Methyl jasmonate mitigates Fusarium graminearum infection in wheat by inhibiting deoxynivalenol synthesis
Abstract
Methyl jasmonate (MeJA), a plant growth regulator, coordinates a diverse array of physiological responses, including the inhibition of seed germination, modulation of secondary metabolite biosynthesis, and activation of defence responses. The external application of MeJA has been demonstrated to effectively diminish the severity of fungal diseases. Here, we unveil a novel mechanism through which exogenous MeJA alleviates Fusarium head blight (FHB) by inhibiting the synthesis of deoxynivalenol (DON) in Fusarium graminearum, rather than by enhancing the wheat resistance response. MeJA treatment reduced the infection by wild-type F. graminearum in wheat coleoptiles, but exhibited no significant influence on that of the DON-deficient mutant strain (∆Tri5). The production of DON in F. graminearum was significantly inhibited both in vitro and in planta. MeJA affected the expression of genes related to DON biosynthesis, without influencing the formation of toxisomes as observed under microscopic analysis. Exogenous MeJA demonstrated a limited impact on the early genes of plant jasmonic acid signalling pathway, in contrast to the wild-type pathogen strain, which induced the upregulation of these genes. The expression levels of defence marker genes induced by MeJA were notably lower compared to those induced by the pathogen. This study elucidates the molecular mechanisms of MeJA in modulating the wheat-F. graminearum interaction, providing new insights into the development of environmentally friendly strategies against fungi.
1 INTRODUCTION
Fusarium graminearum, a filamentous fungal genus prevalent in tropical and subtropical regions, poses a threat to a variety of food and feed grain crops (Dweba et al., 2017; Johns et al., 2022; Wegulo et al., 2015). Statistical data indicate that the elevated prevalence of F. graminearum has a substantial impact on wheat yields (Reis & Carmona, 2013), resulting in a maximum reduction of up to 70% (Palazzini et al., 2015). More importantly, F. graminearum produces various mycotoxins (Ferrigo et al., 2016; Kolawole et al., 2021), particularly deoxynivalenol (DON), which not only results in significant losses in crop quality but also poses substantial risks to food safety (Chen et al., 2019). DON also acts as a virulence factor that plays important roles in the spread of F. graminearum within cereal heads (Bai et al., 2002; Jansen et al., 2005; Proctor, 1995). Hence, suppressing DON accumulation in plants emerges as an alternative strategy for mitigating FHB in wheat-producing areas and ensuring global food security. In recent decades, extensive studies have been conducted to characterize the molecular pathways of DON biosynthesis in F. graminearum (Gale et al., 2005; McCormick et al., 2011) and to investigate the environmental factors influencing DON accumulation (Hou et al., 2015; Kim et al., 2013; Merhej et al., 2010). Additionally, studies on the interactions between wheat and F. graminearum have demonstrated that host tolerance to mycotoxins or direct detoxification of DON, which reduces the adverse effects of mycotoxin accumulation, are favourable traits in germplasms with scab resistance (Bönnighausen et al., 2019; Brown et al., 2017; Desmond et al., 2008; Diamond et al., 2013).
The chemical and molecular aspects of DON biosynthesis in Fusarium species, including the identification of mycotoxin intermediates, gene clusters, and their regulation, have been well characterized (Chen et al., 2019; Nasmith et al., 2011; Seong et al., 2009). Previous studies have shown that the Tri5 gene encodes trichodiene synthase, which serves as the pivotal enzyme responsible for trichothecene mycotoxin biosynthesis (Boenisch & Schäfer, 2011; Hohn & Beremand, 1989). Deletion of Tri5 in a DON-producing F. graminearum strain resulted in complete cessation of mycotoxin production (Bai et al., 2002) and unhindered growth within the initially infected spikelet, but prevented penetration into the adjacent rachis and colonization of further parts of the spike (Jansen et al., 2005). It has been proposed that enzymes participating in the biosynthesis of secondary metabolites are localized within conserved subcellular sites in fungi (Menke et al., 2013). For F. graminearum, a cellular structure, known as “toxisomes”, has a diameter ranging from approximately 3 to 4 μm, housing the DON biosynthesis enzymes (Chen et al., 2019; Qiu et al., 2019). Various factors, such as humidity, temperature, chemical fungicides, and plant resistance response have been reported to influence DON levels by affecting trichothecene gene expression and toxisome formation (Janaviciene et al., 2023; Kolawole et al., 2021; Kriss et al., 2012). Excessive rainfall either during or after the anthesis stages results in heightened DON production (Hooker et al., 2002; Klem et al., 2007), and the use of quinone outside inhibitors (QoIs) fungicides further stimulates the biosynthesis of DON (Duan et al., 2020). While understanding the impact of plant resistance response on DON production is crucial, most studies fall short of offering mechanistic insights into how this process influences the toxigenic machinery of Fusarium.
Plants are endowed with a sophisticated set of plant defence mechanisms that can be activated upon pathogen infection, including cell-surface-localized pattern-recognition receptor (PRR)-triggered immunity (PTI) and effector-triggered immunity (ETI) (Boller & He, 2009; Jones & Dangl, 2006; Yuan et al., 2021; Zhou & Zhang, 2020). This intricate system is regulated by a complex signal transduction network and involves various responses, such as the generation of reactive oxygen species (ROS) (Wu et al., 2023), activation of mitogen-activated protein kinase (MAPK) (Ding et al., 2022), as well as the induction of salicylic acid (SA) (Filgueiras et al., 2019; Yang et al., 2015) or jasmonic acid (JA)/ethylene (ET) signalling pathways (Huang et al., 2016). It is believed that SA induces hypersensitive response (HR) and programmed cell death (PCD), along with systemic acquired resistance (SAR), providing robust resistance against some biotrophic pathogens (Lee et al., 2015). Conversely, JA/ET signalling pathways mainly regulate plant resistance to necrotrophic pathogens and wounds (Li et al., 2019; Wang et al., 2021). However, DON-producing Fusarium spp. are hemibiotrophic, exhibiting both biotrophic and necrotrophic phases during the colonization of their host (Qiu et al., 2019; Taheri et al., 2017). Therefore, in such interactions, a coordinated and ordered expression of SA- and JA-dependent defence responses in the plant is crucial to halt the fungus (Makandar et al., 2012). Nonetheless, this also presents multiple opportunities for pathogen interference. The infection of F. graminearum during the biotrophic stage stimulates rapid hydrogen peroxide (H2O2) production in the host plant (Lehmann et al., 2015), and the additional H2O2 induced by SA signalling benefits the fungus itself through DON mycotoxin production (Brodersen et al., 2005). During the necrotrophic infection stage, invasive hyphal cells colonize the entire intracellular space, utilizing internal nutrients such as carbon and nitrogen sources to facilitate DON production. This reduces the biological activity of plant cells, hinders the JA defence system in plants, and increases H2O2 accumulation in plant tissues, thereby accelerating the death of wheat heads (Boenisch & Schäfer, 2011; Bönnighausen et al., 2019). Research has shown that exogenous methyl jasmonate (MeJA) enhances plant resistance against pathogens by activating the reinforcement of physical defensive structures, facilitating the production of secondary metabolites, inhibiting pathogen growth through the induction of defensive proteins, and activating the ROS scavenging system (He et al., 2020; Ho et al., 2020; Motallebi et al., 2015; Yu et al., 2018). However, given the intricate role of DON in Fusarium virulence and its interaction with defence response molecules, it is plausible that MeJA-induced plant defence mechanisms extend beyond the outcomes currently reported.
The aim of this study was to explore the potential role of exogenous MeJA in enhancing wheat's resistance against hemibiotrophic F. graminearum and to elucidate the underlying mechanism. Our findings revealed that exogenous MeJA enhanced wheat's resistance to F. graminearum by inhibiting initial DON production, rather than inducing resistance. Additionally, MeJA inhibited DON biosynthesis by regulating the expression levels of genes involved in its biosynthesis and suppressing the production of the precursor compound farnesyl pyrophosphate (FPP). This paper presents a novel model for understanding how MeJA increases wheat's resistance against F. graminearum.
2 MATERIALS AND METHODS
2.1 Compounds, fungal strains and culture conditions
Methyl jasmonate (MeJA, PubChem CID: 5281929), 2,1,3-Benzothiadiazole (BTH, PubChem CID: 67502) and anisodamine (PubChem CID: 6918612) were purchased from Shanghai Macklin Biochemical Co., Ltd. BTH, the active ingredient of the first successfully commercialized plant activator Bion®, is a typical broad-spectrum plant resistance inducer that enhances disease resistance (Kouzai et al. 2018; Ge et al. 2023; Zhu et al. 2023; Abo-Elyousr et al. 2024). Anisodamine is a significant alkaloidal secondary metabolite derived from plants of the Solanaceae family (Xia et al., 2024). MeJA was dissolved in ethanol and further diluted using methanol. BTH was dissolved and diluted in methanol, while anisodamine was dissolved and diluted in sterile water. The wild-type strain PH-1 (NRRL 31084) of F. graminearum and the ∆Tri5 mutant strain (a DON-deficient mutant with Tri5 gene knockout) were cultivated on potato dextrose agar (PDA) (200 g potato, 20 g glucose, 16 g agar and 1 L water) at 25°C under dark conditions.
For conidiation assays, three mycelial plugs (5 mm in diameter) from each strain were taken from the periphery of a 3-day-old colony and inoculated into 20 mL SNA (Spezieller Nährstoffarmer Agar) liquid medium (1 g KH2PO4, 1 g KNO3, 0.5 g KCl, 0.5 g MgSO4•7H2O, 0.2 g glucose, 0.2 g sucrose and 1 L water). After 3 days of cultivation, conidia were collected, and their concentrations were adjusted using a hematocytometer.
2.2 Plant infection
The wheat variety used was Yangmai 25, which is resistant to FHB at the seedling stage (Li et al. 2010). Seeds were sterilized with 75% ethanol for 30 s, rinsed several times with sterile water, and evenly placed in sterile Petri dishes lined with two layers of filter paper. Each Petri dish contained fourteen wheat seeds. The seedlings were grown in a growth chamber at 25°C and 95% humidity. Conidia from each strain, formed in SNA medium, were collected and suspended in sterile distilled water to a final concentration of 106 conidia mL−1. After 2.5 days, when the first wheat leaf emerged from the coleoptile, the tip of the coleoptile was cut off, and a 3 μL suspension of fresh conidia was inoculated. The wheat coleoptiles were treated with 0, 0.2, and 2 mM MeJA, as well as 0.7 mM BTH, either 24 h before or after inoculation. Each treatment consisted of 3 Petri dishes. Incubation was continued for 7 days post-inoculation, after which the length of black lesions and DON production were measured. The experiment was repeated three times.
Powdery mildew infection was conducted following previously described methods with some modifications (Xie et al., 2021). Wheat seeds were surface sterilized by immersion in 75% (v/v) ethanol for 30 s and rinsed several times with sterile water. The sterilized seeds were planted in 15 cm diameter pots filled with commercial peat mix and grown in a growth chamber under a 16/8 h light/dark photoperiod at 24°C and 75% humidity. Seven-day-old wheat seedlings of similar height were selected for treatment. Fresh spores of Blumeria graminis f. sp. tritici (Bgt) at a density of 100–140 conidia mm−2 were inoculated by shaking onto the leaf surfaces of wheat seedlings. Seedlings were treated with 0, 0.2, and 2 mM MeJA, as well as 0.7 mM BTH, either 24 h before or after inoculation. During spore shaking, a slide was placed next to the leaves to quantify the inoculated spore count under an optical microscope (10 × 10 magnification). Each treatment was replicated three times with ten plants per replicate. Disease severity was assessed 7 days post-inoculation and classified according to the ‘Pesticide Guidelines for Field Efficacy Trials (GB/T 17980–22-2000)’. The disease index was calculated using the formula: Disease index (%) = Σ (number of diseased leaves per grade × corresponding grade) / (total number of diseased leaves × 9).
2.3 DON production assays
To quantify the production of DON in diseased wheat coleoptiles, the coleoptiles were carefully excised and pulverized in liquid nitrogen. One gram of each sample was re-suspended in 2 mL of water and thoroughly mixed using a vortex shaker for 1 min. The mixture was then incubated at 30°C for 30 min, centrifuged, and the supernatant was collected for analysis using a competitive ELISA-based DON Quantification Kit (Weisai-bio). Mature wheat kernels harvested from the field experiment were ground into flour, and DON production was measured using the aforementioned method. DON levels were expressed as a ratio of DON production to dry wheat powder (μg g−1).
To investigate the effect of MeJA on DON production in mycelium in vitro, 1.5 × 105 spores were inoculated into 30 mL of trichothecene biosynthesis-inducing (TBI) media (Duan et al., 2020). The cultures were incubated in the dark at 28°C with agitation at 175 rpm for 24 h. MeJA was then added to final concentrations of 0, 0.1, 0.2, 0.5, 1 and 2 mM, followed by further incubation for 6 days. The filtrate was collected for measurement, and the dry weight of mycelium was used as an internal reference. DON production was also assayed using the DON Quantification Kit (Weisai-bio).
2.4 Observation of toxisomes, TRIs gene relative expression, and determination of FPP and acetyl-CoA content in F. graminearum
To characterize the pattern and subcellular localization of toxisomes under various conditions, the Tri1 gene tagged with a green fluorescent protein (GFP) was introduced into an F. graminearum-ΔTri1 background strain. The Tri1-GFP strain was grown in triangular flasks containing TBI medium. After incubating for 24 h, 1 mM MeJA was added to the flasks. Following an additional 24 h incubation, the fluorescent intensity was observed using a microscope.
To examine the transcription of DON biosynthesis genes (FgTRIs), mycelium was initially incubated for 24 h, then treated with 1 mM MeJA. Anisodamine, at a final concentration of 0.15 mM, served as the control. After an additional 12 h incubation, the mycelium was harvested, and RNA was extracted using the Ultrapure RNA kit (Cwbio). The isolated RNA was reverse transcribed into complementary DNA using reverse transcriptase (Cwbio). For quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis, SYBR Green mixed mother liquor (Cwbio) was employed. Primers are listed in Table S1 and the FgGAPDH gene was used as the internal control for normalization. Each experiment was repeated three times.
To investigate the impact of MeJA on acetyl coenzyme A (Acetyl-CoA) and FPP biosynthesis in F. graminearum, mycelium was cultured in TBI using standard procedures. After 24 h incubation, MeJA was added at final concentrations of 0, 0.2, and 2 mM. The mycelium was further incubated for 48 h before being harvested. Subsequently, the mycelium was ground using liquid nitrogen, and 0.1 g was weighed and mixed with 1 mL of PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH = 7.4). After vortexing for 1 min, the mixture was incubated for 1 h, followed by centrifugation. The supernatant was collected for analysis using an A-CoA ELISA Kit instruction (JIANXIN) and a Microbial farneside pyrophosphate (FPP) ELISA Kit (Jiaoziteng). Each experiment was repeated three times.
2.5 The mycelia growth, conidia production, and conidia germination in different conditions
The inhibition of MeJA on the mycelial growth in vitro was measured as in previous studies (Song et al., 2018; Xu et al., 2019). Mycelial plugs (5 mm) from the edge of the actively growing colony were transferred into PDA plates containing varying concentrations of 0.1, 0.2, 0.5, 1 and 2 mM MeJA. Methanol without MeJA (0 mM) served as the control. After 3 days of incubation in a growth chamber (25°C), the colony diameters in two perpendicular directions on each PDA plate were measured and averaged.
The synergism between MeJA and tebuconazole was assessed by applying Limpel's criterion, calculated using the following equation (Kartashov et al., 2019): Ee = X + Y − (XY/100), where Ee is an expected summary growth inhibition, and X and Y are the percentage growth suppression values obtained when each compound was used alone. If the observed growth-inhibiting effect (Er) of fungicide co-applied with MeJA exceeded the Ee value calculated using the above formula, chemosensitizing effect was considered. The tested concentrations used were: MeJA at 0 (control), 0.1, and 1 μg mL−1; and tebuconazole at 0 (control), 0.05, and 1 μg mL−1.
For spore germination inhibition tests, WA plates (16 g agar per 1 L water) were supplemented with MeJA at concentrations of 0, 0.1, 0.2, 0.5, 1 and 2 mM. The conidia suspension was adjusted to 106 mL−1 with sterile water. Then, 50 μL of each conidia suspensions was evenly spread onto WA plates. The plates were then incubated in the dark at 25°C for 6 h. Conidia were considered germinated when the germ tube reached at least half the length of the conidium, and 200 conidia were scored for each plate. The spore germination rate was calculated according to the methods described in a previous study (Wang et al., 2018).
For conidia production tests, 500 μL of conidia suspensions at 108 mL−1 were added to 30 mL of SNA medium supplemented with MeJA at concentrations of 0, 0.1, 0.2, 0.5, 1 and 2 mM. All treatments were cultured under light at 25°C with shaking at 175 rpm. After 3 days cultivation, conidia production was quantified using a hemocytometer and light microscope.
2.6 Detection of plant defence responses
Wheat coleoptiles were inoculated with spores of the wild-type (WT) strain and the ∆Tri5 strain 24 h after spraying. Samples were collected either 12 or 24 h post-inoculation to assess reactive oxygen burst, callose deposition, and defence gene expression.
Reactive oxygen species (ROS) were detected using 3,3′-diaminobenzidine (DAB, Coolaber), which produces a brownish-yellow precipitate upon reaction. Wheat coleoptiles were fully submerged in a 4.67 μM DAB solution (pH 3.8) and stained in darkness at 25°C for 12 h. After staining, the wheat coleoptiles were decolourized using 96% ethanol for 12 h to visualize ROS accumulation post-decolourization. The relative intensity of grayscale values was measured using ImageJ software (https://imagej.nih.gov/ij/). To minimize errors from mechanical damage to the ends of the coleoptiles, only the grayscale values of the brown regions in the central portions of the coleoptiles were quantified.
Callose deposition was detected using aniline blue (Ryon), a dye that specifically binds to callose and fluoresces under UV excitation. Wheat coleoptiles were immersed in 5 mL of a decolorizing solution (phenol: glycerol: lactic acid: water: ethanol = 1: 1: 1: 1: 2). After complete decolourization, the coleoptiles were stained in 5 mL of aniline blue solution (150 mM K2HPO4, pH 9.5, containing 0.01% aniline blue) for 4 h in darkness. Following gently rinsing with water, the stained leaves were observed and photographed under UV excitation light using an inverted fluorescence microscope (Axio Observer 3, Zeiss).
Total RNA was extracted from wheat coleoptiles using the OminiPlant RNA kit (Cwbio) according to the manufacturer's instructions. The TaSAR-DBP gene (Scaffold-associated regions DNA binding protein) was used as the internal control for normalization (Long et al., 2010). Primers for qRT-PCR are provided in Table S1.
2.7 Field experiments
The experiment was conducted in a flat wheat field located in Huai'an, Jiangsu Province, China (119.0143°N, 33.8261°E), where the natural incidence of FHB is prevalent. To ensure an accurate assessment of the field efficacy of fungicides against FHB, no artificial inoculation was performed. Each treatment, including the control (no-fungicide), was represented by three replicate plots (20 m2 per plot), which were randomly arranged in the field. Wheat plants were sprayed with fungicides at the onset of anthesis and again 3 to 5 days later. The application doses of the test fungicides are listed in Table 2. After 4 weeks, FHB incidence was evaluated by examining 80 wheat ears per plot. Diseased spikes were categorized into classes (0, 0%; 1, 0–25%; 3, 25–50%; 5, 50–75%; 7, 75–100%) based on the level of incidence (Wu et al., 2023). The disease index was calculated using the equation: Disease index (%) = Σ (number of diseased spikes at per level × relative grade value) / (total number of investigated spikes × 7).
To evaluate grain yield, a 100 g sample of dried wheat grains from each plot was randomly selected to determine the 1000 grain weight, as previously described (Zhang et al., 2010).
2.8 Statistical analysis
Data were expressed as the mean ± standard errors (SE), calculated from three independent biological replicates. Significant differences were indicated by different letters, based on ANOVA analysis followed by Least Significant Difference (LSD) test and Duncan's Multiple Range Test using the SPSS 27 software. Differences were considered statistically significant at p < 0.05. All figures were generated using Origin 2019b.
3 RESULTS
3.1 MeJA suppresses F. graminearum infestation by inhibiting DON biosynthesis in planta
The impact of MeJA on F. graminearum infection was investigated by treating wheat coleoptiles with MeJA at concentrations of 0, 0.2, and 2 mM, either 24 hours before inoculation (24 hbi) or 24 hours after inoculation (24 hai) with F. graminearum. Fusarium seedling blight resistance assays showed that MeJA treatments at 0.2 and 2 mM significantly suppressed the infestation by the WT strain in wheat inoculated 24 hbi. Additionally, the control agent BTH also exhibited inhibitory effects. At 7 days post-inoculation (dpi), the lesion lengths of infected coleoptiles in the two MeJA-treated groups were 19.7 ± 0.41 mm and 19 ± 0.91 mm, respectively, while the lesion length in the H2O-treated group (0 mM) was 24.4 ± 0.75 mm (Figure 1A). As shown in Figure 1A, statistical analyses revealed that coleoptiles treated with 0.2 and 2 mM MeJA, as well as 0.7 mM BTH, exhibited significant reductions in disease severity (p < 0.001) of 19.2, 22.1 and 27.7%, respectively, compared to the control group. However, no significant differences were observed among all treatment groups when MeJA was applied 24 hai with the WT strain (Figure 1A).
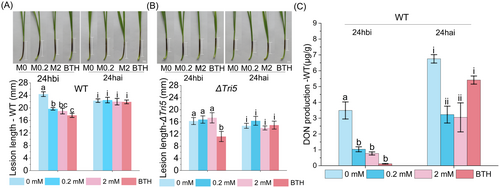
Considering the role of DON as a pathogenic factor, we evaluated the pathogenicity of the DON-deficient strain (∆Tri5) under the same conditions. Interestingly, as shown in Figure 1B, no significant variation in pathogenicity was observed in wheat coleoptiles inoculated with the ∆Tri5 strain, regardless of the timing or concentration of MeJA application. In contrast, applying the control agent BTH 24hbi significantly increased plant resistance (Figure 1B). These results indicated that MeJA enhanced plant resistance to DON-producing strains, while having no effect on DON-deficient strains. In other words, MeJA may influence the production of DON in F. graminearum.
We then measured the DON content in coleoptiles and found that the DON content per unit of coleoptile significantly decreased when coleoptiles were treated with MeJA (Figure 1C). Compared to the untreated group, DON production was reduced by 70.2 and 77.8% in the coleoptiles treated with 0.2 and 2 mM MeJA 24 hbi, respectively. Although no difference in lesion length was observed in coleoptiles treated with MeJA 24 hai, DON production decreased by 52.2 and 54.8% in those treated with 0.2 and 2 mM MeJA, respectively.
To investigate whether wheat's response to MeJA during F. graminearum infestation is related to its facultative parasitic nature, we assessed the effect of MeJA on Bgt. Bgt, a biotrophic fungal pathogen that does not produce mycotoxins as virulence factors, primarily causes wheat powdery mildew. The results showed that MeJA inhibited powdery mildew at a concentration of 2 mM, exhibiting a similar inhibitory effect as the control agent BTH (Figure S1). As shown in Figure S1, among the various treatments inoculated with Bgt 24 h after spraying, 2 mM MeJA exhibited the strongest inhibitory effect, resulting in a disease index of 0.54 and a disease control rate of 36.43%. This finding is consistent with the fact that high concentrations of MeJA can induce resistance against powdery mildew infestation in wheat (Jing et al., 2019). These findings suggest that MeJA primarily prevents pathogen spread by inhibiting DON production during the penetration stage of F. graminearum.
3.2 Regulatory impact of MeJA on the TRI genes and DON biosynthesis in F. graminearum
To clarify the relationship between MeJA and DON biosynthesis, we investigated the impact of MeJA on DON production using TBI medium in vitro. As shown in Figure 2A, MeJA treatment in TBI medium significantly reduced DON production, with an inhibition rate of up to 96.6% compared to the control group (0 mM). Although higher concentrations of MeJA increased the inhibition, no statistically significant difference was observed between the different concentrations. We initially examined the effect of MeJA on the formation of toxisomes. Tri1-GFP localized to spherical structures (toxisomes) in TBI medium, regardless of the presence of 1 mM MeJA. No significant difference was observed, indicating that MeJA did not affect the formation of toxisomes. To further investigate the regulatory mechanism of MeJA on DON biosynthesis, the expression of TRIs was detected. As shown in Figure 2C, qRT-PCR assays revealed diverse alterations in the expression levels of four TRI genes (Tri1, Tri4, Tri6, and Tri101). Tri1, which encodes a P450 oxygenase, showed a 64.4% decrease compared to the control, while Tri4, responsible for catalyzing oxygenations at C-1, C-3, C-12, and C-13, exhibited a 32.1% reduction in expression. Tri101, involved in acetylating the C-3 of DON, showed a 43.7% decrease in transcript levels. However, Tri6, encoding a transcription factor of the Tri gene cluster, increased by 0.5-fold. These transcriptional changes indicate that MeJA may impede DON biosynthesis by modulating the regulatory network of TRIs expression and reducing the transcriptional products of these genes. The results in Figure S3 further indicated that 0.15 mM anisodamine did not significantly change the TRIs expression, suggesting that the effect of MeJA on TRIs expression is specific. At the metabolic level, MeJA significantly reduced the content of FPP (results are displayed in Figure 2D), a key precursor in DON biosynthesis. This reduction in FPP directly constrained DON biosynthesis, suggesting that MeJA may exert its inhibitory effect by altering substrate availability within metabolic pathways. Interestingly, the results shown in Figure S2 indicated that MeJA significantly increased acetyl-CoA levels, a precursor for FPP biosynthesis, in F. graminearum. These findings provide deeper insights into how MeJA impacts DON production at the biochemical level. In summary, MeJA not only downregulated genes involved in DON synthesis but also reduced the availability of precursor compounds like FPP, thereby effectively inhibiting DON production.
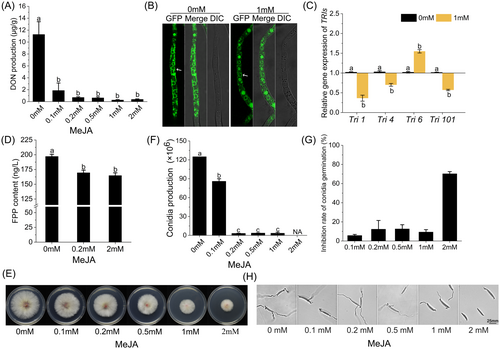
We also measured the impact of MeJA on mycelial growth, conidia germination and conidia production of F. graminearum in vitro. Strains were grown on PDA plates at concentrations of 0, 0.1, 0.2, 0.5, 1 and 2 mM MeJA. After 3 days of cultivation, as shown in Figure 2E, inhibition of mycelia growth was observed at MeJA concentrations between 0.1 and 1 mM, with the slowest growth occurring in the 2 mM treatment group. Conidia production was significantly decreased at all MeJA concentrations, with almost no spore observed when the concentration of MeJA exceeded 0.2 mM (Figure 2F). In contrast, as shown in Figure 2G and H, only the 2 mM MeJA exhibited an inhibitory effect on conidia germination, resulting in a 70.3% inhibition.
3.3 Low plant defence response induced by exogenous MeJA
Given the role of MeJA in inducing plant resistance, we examined the time-dependent biochemical and molecular responses of wheat coleoptiles following the external application of varying concentrations of MeJA, followed by F. graminearum infection. As depicted in Figure 3A, MeJA was applied at 0 h, and pathogen inoculation was performed 24 h later. Samples were collected at 36 and 48 h to evaluate plant defence responses. Wheat coleoptiles treated with 0.2 or 2 mM MeJA remained healthy, exhibiting no discernible morphological alterations. As illustrated in Figure 3B, coleoptiles treated exclusively with MeJA exhibited lower levels of oxidative burst, resulting in less intense brown lesions at 36 h. Additionally, variations in oxidative burst were observed following inoculation with the WT or ∆Tri5 strains 24 h after MeJA application. At 36 h, a noticeable increase in oxidative burst was observed in coleoptiles treated with 0 mM MeJA and inoculated with WT. In contrast, the oxidative burst levels decreased when treated with 0.2 or 2 mM MeJA along with WT. Interestingly, at 36 h, coleoptiles treated with 2 mM MeJA exhibited higher ROS levels when inoculated with the ∆Tri5 compared to the WT strain. This difference no longer existed at 48 h, at which point ROS levels were significantly elevated in all coleoptiles treated with 2 mM MeJA. At 48 h, compared to the non-inoculated controls, a more pronounced oxidative burst was observed in all treatments, including BTH, with either WT or ∆Tri5. There was almost no difference in the treatments between WT and ∆Tri5. In summary, the presence of pathogens and the concentration of MeJA primarily influenced the ROS level in wheat coleoptiles. Moreover, the differences in pathogenicity observed between these two strains upon MeJA application cannot be ascribed to fluctuations in ROS levels.
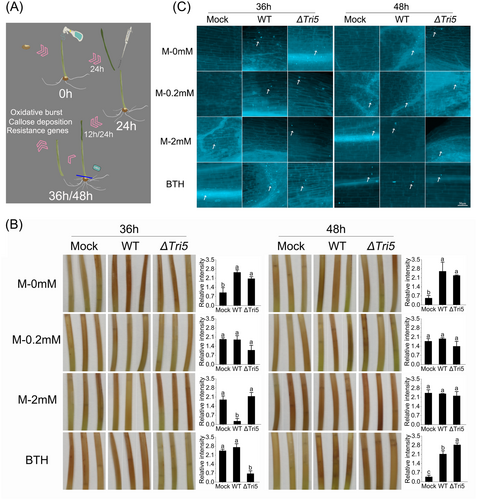
Callose formation, a key part of the plant defence response, occurs following oxidative burst during fungal infection. We analyzed callose deposition in wheat coleoptiles treated with MeJA or infection with F. graminearum. From Figure 3C, we can see increased callose accumulation in WT and ∆Tri5 infected coleoptiles at 36 h, regardless of MeJA treatment. However, no differences were observed in coleoptiles following only 0.2 mM MeJA treatment. When treated with 2 mM MeJA, no callose deposition was observed in the coleoptiles at 36 h. Moreover, at 48 h, all treatment groups showed significant deposition of callose. No significant difference in wheat callose deposition was observed between the treatments inoculated with WT and ∆Tri5 strains. These results did not align with the inhibitory effect of MeJA on F. graminearum, suggesting that the reduction in pathogenicity of the WT strain induced by MeJA was not primarily attributed to its activation of defence responses in wheat.
3.4 Wheat defence marker genes had different responses to exogenous MeJA and F. graminearum
To further analyze the impact of different concentrations of exogenous MeJA on plant disease resistance, we examined the expression of disease-resistance genes in wheat at different stages following MeJA treatment. Four genes involved in the JA signalling transduction process were selected: TaJAZ1, a negative regulator of JA signalling (Wang et al., 2017); TaPDF1.2, an indicator of JA pathway activation (Qi et al., 2016); and TaPR3 and TaPR4, which are JA-induced resistance genes (Ding et al., 2022). We also examined two SA-regulated resistance genes, TaPR1 and TaPR2 (Ding et al., 2022). Additionally, genes associated with ROS, namely TaSOD (superoxide dismutase) and TaPOX2 (peroxidase) (Jing et al., 2019), were included to assess changes in transcriptional levels. Initially, we assessed the effect of MeJA alone on the expression of disease-resistance genes (Figure 4A). Among the JA signalling-related genes, TaPR3 and TaPR4 were significantly induced by MeJA treatment. TaPR3 showed a time- and MeJA concentration-dependent increase, peaking at 48 h with 2 mM MeJA, an 8-fold increase compared to the control. TaPR4 followed a similar pattern, with a 6.1- and 6.4-fold upregulation at 48 h in response to 0.2 and 2 mM MeJA, respectively. However, MeJA had a weak effect on the expressions of TaJAZ1 and TaPDF1.2 at both time points. It appears that MeJA primarily induces late-stage rather than initial-stage JA signalling responses. Among SA-related genes, TaPR1 increased 11.8-fold after 48 h of 0.2 mM MeJA treatment and 4.0- and 13.6-fold after 36 and 48 h of 2 mM MeJA treatment, respectively. TaPR2 exhibited a similar expression pattern. For ROS-related genes, TaPOX2 was significantly induced by 2 mM MeJA, showing 5.1- and 3.1-fold increases at 36 and 48 h, respectively, while 0.2 mM MeJA had no significant effect. TaSOD levels increased 3.0- and 4.5-fold after 36 h of 0.2 and 2 mM MeJA treatment, respectively, and 3.5-fold after 48 h with 2 mM MeJA. These results suggest that MeJA indeed induces plant disease resistance but not through the initial response of the JA signalling pathway.
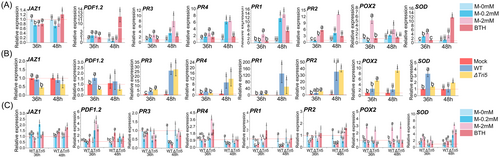
We next investigated the influence of pathogen infection alone on disease-resistance gene expression (Figure 4B). The WT strain had no effect on TaJAZ1 at either 36 or 48 h, but TaJAZ1 expression was significantly reduced 12 h post-inoculation with the ∆Tri5 strain. Concurrently, the expression of TaPDF1.2 was stimulated by the WT strain at 36 h. Moreover, TaPR3, TaPR4 and TaPR2 were significantly upregulated in response to both WT and ΔTri5, with increases ranging from 1.4- to 37.8- fold. Infection with the WT strain resulted in an increase in TaPR1 expression, with the change being more pronounced 24 h post-inoculation. Interestingly, TaPOX2 showed no significant variation following WT inoculation at 12 and 24 h, but was markedly upregulated after ∆Tri5 inoculation, with increases of 5.5- and 9.2-fold at 36 and 48 h, respectively. Both the WT and ΔTri5 strains induced a slight increase in TaSOD expression. Overall, pathogen infection induced significantly greater changes in resistance gene expression than MeJA treatment alone.
The combined effect of MeJA application and pathogen infection on the expression of wheat disease-resistance genes was subsequently assessed. As shown in Figure 4C, the expression profiles of four JA signalling-related genes underwent significant changes when MeJA and pathogen inoculation were applied concurrently, contrasting with the patterns seen with either MeJA or pathogen inoculation alone. Post-inoculation with the strain ΔTri5, TaJAZ1 and TaPDF1.2 expression levels were amplified by 2.0- to 4.4-fold at 36 h, and by 1.6- to 1.8-fold at 48 h. Conversely, the expression of TaPR3 and TaPR4 decreased, in contrast to the upregulation observed with MeJA or pathogen inoculation alone, particularly at 48 h. Similarly, the two SA-regulated resistance genes, TaPR1 and TaPR2, exhibited diminished expression following WT inoculation when combined with MeJA treatment. The expression trends of ROS-related genes (TaSOD and TaPOX2) were in line with previous observations. TaPOX2 expression levels were significantly elevated in all treatments with 2 mM MeJA, with increases ranging from 2.4 to 2.9-fold. Likewise, the 0.2 mM MeJA + ΔTri5 treatment reduced TaPOX2 expression at 36 h, with only 58.1% compared to the control group. Regardless of whether WT or ΔTri5 was inoculated, 2 mM MeJA significantly upregulated TaSOD by 2.1 to 6.7-fold compared to the control. Meanwhile, at 36 h, the 0.2 mM MeJA + WT treatment significantly increased TaSOD expression by 1.8-fold compared to the control, but this effect disappeared at 48 h. Interestingly, 0.2 mM MeJA + ∆Tri5 had no significant impact on TaSOD expression levels at 36 h but induced a 3.7-fold upregulation at 48 h.
In summary, exogenous MeJA has limited impact on the initial genes within the JA signalling pathway, in contrast to the WT strain, which strongly upregulates these genes. This upregulation is notably reduced in the ΔTri5 strain. Concurrent administration of MeJA and ΔTri5 strain results in an observed increase in the expression of TaJAZ1 and TaPDF1.2. While both MeJA and pathogen inoculation alone induced the expression of disease-resistance genes governed by JA and SA (such as TaPR1, TaPR2, TaPR3, and TaPR4), the expression levels induced by MeJA alone were significantly lower than those induced by pathogen. Intriguingly, the simultaneous application of MeJA with pathogen inoculation inhibits the expression of these disease-resistance genes. The expression of ROS-related genes was upregulated by MeJA, pathogen inoculation, and their combined application.
3.5 MeJA coupled with fungicide inhibiting DON biosynthesis in the field
In the process of controlling FHB in the field, the use of most fungicides tends to promote mycotoxin production, which hinders effective disease management. Therefore, we conducted additional experiments to evaluate the combined effects of MeJA with a commonly used chemical fungicide (tebuconazole). The observed growth-inhibitory effect (Er) of tebuconazole co-applied with MeJA against the WT strain exceeded the expected summary effect of their joint application (Ee) calculated by the Limpel's formula. Looking at the calculations in Table 1, for MeJA (0.1 μg mL−1) + tebuconazole (0.1 μg mL−1), the E𝑟 value (40.8%) significantly (p <0.05) exceeded the corresponding E𝑒 value (35.4%). In addition, the E𝑟 value (31.9%) was also higher than the corresponding E𝑒 value (30.2%) in the treatment of MeJA (1 μg mL−1) + tebuconazole (0.05 μg mL−1). These results suggest that the co-application with MeJA could enhance the sensitivity of tebuconazole to F. graminearum.
| Compounds | Mycelia growth inhibition rate (%) |
|---|---|
| MeJA-0.1 μg/mL | 3.1 ± 0.71 |
| MeJA-1 μg/mL | 4.7 ± 0.20 |
| Tebuconazole-0.05 μg/mL | 26.8 ± 0.41 |
| Tebuconazole-0.1 μg/mL | 33.3 ± 1.75 |
| MeJA (0.1 μg/mL) + Tebuconazole (0.1 μg/mL) | 40.8 ± 0.31 |
| MeJA (1 μg/mL) + Tebuconazole (0.05 μg/mL) | 31.9 ± 1.84 |
- All data are expressed as means ± SE.
Next, tebuconazole (commonly used in the field) and metconazole (with a better anti-fungal effect) were selected for use in combination with MeJA, and the effects were evaluated in field trials. As shown in Table 2, the untreated control plots exhibited a mean FHB disease index of 5.08 and DON concentration in harvested grains of 0.629 μg g−1. The application of MeJA resulted in a significant reduction (p < 0.05) in DON concentration (0.131 μg g−1) in harvested grains, while its disease index (4.00) showed no statistically significant difference compared to the untreated control plots. The application of tebuconazole and metconazole resulted in a reduction in the FHB disease index (1.63 and 1.62, respectively) and DON production (0.189 and 0.312, respectively). The co-formulation of MeJA + metconazole demonstrated a dual effect by effectively inhibiting pathogens while simultaneously suppressing DON biosynthesis. This combined approach resulted in the most significant reduction in both FHB disease index (1.54) and DON concentration (0.144 μg g−1), surpassing the reduction achieved by the application of metconazole alone. These results suggest that the implementation of combination fungicide programs holds promise for achieving dosage reduction and impeding resistance development in practical settings. The 1000-grain weight did not exhibit a significant difference across all treatments. Under field conditions, MeJA exhibited promising potential in suppressing DON biosynthesis, making it a valuable candidate for utilization in conjunction with fungicides to effectively suppress fungal growth and DON biosynthesis.
| Treatment | Dosage (g a.i. ha−1) | Disease index | DON production (μg g-1) | 1000-grain weight (g) |
|---|---|---|---|---|
| Control | - | 5.08 ± 0.6657 a | 0.629 ± 0.0145 a | 43.42 ± 1.5491 a |
| MeJA | 7.5 | 4.00 ± 0.3905 a | 0.131 ± 0.0260 c | 43.41 ± 0.5750 a |
| Tebuconazole | 112.5 | 1.63 ± 0.2095 b | 0.189 ± 0.0185 c | 42.98 ± 0.9800 a |
| Metconazole | 90 | 1.62 ± 0.5364 b | 0.312 ± 0.0485 b | 44.97 ± 1.0450 a |
| MeJA+ Tebuconazole | 7.5 + 112.5(1:15) | 1.75 ± 0.3629 b | 0.134 ± 0.0305 c | 44.13 ± 0.8950 a |
| MeJA+ Metconazole | 7.5 + 90(1:12) | 1.54 ± 0.0214 b | 0.144 ± 0.0085 c | 47.21 ± 1.0900 a |
- All data are expressed as the means ± SE. Different letters represent significant differences (p < 0.05). The experiments were repeated twice.
4 DISCUSSION
During the course of evolution, plants have developed multi-level passive and active resistance mechanisms that can be used coordinately against pathogen infection (Mittler et al., 2022; Ngou et al., 2022; Xu et al., 2022). Defense responses, such as HR/PCD and SAR, can be induced by SA, JA or ET, resulting in a strong pathogen resistance (Ding et al., 2022; Shapiro & Zhang, 2001). Previous studies have confirmed that the exogenous application of MeJA can enhance wheat resistance to Fusarium, with the mechanism attributed to the disease resistance response induced by MeJA (Duan et al., 2014; Sun et al., 2016). However, due to the complexity of the interaction between Fusarium and wheat, this may not represent the true mechanism of action. Our research confirms that exogenous MeJA enhances wheat resistance to Fusarium by inhibiting mycotoxin production.
FHB disease, primarily caused by F. graminearum, induces premature senescence of the wheat head and is the most serious and hazardous floral affliction worldwide (Dweba et al., 2017; Johns et al., 2022). The mechanism by which F. graminearum infects wheat heads has unique characteristics in comparison to other fungal–plant interactions. Infections begin with direct entry into plant tissues, with or without the formation of infection cushions (Boenisch & Schäfer, 2011). Epiphytic runner hyphae grow on the outer surface of host cells or in intercellular spaces without causing obvious disease symptoms (Boenisch & Schäfer, 2011). Upon invasion of the host cell by the surrounding fungal mycelium, accompanied by host cell death, the symptomatic phase occurs in the host (Mentges et al., 2020). During the symptomless phase, extensive transcriptional changes occur in F. graminearum, including the rapid activation of various Tri genes responsible for trichothecene and DON biosynthesis (Brown et al., 2017; Lysøe et al., 2011). The production of DON by F. graminearum is known to be essential for the formation of disease in wheat spikes (Cuzick et al., 2008; Wang et al., 2023). In planta, the presence of DON may hinder early induced plant defense responses (Bönnighausen et al., 2019). At higher concentrations, DON induces plant PCD in plants and triggers specific plant defenses (Desmond et al., 2008).
In our findings, the exogenous application of MeJA 24 h prior to inoculation with WT strain significantly enhanced the resistance of wheat coleoptiles to F. graminearum. However, when MeJA was applied 24 h after inoculation, there was no significant improvement in wheat resistance, indicating that the resistance induced by exogenous MeJA is closely associated with the process of F. graminearum infection. Previous studies on Fusarium infection have revealed that within the first 16 h of infection, the pathogen does not penetrate the cell wall of wheat coleoptiles, and the toxisomes responsible for DON production are not fully formed (Qiu et al., 2019). Hence, there was no difference in infection between the WT strain and the DON-deficient strain during this early infection period. Interestingly, pre-treatment with MeJA 24 h prior to inoculation with the DON-deficient strain did not enhance the resistance of wheat coleoptiles against F. graminearum, suggesting that alterations in resistance occur within 16–24 h between WT and DON-deficient strains. During this period, extracellular hyphae began to penetrate the cell wall of wheat coleoptiles and initiate DON production, which plays a crucial role in the process (Mentges et al., 2020; Qiu et al., 2019). Even at low concentrations in vitro, MeJA's inhibitory effect on DON was noticeable, accounting for the significant decrease in the virulence of the WT strain when MeJA was applied externally 24 h before inoculation. Consequently, the application of MeJA 24 h post-inoculation, a point at which mycotoxins had already been synthesized, did not result in a statistically significant reduction in pathogenicity.
Exogenous MeJA treatment resulted in a slight upregulation of plant disease resistance genes, as evidenced by the assessment. However, the level of upregulation observed is significantly lower than that induced by the pathogens. This suggests that the wheat's disease resistance response, induced by exogenous MeJA, is insufficient to confer significant protection against pathogen invasion, resulting in notable differences in resistance levels. Interestingly, the application of exogenous MeJA prior to pathogen inoculation actually suppresses the expression of certain disease resistance genes, such as TaPR1, TaPR2, and TaPR3. This contradicts the idea that MeJA induces the expression of wheat's disease resistance genes to resist F. graminearum infection. On the contrary, the DON-deficient strain and the wild-type strain exhibit only marginal distinctions in their ability to induce the expression of plant disease-related genes. This implies that plants initiate the expression of disease resistance genes in response to pathogen invasion (Ngou et al., 2022), irrespective of mycotoxin production. However, as a resistance gene governed by SA, the expression of TaPR1 was markedly lower 24 h after ∆Tri5 inoculation compared to that following WT inoculation. This finding aligns with a previous report suggesting that plant defense against F. graminearum is sequentially regulated by SA and JA during the early and later stages of infection, respectively (Ding et al., 2022; Makandar et al., 2012). The exogenous application of MeJA does not lead to distinct patterns of disease resistance gene expression in wheat against the wild-type strain and the DON-deficient strain, further emphasizing that the reduction in Fusarium virulence due to MeJA application is not a result of increased expression of wheat disease resistance genes. This may be attributed to the hemibiotrophic nature of F. graminearum, which transitions from parasitic to saprophytic phases during infestation. As depicted in Figure S1, the application of MeJA also exhibits inhibitory effects on the biotrophic fungal pathogen Bgt, which causes powdery mildew in wheat (Jing et al. 2019). Disease resistance development is critical for combating Bgt in wheat (Gottwald et al. 2012). However, MeJA-induced resistance in wheat is inadequate against F. graminearum infestation. The pathogen produces DON, which disrupts plant cellular structures and enhances infestation. Therefore, during late-stage infestations, MeJA primarily suppresses the pathogen's virulence by inhibiting DON biosynthesis. Additionally, gene specificity plays a pivotal role in determining wheat's response to MeJA. Varieties that are disease-resistant or susceptible exhibit distinct genetic profiles affecting their perception and transmission of MeJA signals. Exogenous MeJA notably enhanced disease resistance in susceptible wheat compared to that in resistant varieties (Duan et al. 2014). These differences contribute not only to understanding gene-specific responses of wheat to MeJA but also to comprehending how different wheat varieties perform under biotic stress conditions.
As a plant metabolite, MeJA is accumulated in response to mechanical injury or pathogenic attacks (Gu et al., 2022; Tian et al., 2023; Yu et al., 2018). Actually, MeJA also directly inhibits fungal mycelial growth and regulates the production of secondary metabolites (Tzortzakis et al., 2016), particularly Aflatoxin (AF) B1, which is stimulated at low level of MeJA but inhibited at higher concentrations (Li et al., 2021). The subsequent study confirmed that MeJA can induce changes in the expression of genes related to AFs biosynthesis and some transcription factor genes in Aspergillus flavus (Li et al., 2021). Our results indicate that MeJA significantly inhibits the production of DON at both low and high concentrations, without affecting on toxisome formation. However, notable changes were observed in the expression of DON-related genes. The precise mechanism behind these gene expression changes induced by MeJA remains unclear (Dinolfo et al., 2022), and we speculate that they are linked to MeJA metabolism in fungi. This speculation is supported by the significant reduction in FPP concentration observed after MeJA treatment. Notably, ascomycete pathogens, such as Fusarium fujikoroi and Aspergillus fumigatus, can synthesize and metabolize JA, potentially reflecting the long-term coevolution between plants and pathogens (Cross et al., 1970; Oliw, 2022). While MeJA inhibits the growth of F. graminearum at a concentration of 1 mM, it does not affect spore germination at this concentration, contrasting with previous findings in Fusarium oxysporum (Król et al., 2015).
Currently, sub-lethal doses of most chemical fungicides used for FHB control have been shown to increase DON production. For instance, tebuconazole (0.1 μg mL−1) and difenoconazole (0.1 μg mL−1) increase the production of 3-ADON in cultures of Fusarium culmorum (Felix D'Mello et al., 1998). Recent studies have explored the potential of plant-derived antifungal compounds, including essential oils, melatonin, and quercetin, in inhibiting Fusarium pathogenicity and DON production (Abbas & Yli-Mattila, 2022; Kong et al., 2023; Romoli et al., 2022). In our experiments, we evaluated the efficacy of MeJA applied alone or in combination with fungicides in the field for controlling FHB and suppressing DON production. Although MeJA alone did not significantly prevent FHB occurrence, it demonstrated a substantial reduction in mycotoxin levels. Interestingly, the reduction in DON did not correspond to a decrease in pathogenicity, possibly due to F. graminearum invasion causing wheat spike death. The simultaneous application of MeJA and fungicides demonstrated a synergistic effect in reducing DON content, offering a potential improvement to fungicide applications.
In summary, our research reveals the underlying mechanism by which exogenous MeJA enhances wheat resistance against F. graminearum, challenging conventional perspectives on the role of MeJA in inducing wheat disease resistance. We discovered that MeJA inhibited DON production in F. graminearum by regulating the expression of DON-related genes and reducing the production of its precursor FPP. Field studies have demonstrated the effectiveness of MeJA in suppressing DON and managing FHB, presenting new potential compounds for tackling this disease.
AUTHOR CONTRIBUTIONS
J.G.: methodology, investigation, writing-original draft. Y.S. and W.J.: methodology, investigation. F.Z. and M.Z.: writing-review & editing. X. S.: conceptualization, methodology, supervision, resources, funding acquisition, writing-review & editing.
ACKNOWLEDGEMENTS
This research was funded by the National Key Research and Development Program of China (2022YFD1700401 and 2023YFD1400901).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open Research
DATA AVAILABILITY STATEMENT
All data that support the findings of this study are available in the Supporting Information of this article.




