopied the multiple gh3 mutants currently available (Casanova-Sáez et al. 2022; Guo et al. 2022), indicating that GH3 inhibitors may serve as a promising chemical tool for elucidating auxin homeostasis in a range of plant species.
The objective of this study was to identify novel inhibitors of group II GH3 enzymes that enhance endogenous levels of IAA, with the potential for application as plant growth regulators in agriculture. To this end, we developed an in silico procedure based on molecular docking analysis and employed it to screen a database of food-derived metabolites. This approach has enabled the identification of a series of flavonoid compounds with the capacity to interfere with the enzymatic activity of group II GH3 enzymes. In vivo assays using fluorescent lines and mutants of A. thaliana demonstrated that some of these compounds elicit a response comparable to the exogenous addition of auxin, likely due to their impact on the inhibition of group II GH3 enzymatic activity.
2 MATERIALS AND METHODS
2.1 Structure selection and docking analyses
The selected structures for docking analysis were obtained from RCSB Protein Data Bank (PDB) (https://www.rcsb.org/). The GH3-1 enzyme of Vitis vinifera at 2.4 Å resolution was found in complex with the inhibitor AIEP (RCBS PDB code: 4B2G) (Peat et al. 2012). The structure of the isoform GH3.5 from A. thaliana was directly taken from the RCBS PDB database, with code 5KOD (2.2 Å resolution). Isoforms GH3.1, GH3.2, GH3.3 and GH3.4 from A. thaliana were modeled using the structure of GH3-1 from V. vinifera, while isoforms GH3.6, GH3.9, and GH3.17 were modeled using the crystal structure of the A. thaliana GH3.5 isoform. The GH3.1 protein from Solanum lycopersicum Solyc01g107390.4.1 gene was modeled using the structure of GH3-1 from V. vinifera.
Homology modeling was performed with Yasara version 22.5.22 (http://www.yasara.org), using standard modeling protocols (Krieger et al. 2005), including manually provided alignments. Loops are optimized by trying a large number of different conformations, and side chain rotamers are fine-tuned considering electrostatic and knowledge-based packing interactions, as well as solvation effects. Similarly, the hydrogen bonding network is optimized, including pH-dependence and ligands. Finally, a high-resolution energy minimization with explicit solvent is run using the latest knowledge-based force field implemented in the Yasara web application, and the quality of the models is evaluated by calculating Z-scores.
Docking procedures were conducted by means of the following programs: DOCK6 (https://dock.compbio.ucsf.edu/DOCK_6/index.htm), based on an anchor-and-grow algorithm (Allen et al. 2015), AutoDock4 (https://autodock.scripps.edu), a classical genetic algorithm (Morris et al. 2009), Yasara-Vina, a modern algorithm based on Monte Carlo sampling (Trott and Olson 2010), X-Score (https://www.ics.uci.edu/~dock/manuals/xscore1.1_manual/intro.html) (Wang et al. 2002; Obiol-Pardo and Rubio-Martinez 2007) and DSX (https://agklebe.pharmazie.uni-marburg.de/?id=11) (Neudert and Klebe 2011), which evaluate the poses obtained with AutoDock4 in different ways. For docking purposes, the reference ligand is used to size and center the simulation box in the cavity. The simulation box (a cube) restricts the simulation space.
In this way, the ligand reference resulted in 1, and any value above 1 indicates that the compound bounds better than the reference ligand. The screening collection used was Phenol Explorer (http://phenol-explorer.eu/downloads), a comprehensive web-based database on polyphenol content in foods containing 921 molecules (Neveu et al. 2010).
2.2 Plant material and growth conditions
The plant genotypes used in these experiments were wild type Arabidopsis thaliana (L.) Heyhn. Columbia-0 (Col-0), PIN1-GFP (Benková et al. 2003), PIN2-EGFP (Xu and Scheres 2005), DII-VENUS (Brunoud et al. 2012), gh3.1,2,3,4,5,6,9,17 (Casanova-Sáez et al. 2022), and dao1-1 (Porco et al. 2016). Seeds were disinfected in 50% (v/v) commercial bleach with 1% Triton. They were then rinsed with several washes of sterile water before being incubated for 2 days at 4°C in the dark for stratification.
The seeds were sown in 120 mm × 120 mm × 10 mm square Petri dishes containing 75 mL of germination medium (GM medium) with ½ Murashige & Skoog (MS) salts (Duchefa Biochemie), 1% sucrose, 8 g L−1 plant-agar (Duchefa Biochemie), and 1 × Gamborg B5 vitamin mix (Duchefa Biochemie). The plates were placed in an MLR-352-PE growth chamber (Panasonic) at 22 ± 1°C and continuous light (50 μmol m−2 s−1) for 6 days in a nearly vertical position. For each genotype, 14 to 16 seedlings at a similar developmental stage were transferred to 90 mm × 14 mm round Petri dishes with (1) GM medium (mock treatment), GM medium supplemented with (2) apigenin (40 and 100 μM), (3) apigenin 7-O-glucuronide (40 and 100 μM), (4) luteolin 7-O-glucuronide (40 and 100 μM), (5) dihydroquercetin (40 μM), (6) dihydromyricetin (10 and 20 μM), (7) indole-3-acetic acid (20 nM), or (8) indole-3-acetic acid (20 nM) and dihydromyricetin (10 μM). The experiments were conducted in triplicate. The plates were incubated for four days (two days in light and two days in dark) in the growth chamber, as described above.
2.3 Image acquisition, processing, and parameter measurement
The images were obtained from Petri dishes at 0, 2, and 4 days after transfer to supplemented media using an Epson Perfection V330 photo scanner at a resolution of 1200 dpi and using the film option at a resolution of 2400 or 3200 dpi. Subsequently, the images were saved in the RGB color format with the JPEG file format. The PR and root hairs were quantified using a Fiji ImageJ plug-in. The number of LRs was determined by visual observation at the indicated times using a Motic SMZ-168 stereomicroscope.
Microscopic images were acquired using a TCS SP8 confocal microscope (Leica Microsystems) controlled by LAS X v3 software. The seedlings were stained in 10 μg/mL of propidium iodide for one minute, after which they were washed in water for 30 seconds to visualize the cell walls. The samples were imaged using a 20×/0.75 air immersion objective for observation and image acquisition. The excitation (Ex.) and emission (Em.) wavelengths utilized were as follows: GFP (Ex./Em.): 488/498–530; Venus (Ex./Em.): 514/493–578, propidium iodide (Ex./Em.): 561/578–718. During image acquisition with the confocal microscope, the laser intensity and gain values were maintained at constant levels for each untreated fluorescent line, ensuring accurate and subsequent analysis of the fluorescent signal.
The analyses conducted on the PRs (root meristem length, fluorescent signal intensity value and number and length of cortex cells within the root meristem) were performed using the Fiji software (Schindelin et al. 2012). The meristem of each PR was defined as the longitudinal region extending from the quiescent center cells to the first elongated cell in the cortex. Consequently, a line was drawn from both defined endpoints and its length was measured with the Fiji software. To analyze signal intensity, the same reference was utilized, and a rectangle was drawn around the meristem to measure the intensity values with ImageJ. The number of cortex cells was manually counted, and the length of each cortex cell was measured along the longitudinal axis using the line tool from the Fiji software.
2.4 Hormone analysis
A total of 14 to 16 five-day-old seedlings were transferred to plates containing GM medium, which had been supplemented with either a control solution or a solution containing 20 μM dihydromyricetin. The plates were incubated for a period of two days under continuous illumination. Following the harvest, the samples were frozen in liquid nitrogen and stored at a temperature of −80°C. The experiments were conducted in triplicate. The analysis of hormonal metabolites was conducted in accordance with the methodologies outlined by Albacete et al. (2008) and Großkinsky et al. (2014), with certain modifications. In summary, 40–100 mg of frozen plant material was homogenized and poured into variable volumes (170–500 μL) of a cold (−20°C) methanol/water (80/20, v/v) extraction mixture. The solids were separated by centrifugation (20,000 g, 15 minutes) and re-extracted for 30 minutes at 4°C in an additional volume of the same extraction solution. The combined supernatants were passed through a Sep-Pak Plus C18 cartridge to remove interfering lipids and some of the plant pigments. Subsequently, the organic solvent was evaporated at 40°C under vacuum until the sample was in a near-dry state. Subsequently, the residue was dissolved in 0.5 mL of a methanol/water solution (20/80, v/v) using an ultrasonic bath. The dissolved samples were filtered through 13 mm diameter Millex filters with 0.22 μm pore size nylon membranes (Millipore). Ten microliters of the filtered extract were introduced into a U-HPLC-MS system comprising an Accela Series U-HPLC (ThermoFisher Scientific) linked to an Exactive mass spectrometer (ThermoFisher Scientific) with a heated electrospray ionization (HESI) interface. Mass spectra were obtained using the Xcalibur software, version 2.2 (Thermo Fisher Scientific). Calibration curves were constructed for each phytohormone component analyzed (1, 10, 50, and 100 μg L−1), with the data corrected for deuterated internal standards at 10 μg L−1. The recovery rate was determined to be between 92 and 95%.
2.5 Statistical analyses
Statistical analyses of the measured parameters were conducted using StatGraphics Centurion XV (StatPoint Technologies) and GraphPad Prism 10.1.2 (GraphPad Prism Inc.). Outlier data were identified based on aberrant standard deviation values and were excluded from further analysis (Aguinis et al. 2013). One-sample Kolmogorov–Smirnov tests were conducted to evaluate the fit between the data distribution and theoretical normal distribution. Non-parametric tests were used when necessary. To compare data for a specific variable, we conducted multiple test analyses using the ANOVA F test, Student's T-test or Fisher's least significant difference (LSD) methods. Significant differences were recorded at a significance level of 1% (P-value <0.01) unless otherwise specified.
3 RESULTS AND DISCUSSION
3.1 In silico search for novel inhibitors of group II GH3 IAA-amido synthetases
The structure of GH3.1 from V. vinifera (RCBS PDB 4B2G) was first selected and used for the identification of putative inhibitors. The in-silico approach started with the localization of the receptor area for the binding site since the cavity of the GH3.1 to allocate ligands is very large, and the indole-3-acetic acid (IAA) binding site was not well defined in this structure. For that, the AIEP ligand present in the original structure was first split into AMP and indol molecules. The AMP was retained to emulate the original AMP binding site (Figure 1A, green), and the indol moiety was fused to the malonate molecule to recreate the amino acid derivative of IAA bound to the IAA binding site, which is then used as the reference ligand. Given that some GH3 enzymes have been observed to bind chorismate (Holland et al. 2019), a molecule of chorismate was incorporated into a cavity situated near the AMP site (Figure 1A, yellow) to restrict the internal space available for searching the inhibitors. Finally, the AMP and chorismate molecules were fixed, whereas the artificial derivative of IAA molecule (reference ligand) was removed to allow the docking algorithm to search for candidate compounds in this site (Figure 1A, cyan). Docking programs (DOCK6, AutoDock4 and Yasara-Vina) were started using the receptor (GH3-AMP-chorismate), while X-Score and DSX used the poses obtained with Autodock4 to evaluate the binding energy of interaction with different force fields.
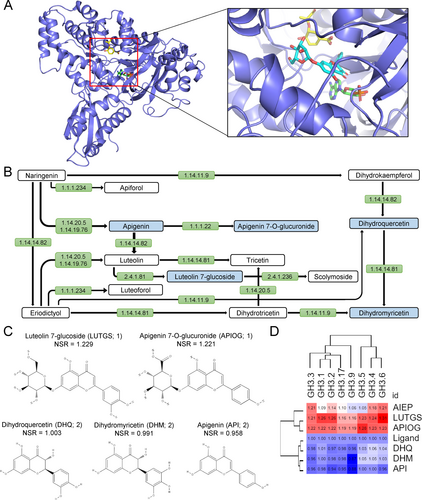
The screening of the Phenol Explorer library (Neveu et al. 2010) over GH3-1 of V. vinifera, S. lycopersicum and the different isoforms of A. thaliana enzymes were analyzed with the NSR consensus docking method (Blanes-Mira et al. 2022), which resulted in a sorted list of compounds (Tables S1 and S2), which were then studied in the context of the metabolic pathway of flavonoid and flavonol biosynthesis in A. thaliana (Figure 1B, see below).
3.2 Flavonoid derivatives are candidates for the inhibition of group II GH3 enzymes
Flavonoids are a relatively diverse family of low molecular weight polyphenolic secondary metabolites derived from plants that play an important role in numerous biological processes (Winkel-Shirley 2001). Several compounds that map to the flavonoid biosynthesis pathway were identified among those exhibiting the highest NSR values for A. thaliana and S. lycopersicum GH3 enzymes (Table S1). It has been postulated that endogenous flavonoid compounds act as negative regulators of polar auxin transport (PAT) in young seedlings and roots (Kuhn et al. 2011; Yin et al. 2014). Other studies have demonstrated that some flavonoid compounds enhance the endogenous concentration of auxin at the root apical meristem (RAM) and can counteract the effect of known PAT inhibitors (Zhang et al. 2021). Nevertheless, our docking analysis suggests an intriguing possibility whereby certain flavonoids may regulate endogenous IAA levels through their inhibitory capacity on the GH3 enzymes, which potentially influence PAT through known cross-regulation between IAA signaling and PAT (Sauer et al. 2006). Apigenin 7-O-glucuronide (APIOG) and luteolin 7-O-glucuronide (LUTGS; KEGG: C03515) were mapped to a specific branch of the flavonoid biosynthesis pathway (Figure 1B). We retrieved other compounds in this pathway, such as apigenin (API; KEGG: C01477), dihydroquercetin (DHQ; KEGG: C01617), and dihydromyricetin (DHM; KEGG: C02906), which also showed high NSR values in our docking assay (Table S1). Flavonols, such as DHQ and DHM, are unique among flavonoid groups due to the hydroxylation of one of the benzene rings. Furthermore, they are distinguished from one another by their differing hydroxylation patterns (Daryanavard et al. 2023). Figure 1C shows the molecular structure of the putative GH3 inhibitors used in this study.
The inhibition of A. thaliana GH3 enzymes with these flavonoid compounds showed strong preferences for LUTGS and APIOG over DHQ, DHM or API. LUTGS and APIOG are glycosyloxyflavones, containing glucopyranosyl and glucuronide groups at position 7, respectively. The analysis of interactions of LUTGS bound to GH3.2 isoform (Figure S1A) showed up to 13 hydrogen bonds involving seven residues (R116, R237, E447, S448, D533 with side chains, and F157 and A339 with main chain), a salt bridge involving K159, four hydrophobic interactions (K159, V173, L174 and V230), and a pi-stacking affecting F231. On the contrary, API, which is the aglycon of several naturally occurring flavones, showed fewer interactions with GH3.2 (Figure S1B), including six hydrogen bonds (K159, R237 and S448 with side chains; and V230, F231 and A339 with main chain), and three hydrophobic interactions (L174, V230, and F231). Similar interactions were observed for flavononols DHM and DHQ (Figure S1C,D). Clearly, the glycosylated compounds favored stronger interactions with the GH3 enzymes, facilitating the inhibition of the enzyme by the establishment of a large hydrogen bonding network, which confers specificity to the interaction.
3.3 Expression and function of A. thaliana group II GH3 enzymes
In A. thaliana, the group II GH3 proteins consist of eight members that mediate the conjugation of IAA to different amino acids, including the irreversible conjugates IAA-aspartic acid (IAA-Asp) and IAA-glutamate (IAA-Glu), among others (Staswick et al. 2005; Brunoni et al. 2019). These GH3-encoding genes showed specific tissue expression patterns in the roots, such as trichoblast/atrichoblast cells during root hair differentiation (GH3.2/BRU6/YDK1, GH3.4, GH3.6/DFL1, and GH3.17/VAS2), columella/lateral root cap (LRC) cells GH3.2/BRU6/YDK1, GH3.5/WES1, GH3.6/DFL1, and GH3.17/VAS2), and the vasculature (GH3.5/WES1, and GH3.6/DFL1) (Figure S2A, B). On the other hand, GH3.1 and GH3.9 showed very low or no expression levels in roots (Figure S2A, B). To circumvent the redundancy in GH3-mediated IAA metabolism, the gh3.1,2,3,4,5,6,9,17 octuple mutant (referred to as gh3oct) was obtained (Casanova-Sáez et al. 2022). The gh3oct mutant plants exhibited pleiotropic high auxin-related phenotypes, including a more branched root system and short PRs (Hayashi et al. 2021; Guo et al. 2022). A novel GH3 inhibitor, kakeimide, which competitively inhibits IAA conjugation, phenocopied GH3-loss-of-function multiple mutants, which were insensitive to kakeimide treatment (Hayashi et al. 2021). Conversely, the addition of exogenous auxin at low concentrations has been demonstrated to inhibit PR growth and promote LR formation (Ivanchenko et al. 2010). Consequently, the elevation of endogenous IAA levels, through the inhibition of IAA turnover via GH3-mediated amino acid conjugation or the exogenous application of IAA elicits specific phenotypes in the PR. The NSR values of the five selected flavonoid derivatives were calculated with respect to the sequences of the A. thaliana group II GH3 proteins (see Materials and methods). In silico studies have revealed that LUTGS and API are the most and the least potent GH3 inhibitors, respectively (Figure 1D).
3.4 Dihydromyricetin mimics the effect of exogenous auxin in root growth and development
To validate the ability of the selected compounds to inhibit group II GH3 enzymes and, consequently, increase the level of endogenous auxin, we have implemented a rapid bioassay to characterize the PR response after incubating young A. thaliana seedlings in media supplemented with these compounds (see Materials and methods, and Figure S3A). Our findings indicate that LUTGS and DHM had a significant effect on the inhibition of root growth, comparable to that observed in the presence of a low concentration of exogenous auxin, 20 nM IAA (Figure 2A). This reduction in the growth of the PR may be attributed to the premature differentiation of cells within the RAM, which would result in a shortening in the elongation zone of the root, which is the region of the root apex that lacks root hairs. A reduction in the size of the root elongation zone was observed in seedlings treated with all five compounds tested. However, the inhibition levels were found to be lower than those caused by the exogenous IAA in all cases (Figure 2B).
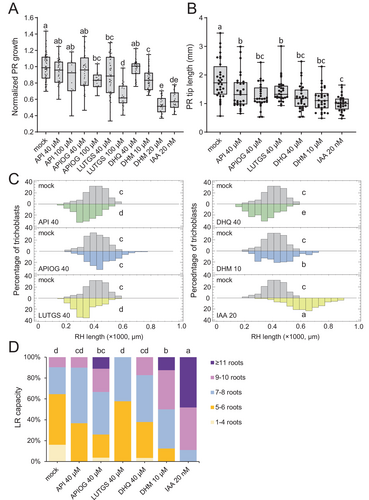
The exogenous addition of auxin also resulted in a significant increase in the length of root hairs in the differentiation zone of the PR (Figures 2C and S3B). This is due to the positive effect of exogenous auxin on the premature differentiation of these epidermal cells (Rahman et al. 2002). Of the compounds tested, only DHM showed a statistically significant increase in root hair length compared to the control treatment. In contrast, API, LUTGS, and DHQ were found to decrease root hair length compared to that of the control treatment (Figure 2C). It was observed that, at a concentration of 20 μM, DHM caused an altered bending of the root tip, which was found to be associated with defects in the columella/LRC cells (Figure S3C). The observed root phenotype of DHM-treated seedlings exhibited a striking resemblance to that of the triple mutant of SOMBRERO (SMB), BEARSKIN1 (BRN1), and BRN2, which are members of the class IIB NAC transcription factor family that regulate secondary cell wall synthesis (Bennett et al. 2010). A steep auxin response gradient from the quiescent center cells to the columella/LRC cells has been proposed to regulate root cap growth dynamics (Dubreuil et al. 2018), and this model has recently been extended to include additional regulators (Possenti et al. 2024). It is therefore plausible that a local increase in auxin levels in the columella/LRC cells of seedlings, as that induced by local GH3 inhibition caused by DHM, might disrupt endogenous auxin gradients leading to SMB, BRN1 and BRN2 inactivation in these tissues.
The exogenous application of IAA was found to significantly enhance the production of LRs to a greater extent than the treatment with DHM (Figure 2D). Among the other compounds tested, only APIOG exhibited a slight increase in the number of LRs produced following the treatment (Figure 2D). Genetic studies using the flavonoid-deficient mutant transparent testa4 (tt4) indicate that flavonol derivatives repress root hair development (Gayomba and Muday 2020). Other studies indicate that some flavonols act as negative regulators of LR emergence (Chapman and Muday 2021), in both cases through modulation of reactive oxygen species levels. Indeed, the results obtained in our study with API, LUTGS, and DHQ are in clear favor of a negative role of these compounds in root hair development and LR formation. Conversely, the phenotypic effects of exogenous DHM on root growth and development, including reduced PR growth, increased root hair length, defects in columella/LRC cells, and enhanced LR formation, are consistent with a specific role of DHM in increasing endogenous IAA levels through inhibition of group II GH3 enzymatic activity.
3.5 Dihydromyricetin affects root growth and development
To determine the effect of exogenous DHM on auxin homeostasis and cellular activity in the RAM, we conducted a comprehensive analysis of the PR tip structure and auxin response in the presence of DHM, IAA, and the combined effect of DHM and IAA (see Materials and methods). Our findings indicate that the length of the RAM, as measured by the distance between the quiescent center cells and the first elongated cell of the cortex, is significantly reduced in the presence of both IAA and DHM (Figure 3A, B). Moreover, the combined application of DHM and IAA results in an additive effect, whereby the meristem length is further reduced (Figure 3A, B). It is noteworthy that the smaller size of the meristem is not correlated with a lower number of cells in the apical-basal axis of the RAM (Figure S3D). Conversely, it is associated with a reduction in the height of the cells in the division zone of the RAM (Figure S3E-F).
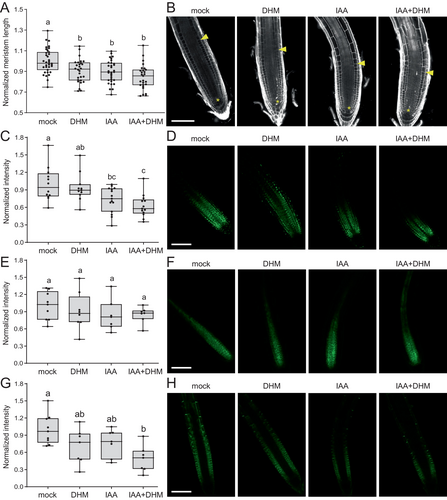
To ascertain the influence of DHM on auxin signaling in the RAM, we employed the DII-VENUS sensor, which generates a map of relative auxin distribution within the RAM (Brunoud et al. 2012). The results demonstrated that IAA treatment significantly reduced the DII-VENUS signal within the RAM (Figure 3C, D), whereas the addition of DHM resulted in a slight reduction in DII-VENUS levels (Figure 3C, D). Notably, the combined effect of DHM and IAA on DII-VENUS levels was additive (Figure 3C, D), indicating that these two compounds act in a functionally redundant manner. This finding is consistent with the hypothesis that DHM acts by inhibiting GH3 function, thereby enhancing endogenous IAA levels. Alternatively, DHM could act primarily on PAT, resulting in a significant alteration of endogenous IAA levels and the associated response, as previously observed for other flavonoid-related compounds. The levels and subcellular localization of PIN-FORMED1 (PIN1) protein in the RAM were not modified by the treatment with either IAA, DHM, or the combination of both compounds (Figure 3E, F). Therefore, it can be concluded that PIN1 protein is not a primary target of DHM. It has been shown that prolonged auxin treatment led to a decrease in the plasma membrane fraction of PIN2 protein, but not PIN1, due to enhanced endocytosis (Narasimhan et al. 2021). Consistent with these results, we have determined that IAA treatment significantly reduces PIN2-GFP protein levels in the RAM, like that produced by the addition of DHM (Figure 3G, H). Furthermore, combined treatment with IAA and DHM further reduced PIN2-GFP fluorescence (Figure 3G, H), confirming the hypothesis that DHM could function to enhance endogenous IAA levels directly.
3.6 Differential effects of dihydromyricetin on mutants affected in the degradative conjugation of IAA or its oxidation
To determine whether the effect of DHM on endogenous IAA levels is attributable to its capacity to competitively inhibit group II GH3 enzymes, we have conducted an analysis of the response of the recently described gh3oct mutants (Casanova-Sáez et al. 2022). In A. thaliana, the degradation of endogenous auxin is dependent upon a precise equilibrium between amino acid conjugation by GH3 enzymes and oxidation by DIOXYGENASE FOR AUXIN OXIDATION1 (DAO1) (Mellor et al. 2016). In the dao1-1 mutant, IAA-Asp and IAA-Glu conjugates accumulate at higher levels due to enhanced expression of some GH3-encoding genes, such as GH3.3 (Mellor et al. 2016; Porco et al. 2016), or simply result from substrate accumulation, as DAO1 has been shown to oxidize these IAA-amino acid conjugates (Müller et al. 2021). In comparison to the Col-0 background, the gh3oct mutant exhibited significantly shorter PRs in both the control conditions and in the medium supplemented with IAA. However, the addition of DHM did not significantly enhance the effect of IAA, in contrast to the results observed in Col-0 (Figure 4A). Conversely, the dao1-1 mutant exhibited a hypersensitive response to DHM in the presence of IAA regarding the PR growth response (Figure 4A). The lack of sensitivity of the gh3oct mutants to DHM is consistent with the inhibitory function of DHM on group II GH3 enzymes. Conversely, the enhanced effect of DHM on the dao1-1 mutant may be attributed to the positive feedback loop between GH3-mediated degradative conjugation of IAA that has been recently proposed (Hayashi et al. 2021).
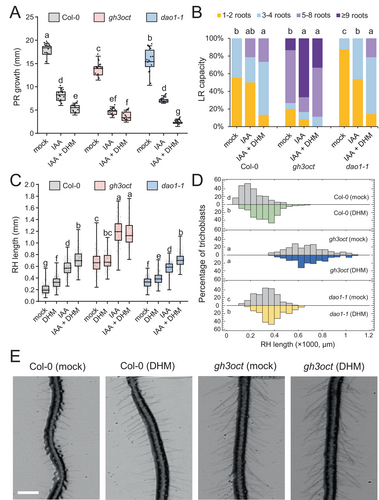
One of the most evident phenotypes of the gh3oct mutants is the increased LR density compared to that of the Col-0 background (Casanova-Sáez et al. 2022). Interestingly, this phenotype associated with the full inactivation of the group II GH3 pathway in A. thaliana seedlings has been demonstrated to result in increased tolerance to different osmotic stresses, including salinity and drought (Casanova-Sáez et al. 2022). In the Col-0 background, the addition of DHM resulted in a heightened impact of IAA on LR formation, whereas no discernible alterations in the influence of IAA on gh3oct were observed after the addition of DHM (Figure 4B). Conversely, the combination of IAA and DHM resulted in an additive increase in the number of LRs in the dao1-1 mutant (Figure 4B). Regarding the length of the root hairs in the differentiation zone of the PR, a significant increase was observed in Col-0 and in the dao1-1 mutant as a result of the effect of either DHM or IAA. The effect was additive in the combination of DHM with IAA (Figure 4C-E). In contrast, the addition of DHM did not result in an increase in root hair length in the gh3oct mutant, either independently or in the presence of IAA (Figure 4C-E). This finding confirms that the root hair elongation phenotype caused by DHM depends on the enzymatic activity of the group II GH3 proteins that are required for IAA inactivation through amino acid conjugation.
3.7 Dihydromyricetin alters the homeostasis of endogenous auxins
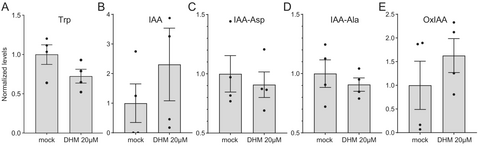
All Arabidopsis group II GH3 enzymes have the capacity to conjugate IAA to different amino acid residues, though with varying degrees of preference (Staswick et al. 2005). Some of these enzymes exhibit promiscuity with respect to the acyl acid substrate. For example, GH3.5 has been demonstrated to facilitate the conjugation of jasmonic acid (Gutierrez et al. 2012) as well as phenylacetic acid (an auxin) and the benzoates salicylic acid and benzoic acid (Westfall et al. 2016). To assess the impact of DHM on auxin homeostasis, the levels of IAA, its biosynthetic precursors (tryptophan [Trp] and indole-3-pyruvic acid [IPyA]), and the primary inactive metabolites, including oxIAA and IAA conjugated to several amino acids, were quantified. In comparison to the control treatment, the levels of Trp were slightly reduced following treatment with 20 μM DHM (Figure 5A). Conversely, DHM was observed to elevate the levels of IAA (Figure 5B) and reduce the levels of IAA-Asp and IAA-Ala by 10% (Figure 5C-D). It is noteworthy that our findings indicate a slight increase in the levels of the oxidized form of IAA, designated as oxIAA (Figure 5E), which is mediated by DAO1 and IAA-LEUCINE RESISTANT 1 (ILR1) amidohydrolase (Hayashi et al. 2021). These results are consistent with the hypothesis that auxin homeostasis in Arabidopsis is subject to highly nonlinear regulation involving GH3, DAO1Trp, and ILR1 (Hayashi et al. 2021). Our findings align with the hypothesis that DHM exerts a selective inhibitory effect on the enzymatic activity of group II GH3, leading to a reduction in endogenous levels of IAA conjugates and an accompanying increase in active auxin (IAA). Nevertheless, we recognize the potential for DHM to exert non-specific effects on other hormones, which warrants further investigation.
4 CONCLUSION
In contrast to several recent studies that have employed a phenotype-based chemical screen (Fukui et al. 2022; Xie et al. 2022), we have implemented a consensus docking analysis method (Blanes-Mira et al. 2022) to search in defined chemical libraries for inhibitors of the IAA-amidase activity of group II GH3 enzymes in plants. The present study was based on the known crystal structure of the grapevine GH3-1 enzyme with the inhibitor adenosine-5′-[2-(1H-indol-3-yl)ethyl]phosphate (AIEP), as well as the existing homology between the A. thaliana and tomato GH3 enzymes. In order to enhance the usefulness of our analytical methodology, we plan to undertake an in-depth comparison of the docking effect of known group II GH3 inhibitors, including AIEP, DHM, nalacin and kakeimide, across a range of plant species of interest. Moreover, the availability of a vast array of chemical libraries will facilitate the performance of high-throughput structure-based virtual screens (Kuan et al. 2023) for the identification of cost-effective pan-inhibitors of group II GH3 enzymes. The bioassay developed in this study for young A. thaliana seedlings will be employed to identify those compounds that exhibit the most promising inhibitory activity of GH3 enzymes. The ultimate objective is to employ these compounds as biostimulants under conditions of moderate stress.
AUTHOR CONTRIBUTIONS
AL, CB-M, PAM-M, LC, MN-A, FP-A, GF-B and JMP-P designed and performed all the experiments. AL, CB-M, PAM-M and MN-A analyzed the data. The first draft of the manuscript was written by GF-B and JMP-P. JMP-P wrote the final manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank Dr. Rubén Sáez-Casanova and Prof. Karin Lung (Umeå Plant Science Centre, Sweden) for sharing seeds of the gh3oct and dao1-1 mutants, Dr. Taras Pasternak (Instituto de Bioingeniería, UMH, Spain) for critical comments and providing seeds of auxin sensors, and Profs. José Luis Micol and María Rosa Ponce (Instituto de Bioingeniería, UMH, Spain) for sharing their confocal equipment.
FUNDING INFORMATION
This research is supported by the grants RTI2018-097189-B-C21 and PID2021-126840OB-I00 funded by MCIN/AEI/10.13039/501100011033, and by the “ERDF A way of making Europe”. MNA held a research contract of the Conselleria d'Educació, Cultura i Sport of the Generalitat Valenciana, grant number EDGJID/2021/025. LC held a “Programa INVESTIGO” contract (INVEST/2022/247) from the Generalitat Valenciana.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are provided in this published article and its supplementary data files or will be provided upon reasonable request.




