“Metabolight”: how light spectra shape plant growth, development and metabolism
Abstract
Innovations in light technologies (i.e. Light Emitting Diodes; LED) and cover films with specific optical features (e.g. photo-selective, light-extracting) have revolutionized crop production in both protected environments and open fields. The possibility to modulate the light spectra, thereby enriching/depleting cultivated plants with targeted wavebands has attracted increasing interest from both basic and applicative research. Indeed, the light environment not only influences plant biomass production but is also a pivotal factor in shaping plant size, development and metabolism. In the last decade, the strict interdependence between specific wavebands and the accumulation of targeted secondary metabolites has been exploited to improve the quality of horticultural products. Innovation in LED lighting has also marked the improvement of streetlamp illumination, thereby posing new questions about the possible influence of light pollution on urban tree metabolism. In this case, it is urgent and challenging to propose new, less-impacting solutions by modulating streetlamp spectra in order to preserve the ecosystem services provided by urban trees. The present review critically summarizes the main recent findings related to the morpho-anatomical, physiological, and biochemical changes induced by light spectra management via different techniques in crops as well as in non-cultivated species. This review explores the following topics: (1) plant growth in monochromatic environments, (2) the use of greenhouse light supplementation, (3) the application of covering films with different properties, and (4) the drawbacks of streetlamp illumination on urban trees. Additionally, it proposes new perspectives offered by in planta photomodulation.
1 INTRODUCTION
Sunlight is the energy that powers life on our planet as photosynthesis is the “green engine” that converts the electromagnetic energy of sunlight into chemical energy by fixing carbon dioxide and water into sugars. Wavelengths of the electromagnetic spectrum absorbed by plants and used to drive photosynthesis can be grouped into (1) ultraviolet (UV) ranging from 100–400 nm (i.e. UV-A 100–280 nm; UV-B 280–315 nm; UV-C 315–400), a region in which photons are associated with potentially damaging high energy, (2) photosynthetically active radiation (PAR; 400–700 nm), corresponding to the wavebands that are principally absorbed by plant pigments to fuel the photosynthetic machinery, and (3) far-red (FR) light, associated with lower-energy photons.
From the first water-to-land transition and the consequent land colonization, plants had to face a set of variable life conditions, including the light environment. Being sessile organisms, they have adapted to light intensity (low/high), light fluctuation, and continuous light (at high latitude) and have evolved to efficiently perceive light quality, intensity, direction, and timing, then transmit and process the information received from light stimuli to optimize the plant development and behaviour (Carvalho et al., 2011). Through photoreceptors, namely phytochromes (dimeric chromopeptides that possess two photoconvertible forms for R and FR perception), cryptochromes (devoted to blue and UV absorption), and phototropin (flavoproteins mediating blue, UV and green light absorption), as well as UV-dedicated receptors (i.e., UV Resistance Locus 8 – UVR8, for sensing UV-A and UV-B) plants regulate photomorphogenesis, photoperiodicity and phototropism, but also maximize light harvesting in the context of competition with neighbouring plants or in shade condition in the understorey. (Rizzini et al., 2011; Landi et al., 2020; Rai et al., 2021). For example, phytochromes that are sensitive to variation in red/far red (R/FR) ratio (Falciatore & Bowler, 2005) are determinants in germination, de-etiolation, shade avoidance, and flowering responses in land plants (Mathews, 2006).
In terms of light harvesting and utilization, plants have evolved finely tuned and well-orchestrated mechanisms aimed at, on the one hand, maximizing light harvesting under suboptimal light conditions and, on the other hand, protecting the photosynthetic apparatus when light is in excess of what can be used for photosynthetic requirements (namely, photoinhibition) (Baker, 2008). In plants, light-harvesting pigments, chlorophyll a and b (which absorb principally in the red and blue regions of the solar spectrum) and the accessory pigments carotenoids (which extend the absorbed light to other wavelengths in the visible spectrum) are devoted to intercepting the sunlight and transferring electromagnetic energy to the reaction centers of both photosystems. The red and blue portions of the spectrum are the most absorbed by chlorophylls, but regardless of all the wavelengths of light they absorb, chlorophylls exclusively utilize red photons to drive water-splitting and ferredoxin-reducing photochemistry. (Björn et al., 2009). Indeed, the extra energy of a blue photon, according to the Planck equation (75% higher comparing a 400 vs a 700 nm photon), is dissipated as heat within subpicoseconds to the same energy level as red (Björn et al., 2009).
Curiously, the leaf's green appearance has spread the misconception that green light is poorly absorbed and utilized by the plant. Actually, green light (GL) plays a pivotal role in photosynthesis in the deepest mesophyll layers as well as in the understorey, where light is depleted in the red and blue regions due to absorbance by the overhanging leaves (Folta & Maruhnich, 2007; Brodersen & Vogelmann, 2010). Indeed, though red and blue are peaks for chlorophyll absorption, leaf reflectance, light scattering and energy loss via thermal dissipation dramatically reduce the efficiency of energy conversion to 4.6% in C3 and around 6% in C4 species (Zhu et al., 2008). Moreover, GL is also perceived by phytochromes and cryptochromes, reversing the effect of blue light, for example, in terms of stomatal regulation (Frechilla et al., 2000; Kim et al., 2004). Additionally, in red-leafed species, the effect of GL might be different due to the presence of red pigments (i.e., anthocyanins), which absorb preferentially (but not exclusively) in the green portion of the solar spectrum (Landi et al., 2021; Simkin et al., 2022).
In view of the above, in the last decade the management of light regimes and spectra has been explored to (1) promote plant growth and maximize biomass production in indoor cultivation (Jones, 2018; Stamford et al., 2023), (2) shape plant size and architecture (Li et al., 2000; Stapel et al., 2011), (3) improve the nutritional value of fruit and vegetables (Jones, 2018; He et al., 2022; Sarabi et al., 2022), (4) modulate the biosynthesis of targeted secondary metabolites (Lobiuc et al., 2017; Landi et al., 2020; Morello et al., 2022). Technical advancements in artificial lighting, i.e. the development of LED technology (Singh et al., 2015; Stamford et al., 2023) and new material developed for cover films (Cerny et al., 2003; Manja & Aoun, 2019; Zheng et al., 2020; Liu et al., 2022) have further improved the capacity of modifying the light environment perceived by the plant. In addition, there are some cases in which the modulation of the light regime may exert unwanted effects on plant performance, for example, the possible side effect of continuous light from streetlamps in urban environments (Matzke, 1936; Chaney, 2002; Ffrench-Constant et al., 2016; Massetti, 2018; Lo Piccolo et al., 2023); thus the new LED technology can be employed to minimize the light disturbance on urban trees physiology and phenology.
The present review summarizes the main findings related to morpho-anatomical, physiological and biochemical changes promoted by specific light regimes (Figure 1), i.e., the growth of plants in monochromatic environments (section 2), the use of light supplementation (section 3) as well as covering films with different features (photo-selective, light-extracting, diffusive, mulching films – section 4). In addition, in section 5, we depicted how streetlamp illumination may influence morpho-anatomy and physio-chemical attributes of urban trees with the aim of increasing awareness of the necessity of less-impacting streetlamp solutions.
2 MORPHO-ANATOMICAL TRAITS AND PLANT SECONDARY METABOLITES IN MONOCHROMATIC ENVIRONMENTS
The effect of monochromatic light (ML) on plant biomass, morpho-anatomical traits, and secondary metabolite production has been extensively investigated in the last decade. Table 1 and Figure 2 summarize the results from recent studies dealing with the effect of ML environments to unveil ML-triggered in planta metabolic changes, whose comprehension is crucial not only for improving horticultural crop yield and quality, as reviewed by Jones (2018), but also to photomodulate the synthesis of targeted bioactive compounds in medicinal plants, such as Cannabis sativa (Danziger & Bernstein, 2021; Wei et al., 2021; Morello et al., 2022), Withania somnifera (Adil et al., 2019), Scutellaria baicalensis (Zhang et al., 2022a), Aronia spp. (Szopa et al., 2018), Artemisia absinthium (Tariq et al., 2014), Moringa oleifera (Bajwa et al., 2023).
| Species | Light recipe | Intensity | Duration | Effects | Reference |
|---|---|---|---|---|---|
| Aronia melanocarpa, Aronia arbutifolia, Aronia × prunifolia in vitro seedlings | W, UV-A irradiation (315–400 nm), B (450–492 nm), R (647–770 nm), FR (770–800 nm), dark condition | 20 and 60 μmol m−2 s−1 | Photoperiod 16 h/8 h, 4 weeks | B ↑ shoot biomass, phenolic acid and flavonoid content | Szopa et al. (2018) |
| Artemisia absinthium callus cultures | Cool-W light (380–780 nm), B (380–560 nm), G (480–670 nm), Y (530–780 nm), R (610–715 nm), dark condition | 40–50 μmol m−2 s−1 | Photoperiod 16 h/8 h, 3 weeks | R ↑ peroxidase activity, protease activity, total protein content, and chlorophyll a/b ratio G ↑ chlorophyll and carotenoid content, TPC, TFC, and antioxidant activity Y ↑of malondialdehyde content |
Tariq et al. (2014) |
| Artemisia argyi seedlings | W, B (455 nm), R light (660 nm), R:B 1:3 | 160 μmol m−2 s−1 | Photoperiod 14 h/10 h, 21 days | R ↑ root number, root length, plant height, chlorogenic acids content. B ↑ leaf area, TPC in the first sampling (7th day; decreasing with time), TFC; ↓ plant height | Su et al. (2023) |
| Broccoli (Brassica oleracea) spouts | B (450 nm), R (660 nm), R + B, R + UVA (for UVA peak at 365 nm) |
50 μmol m−2 s−1 | Photoperiod: 16 h/8 h, 5 days | B ↑ cotyledon anthocyanin content, TPC, TFC, and ascorbic acid content R + B ↑ hypocotyl anthocyanin content, TPC, TFC, and ascorbic acid content |
Yang et al. (2021) |
| Red Brassica oleracea var. gongylodes sprouts | W light (449–551 nm), R (636 nm), B | 90 μmol m−2 s−1 | Photoperiod 16 h/8 h, 10 days | R ↑ fresh weight. B ↑ shoot and root lengths, phenylpropanoid and glucosinolates contents | Sathasivam et al. (2023) |
| Cannabis sativa inflorescences | HPS W light, B (430 nm), R (630 nm), Rose (430 + 630 nm, ratio 1:10), V (430 + 630 nm, ratio 2:1), and Amber (595 nm) | 250–270 μmol m−2 s−1 | Vegetation stage: photoperiod 18 h, 0–13 days. Flowering stage:12 h, 8 weeks |
Amber and R ↑ plant height; ↓ CBD concentrations. B ↑ THC, CBD and terpene concentration; ↓ fresh inflorescence biomass | Morello et al. (2022) |
| plants | HPS W light, R:B 9.30:1, R:B 9.20:1, R:B 1.61:1, R:B 6.47:1, R:B 7.15:1, R:B 16.8:1 | R:B 9.30:1, 191 μmol m−2 s−1, R:B 9.20:1, 129 μmol m−2 s−1, R:B 1.61:1, 540 μmol m−2 s−1, R:B 6.47:1, 28.2 μmol m−2 s−1, R:B 7.15:1, 41.7 μmol m−2 s−1, R:B 16.8:1, 252 μmol m−2 s−1 | Photoperiod 16 h/8 h, 110 days | R:B 1.61:1, R:B 6.47:1 ↑stem diameter (+50%). R:B 9.20:1 and R:B 16.8:1 ↑stem and root biomass (due to light intensity), cannabinoids content. B ↑ hemp flower biomass; ↓ biometrical parameters | Wei et al. (2021) |
| W HPS light, W + R:B 1:1, W+ B:R 1:1, W+ B:R 1:4 | Up to 950 μmol m−2 s−1 | Photoperiod 12 h, 12 days for more vigorous varieties and 24 days for the less vigorous variety | R:B 1:1 ↑ inflorescence yield and height. B:R 1:4 ↓ cannabigerolic acid accumulation | Danziger and Bernstein (2021) | |
| Citrus reticulata callus culture | W light, R, B, G, Y | Photoperiod 16 h/8 h for W, 24 h for the other light treatments, 4 weeks | W ↑ fresh biomass followed by G and B. R ↑ values for TPC, TFC, and antioxidant activity. B ↑ activity of antioxidant enzymes | Anum et al. (2021) | |
| Coriandrum sativum microgreens | B, G, RB 87:13 and RBFr 81.5:12.5:6 | 200 ± 15 μmol m−2 s−1 | Photoperiod 16 h/8 h, 18 days | R, RB, and RBFr ↑ stem and leaf fresh weight. R ↑ ascorbic acid content. B ↑ antioxidant capacity and TPC. G ↓ leaf and steam fresh weight, antioxidant capacity and ascorbic acid content | Nguyen et al. (2020) |
| Crocus sativus plantlets | W light, 100% B, 75%B (75%B + 25%R), 50%B (50%B + 50%R), 25%B (25%B + 75%R), 100%R | 150 ± 10 μmol m−2 s−1 | Photoperiod 11 h/13 h, 18–22 days (until flowering) | R ↑ carotenoids concentration in petals. B ↑ flower number, flower fresh weight, anthocyanin, safranal and crocin content; earliest flowering | Moradi et al. (2022) |
| W (400–700 nm), B (465 nm), R (660 nm), and R:B 1:1 combined with GABA or GA3 treatment(multifactorial experiment) | 80 μmol m−2 s−1 | Photoperiod 16 h/8 h, until the end of the flowering period | B and R:B ↑ phytochemical content. B ↑ flower biomass, enhanced with GABA or GA3 treatment | Eftekhari et al. (2023) | |
| Eclipta alba callus culture | Cool W florescent tubes (400–700 nm), continuous Y (570 nm), G (510 nm), W (400–700 nm), B (460 nm), R (660 nm), dark conditions | 40–50 μmol m−2 s−1 | Photoperiod 16 h/8 h for Cnt W and 24 h for the other light treatments, 28 days | R ↑ TPC, TFC, antioxidant activity and secondary metabolites (amyrin, stigmasterol, luteolin, coumarin, eclalbatin, wedelolactone, wedelolactone) | Khurshid et al. (2020) |
| Ipomoea aquatica plantlets | R:B (87:13), R, B, G, R:B:FR (81.5:12.5:6) | 200 ± 15 μmol·m−2·s−1 | Photoperiod 14 h/10 h, 14 days |
R ↑ stem length and fresh weight. B ↑ antioxidant activity capacity in leaves and stems; ↓ stem elongation | Kitayama et al. (2019) |
| Lactuca sativacv. ‘Banchu Red Fire’ seedlings | W fluorescent light, G (510–520–530 nm) | 100, 200 and 300 μmol m−2 s−1 | Photoperiod 24 h light, 10 days |
G ↓ biomass; ↑ leaf area (+71%), fresh weight (+59%) in plants G510 PPFD300 than plants under PPFD200; ↑ petiole length at PPFD100 | Johkan et al. (2012) |
| seedlings | W fluorescent light, B (470 nm), G1 (510 nm), G2 (520 nm), R (680 nm) | 100 and 300 μmol·m−2·s−1 | Photoperiod 24 h, 7 days | R ↑ sucrose. B ↑ amino acids, fatty acids, lipids, alpha-tocopherol, anthocyanins. G1 and G2 downregulation of flavonoids and the expression of genes involved in flavonoid biosynthesis and PAP2 gene. | Kitazaki et al. (2018) |
| cv. ‘Waldmann's Green’ and ‘Outredgeous’ plantlets | Fluorescent W lamp, 100% R, B:R (7:93), B:R (26:74), B:R (66:34), 100% B | 200 μmol m−2 s−1 | Photoperiod 16 h/8 h, 18 days | 100% R ↑ shoot fresh mass. B 66% ↑ in leaf thickness and chlorophyll percentage | Izzo et al. (2021) |
| cv. ‘Batavia’ plants | W fluorescent light, R:B 3:1, R:B 2:1, R:B:FR 3:1:0.5, R (660 nm), B (450 nm), and FR (730 nm) | R:B 3:1 - R 60 μmol m−2 s−1 and B 20 μmol m−2 s−1. R:B 2:1 - R 40 μmol m−2 s−1, B 20 μmol m−2 s−1. R:B:FR 3:1:0.5 - R: 60 μmol m−2 s−1, B 20 μmol m−2 s−1, FR 10 μmol m−2 s−1 |
Photoperiod 16 h/8 h, 3 weeks | R ↑ leaves and petioles. B ↑ leaf area, ↓ petioles. R:B ↑ anthocyanins and carotenoids content |
Rong Sng et al. (2021) |
| Lepidium sativum callus cultures | W light, R (660 nm), G (510 nm), B (460 nm), Y (570 nm), W (400–700 nm), dark condition | 40–50 μmol m−2 s−1 | Photoperiod 16 h/8 h for W, 24 h for the other light treatments, 28 days | W ↑ fresh and dry biomass, caffeic acid, ferulic acid, vanillic acid, sinapic acid, and protocatechuic acid content. W, B, and dark condition ↑ antioxidant activity (95.1%, 94.2%, and 93.2%, respectively), quercetin and kaempferol concentrations. B ↑ p-coumaric acid content | Asad Ullah et al. (2019) |
| Linum usitatissimum sprouts and microgreens | R:G:B 1:1:1, B (peak at 450 nm), R (peak at 660 nm), G (peak at 520 nm) | 200 μmol m−2 s−1 | Photoperiod 24 h light for 4 days, and 16 h/8 h for the next 7 days | B ↑ TPC, TFC, chlorogenic acid content and antioxidant activity | Puccinelli et al. (2022) |
| Mesembryanthemum crystallinum plantlets | R (660 nm), B (450 nm) | 120 or 150 μmol m−2 s−1 | Photoperiod 14 h/10 h, 4 weeks | R ↑ biomass and leaf area. B (150 μmol m−2 s−1) ↑ phytochemicals concentrations and antioxidant activity | Kim et al. (2018) |
| Moringa oleifera callus culture | W light (400–700 nm), continuous W (400–700 nm), Y (570 nm), R (660 nm), G (510 nm), and B (460 nm), dark conditions | 40–50 μmol m−2 s−1 | Photoperiod 16 h/8 h for Cnt W and 24 h for the other light treatments, 28 days | B ↑ biomass and photosynthetic pigment content, chlorogenic acid accumulation | Bajwa et al. (2023) |
| Operculina turpethum callus culture | Cool W fluorescent light, dark conditions, continuous W (400–700 nm), R (630 nm), G (520 nm), B (460 nm), and Y (570 nm) | 40–50 μmol m−2 s−1 | Photoperiod 16 h/8 h for Cnt W and 24 h for the other light treatments, 35 days | B ↑ DPPH antioxidant activity, followed by R and G light, gallic acid, quercetin, coumarin, salicylic acid. Dark and R ↑ SOD and CAT activities |
Biswal et al. (2022) |
| Petroselinum crispum cv. microgreens | RGB: 45% R (600–700 nm, peaking at 650 nm), 10% G (500–600 nm), 45% B (400–500 nm, peaking at 460 nm), B: 0% R, 10% G, 90% B; R: 90% R, 10% G, 0% B | 300 ± 15 μmol m−2 s−1 | Photoperiod 12 h, 11 days | R ↑ biomass, cotyledon, and petiole length; ↓ thickness of spongy parenchyma tissue, number of intercellular spaces. B ↑ compactness, higher ascorbic acid content and antioxidant activity; ↓ height. RGB ↑ polyphenol content; ↓ photosynthetic pigments | Carillo et al. (2022) |
| Pistacea vera in vitro plantlets (micro propagated by callus culture) | W fluorescent light, B, R, and BR (1:1) | 40 μmol m−2 s−1 | Photoperiod 16 h/8 h, 6 weeks | R ↑ biomass and stem elongation, ↓ antioxidant activity. B: ↑ TFC than R and BR treatments. BR ↑ chlorophyll content, TPC | Abdouli et al. (2023) |
| Raphanus sativus, Brassica rapa cv. ‘Nipposinica’, Cichorium intybus, Medicago sativa, Tagetes tenuifolia and Celosia plumosa argentea microgreens | R6:B (65:35) and R:G:B (47:19:34) | 110, 220 and 340 μmol m−2 s−1 | Photoperiod 16 h/8 h, 7 days | RGB ↑ biomass and TPC than RB treatment | Orlando et al. (2022) |
| Scutellaria baicalensis seedlings | W light, B (460 nm), R (660 nm), and different combinations of R and B (R9B1, R7B3, R5B5, R3B7, and R1B9) | 50 μmol m−2 s−1 | Photoperiod16 h/8 h, 15 days | R ↑ biomass of whole plant and root, increasing with the enhancement of R portion, ↑ baicalin and wogonoside content. B ↓of plant growth, bacalein and wogonoside content. R9B1, R7B3 ↑ accumulation of flavonoids | Zhang et al. (2022a) |
Solanum xanthocarpum callus cultures |
W, B (460 nm), W (400–700 nm), G (510 nm), R (660 nm), Y (570 nm), dark condition | 45–50 μmol m−2 s−1 | Photoperiod 16 h/8 h for Cnt W and 24 h for the other light treatments, 5 weeks | Continuous W and B ↑ biomass accumulation. B ↑ TPC, methyl-caffeate, efficiency against alpha-glucosidase (41.92%) and alpha-amylase (29.63%) inhibition, more practical for enhanced anti-AGEs formation ability, ↑ COX-1 inhibition %. W ↑ flavonoid content. B and R ↑ scopoletin and esculetin concentrations. R ↑ 15-LOX inhibition %. Dark ↑ antioxidant activity. | Usman et al. (2020) |
| Stevia rabaudiana callus cultures | W light (380–780 nm), G (480–670 nm), Y (530–780 nm), B (380–560 nm) and R (610–715 nm) | 40–50 μmol m−2 s−1 |
Photoperiod 16 h/8 h, 30 days | R ↑ biomass accumulation. B ↑ TPC, TFC and antioxidant activity | Ahmad et al. (2016) |
| Vaccinium corymbosum cv. ‘Sunt Blue Giant’ callus cultures from root or leaf origin | Dark, W, B and R | W 150 μmol m−2 s−1; B 120 μmol m−2 s−1 and R 180 μmol m−2 s−1 |
Photoperiod 16 h/8 h, 12 days | R ↑ anthocyanin content from root and leaf origin (5.7- and 4.9-fold higher respectively), qualitative composition of anthocyanins in callus obtained from leaf origin largely than in callus obtained from root origin, accumulation of flavonols, PAL activity | Abou El-Dis et al. (2021) |
| Withania somnifera callus culture | W fluorescent light, V (350–400 nm), B (380–560 nm), G (480–670 nm), Y (530–780 nm), R(610–715 nm) | 50 μmol m−2 s−1 | Photoperiod 16 h/8 h, 5 weeks | R ↑ callus biomass, TPC and TFC, chlorogenic acid and withaferin A content; V ↑ TPC and TFC. G ↑ withaferin A | Adil et al. (2019) |
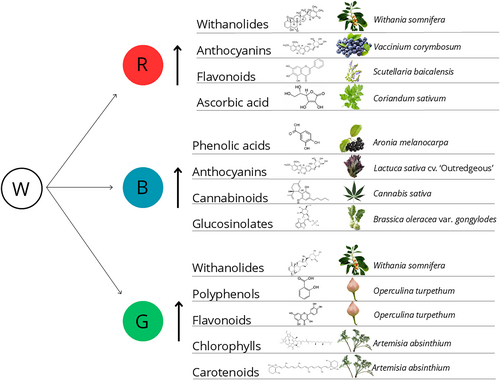
The effects of monochromatic red light (RL) and blue light (BL) environments have attracted intensive research. Under short photoperiods of red and blue light, cryptochromes and phyB act synergistically, but under continuous exposure to the same light field, the actions of phyB and cryptochrome can become independent and additive (Casal, 2000). Therefore, the behaviour of plants under monochromatic RL or BL is not easily predictable and, as depicted below, may lead to species-specific as well as dose-dependent responses (Ahmad et al., 2016; Kitayama et al., 2019; Khurshid et al., 2020; Izzo et al., 2021; Rong Sng et al., 2021; Wei et al., 2021; Carillo et al., 2022; Zhang et al., 2022a; Su et al., 2024). RL is able to enhance plant/callus biomass production, as observed in plants of Withania somnifera (Adil et al., 2019), C. sativa (Wei et al., 2021), Coriandum sativum (Nguyen et al., 2020), Lactuca sativa (Izzo et al., 2021), Mesembryanthemum crystallinum (Kim et al., 2018), I. aquatica (Kitayama et al., 2019), Scutellaria baicalensis (Zhang et al., 2022a), Brassica oleracea, (Sathasivam et al., 2023), callus of S. rabaudiana, (Ahmad et al., 2016) and in vitro cultures of Pistacea vera (Abdouli et al., 2023). Greater stem diameter, leaf area, leaf length, and overall plant weight are the most common morpho-anatomical traits observed after red light (RL) exposure, which explains the higher biomasses seen in plants exposed to this light condition. (Kim et al., 2018; Kitayama et al., 2019; Nguyen et al., 2020; Rong Sng et al., 2021; Carillo et al., 2022; Morello et al., 2022; Abdouli et al., 2023; Su et al., 2024; Wei et al., 2021). Given that phytochromes are present in two forms: the inactive form, which absorbs maximally in RL (Pr) and the active form, which absorbs maximally in far-red (FR) light (Pfr), the increase in plant biometric traits is conceivably related to a combination of increment of the active form of phytochromes as well as the interception of the most efficient wavebands for photosynthesis (McCree, 1972). RL is principally absorbed by PSII, whilst FR light is preferentially absorbed by PSI, so the use of monochromatic RL may lead to overexcitation of PSII. Therefore, neither of the two lights would be optimal for photosynthesis when applied alone, and the interaction between the two lights would be synergistic and has to be considered when growing plants under monochromatic RL (Zhen & van Iersel, 2017). Indeed, the supplementation of FR to white light (up to 40% of background light) can increase the canopy's gross photosynthesis by adding an equivalent amount of white photons (Zhen &Bugbee, 2020).
Though a huge body of evidence supports the accumulation of biomass in plants exposed to RL, contrasting results have emerged regarding the RL-dependent biosynthesis of secondary metabolites that are of utmost importance for human health. For example, Adil et al. (2019) found the highest content of chlorogenic acid and withaferin A in callus cultures of W. somnifera grown in an RL environment. These authors hypothesized that withanolides, such as withaferin A, are involved in the conversion of phenylalanine to cinnamic acid catalyzed by phenylalanine ammonia-lyase (PAL). This enzyme is activated under red light (RL), signalling the phenylpropanoid pathway to counteract oxidative stress by accumulating higher levels of withanolides (Adil et al., 2019). Enhancement of PAL activity, the key enzyme of the phenylpropanoid branch metabolism, promoted by RL might also explain the increase in anthocyanin and flavonol content in Vaccinium corymbosum callus cultures (Abou El-Dis et al., 2021). Zhang et al. (2022a) also observed an increased level of two other flavonoids, namely baicalein and wogonoside, in S. baicalensis seedlings supplied with RL. In the same study, a punctual transcriptome sequencing analysis demonstrated that RL induced a higher accumulation of flavonoids than other ML treatments. This effect was attributed to the upregulation of genes involved in flavonoid biosynthesis, such as PAL, cinnamate CoA ligase (CLL), chalcone synthase (CHS), flavonol synthase (FNS) and 8-O-methyl transferase (OMT). Moreover, the RL-promoted increased level of cytokinins may also contribute to the accumulation of phenolic compounds (Khurshid et al., 2020).
RL also promoted the accumulation of ascorbic acid in C. sativum microgreens (Nguyen et al., 2020), targeted secondary metabolites in Eclipta alba such as amyrin, stigmasterol, luteolin, coumarin, eclalbatin, wedelolactone and derivatives (Khurshid et al., 2020) as well as carotenoids in petals of Crocus sativum (Moradi et al., 2022). Despite the generally positive impact of RL in terms of cannabinoid accumulation (Wei et al., 2021), some authors reported a reduction of this class of compounds in some C. sativa varieties after RL treatment, highlighting the importance of considering the genotype-dependent responses in terms of secondary metabolism modulation (Danziger & Bernstein, 2021; Morello et al., 2022).
Several studies have also analysed the effects of BL on plants, yielding interesting but occasionally contrasting results (Table 1). In terms of morpho-anatomical traits of plants treated with monochromatic BL, short petioles and shoots (with a reduction of plant height), leading to more compact plants characterized by increased leaf area and leaf thickness were observed (Izzo et al., 2021; Rong Sng, 2021; Carillo et al., 2022). In some studies, these changes translated into a reduction of the overall plant biomass (Zhang et al., 2022a; Wei et al., 2021). It is worth noting that such treatment led to enhanced inflorescence biomass in flowering crops (Wei et al., 2021; Orlando et al., 2023; Moradi et al., 2022). Rong Sng et al. (2021) analysed the morpho-anatomical traits of Lactuca sativa cv. Batavia plants grown for three weeks in a BL environment were found to have a higher leaf area and shorter petioles compared to lettuce grown in polychromatic or other MLs, such as RL and FR light. Accordingly, Carillo et al. (2022), analysing Petroselinum crispum microgreens grown in a chamber illuminated with BL, observed shorter internodes and more compact plants compared to those grown with other MLs or polychromatic lights. These authors attributed the inhibition of hypocotyl growth in plants exposed to BL to modifications in the cell wall structure, resulting in reduced turgor and finally leading to a slower plant growth rate (Carillo et al., 2022).
Another topic of research on the effect of a BL environment on plants is the possibility of increasing the inflorescence biomass. This phenomenon has been specifically observed in Cannabis sativa and C. sativus (Wei et al., 2021; Eftekhari et al., 2020; Moradi et al., 2022), suggesting a species-specific explanation for this feature. In C. sativa, it was observed that BL increased the photosynthate accumulation in the inflorescence, thereby reducing the vegetative growth and promoting inflorescence development and yield (Wei et al., 2021). Moradi et al. (2022) provided new insights, elucidating that the BL photoreceptor CRY is capable of initiating flowering by controlling the CRY2/coat protein 1(COP1) complex. COP1, in turn, affects the dehydration of CONSTANS (CO), a zinc-finger transcription factor able to trigger plants to flower.
It has long been known that in response to biotic and abiotic stressors, plants synthesize an arsenal of secondary metabolites. Exposure to a monochromatic BL can represent a “eustress” for plants, thus a stress that activates and stimulates plant responses, with positive consequences for plant development (Lichtenthaler, 1996), for example, the stimulation of the biosynthesis of those targeted secondary metabolites whose accumulation is strictly dependent on the applied narrowband light. As illustrated in Table 1, several studies reported an increase in the concentrations of key antioxidant molecules and enhanced antioxidant activity in seedlings and/or callus cultures grown under monochromatic BL (Ahmad et al., 2016; Kim et al., 2018; Kitazaki et al., 2018; Szopa et al., 2018; Asad Ullah et al., 2019; Kitayama et al., 2019; Nguyen et al., 2020; Usman et al., 2020; Anum et al., 2021; Yang et al., 2021; Biswal et al., 2022; Carillo et al., 2022; Moradi et al., 2022; Morello et al., 2022; Puccinelli et al., 2022; Bajwa et al., 2023; Eftekhari et al., 2023; Sathasivam et al., 2023; Su et al., 2024). The highest observed total phenolic content (TPC) and total flavonoid content (TFC) in plants exposed to BL can be attributed to the proximity of B and UV spectra wavelengths, thus promoting similar stimulatory effects on secondary metabolite biosynthesis (Abdouli et al., 2023). This observation is corroborated by Szopa et al. (2018), who noted the highest accumulation of neochlorogenic and protochatecuic acids in Aronia melanocarpa grown under BL and UV-A monochromatic environment. Simultaneously, other researchers confirmed that cryptochromes (blue and UV receptors) play a pivotal role in regulating the biosynthesis of secondary metabolites such as anthocyanins, cannabinoids and glucosinolates (Mickens et al., 2018; Wei et al., 2021; Yang et al., 2021; Morello et al., 2022; Sathasivam et al., 2023).
Although fewer studies are available on the use of monochromatic green (GL) environments (compared to those dealing with BL and RL), GL has been generally proven to exert negative effects on plant growth, as revealed by the impairment of several morpho-anatomical parameters, including a reduced leaf number and area in relation to stem weight as well as lower stomata density (Table 1). Furthermore, starch granules in the chloroplasts were fewer in number and smaller in size in plants grown under GL (Su et al., 2014). The biochemical parameters which have been most strongly affected by GL (compared to RL or BL) and contribute to the impairment of plant growth include lower chlorophyll content, reduced ribulose-1,5 bisphosphate carboxylase/oxygenase (Rubisco) activity and consequently, a decline in ETR and PSII efficiency (Landi et al., 2020).
In addition to this generalization, it is important to recognize that what is often described as a “monochromatic environment”, should more accurately be defined as a “narrowband light”, particularly for green light (GL). Indeed, supplementation with short- and long-wavelength GL may elicit different responses in plants (Dougher & Bugbee, 2007). Depending on the peak of the GL (usually ranging from 550 to 570), the absorbance by the inactive form of phytochrome (Pr) could be higher than that of Pfr. Therefore, the plant's responses may be derived from a trade-off between the effect of cryptochrome and phytochrome, which might explain why, in some cases, plants grown under green light have similar or even higher biomass yield (Pedroso et al., 2017; Amaki et al., 2011, respectively).
Biochemical modification triggered by GL also includes the secondary metabolism; for example, Adil et al. (2019) found that W. somnifera callus cultures grown in a monochromatic GL accumulated a higher withaferin A level than those grown with a cool white light, used as a control. Tariq et al. (2014) reported an increase in TPC and TFC, along with enhanced antioxidant activity in A. absinthium callus culture exposed to monochromatic GL compared to controls, i.e. callus culture grown in a cool white environment.
3 SUPPLEMENTAL LIGHTING WITH TARGETED WAVEBANDS
If monochromatic environments are used to stimulate targeted secondary metabolisms or are relevant in a vertical farming context for short-term applications (i.e., spouts, microgreens), the supplementation of solar light with narrowband lamps represents a feasible technique in greenhouse cultivation to be applied transiently or during a whole cultivation cycle. Nowadays, LEDs are the leading source of light in agriculture. Compared to traditional lighting methods, LEDs have energy efficiency implications as they (1) have up to 2.5-fold longer life span than HPS lamps, (2) have low energy consumption due to the low heat emissions and (3) can be efficiently designed for controlling the light spectrum, the intensity and the scheduling to fine-tuned their application based on the plant's needs (Bourget, 2008; Stamford et al., 2023).
In greenhouses, ML supplementation can increase the daylight intensity where daily light integral (DLI) is insufficient or extend the photoperiod by providing additional hours of illumination. The lighting enrichment by narrowband lamps can alter the spectral composition, capitalizing on the morphological, biochemical, and physiological responses connected with the exposure to specific monochromatic wavelengths. However, it is crucial to maintain a full-spectrum solar light background to ensure that plants receive all the necessary portions of the light spectrum. Selected wavebands can be applied individually or in combination, affecting plant productivity and morphology (Stamford et al., 2023). The consequences of light supplementation are not always consistent; in most cases, results are genotype-dependent but also attributable to the DLI provided, as well as the season/latitude where light supplementation is employed.
The impacts of narrowband light supplementation on plant development, physiology, and biochemistry are summarised in Table 2. Effects which are not related to the alteration of light quality by narrowband lights (i.e., effects merely related to increased light flux) have been omitted. In all the studies reported in Table 2, narrowband lights provided by LED lamps were employed because of their advantages over older light sources (Morrow, 2008) and, generally, a maximum intensity of up to 250 μmol m−2 s−1 was provided.
| Daylight supplemental irradiances | |||||
|---|---|---|---|---|---|
| Species | Light recipe | Intensity | Duration | Effects | Reference |
| Solanum lycopersicum | R + 0, 6, 12, 24%B | 99 μmol·m−2 s−1 | Photoperiod 16 h for 111 days | R ↑ plant height, leaf area, rates of day respiration; ↓ biomass, Fv/Fm, TPU. R + 24B ↑ photosynthetic capacity, rate of day respiration; ↓ biomass, leaf area, stem length, TPU | Kaiser et al. (2019) |
| FR | 30 or 50 μmol·m−2 s−1 | Photoperiod 16 h long term | FR ↓ weight loss, pitting, softening in mature green tomatoes; ↑ firmness, ↓ weight loss and decay during shelf-life in red tomatoes | Affandi et al. (2020) | |
| B (increasing percentage up to 61%) | 100 μmol·m−2 s−1 | Photoperiod 16 h for 30 days | Increasing B ↑ ΦPSI; ↓ leaf area, height, biomass | Kalaitzoglou et al. (2021) | |
| B (supplied intermittently) | 100 μmol·m−2 s−1 | Photoperiod 12 h for 46 days | ↑ earlier flowering, ethylene, lycopene, phenolics and flavonoids, DPPH, FRAP, free amino acids, soluble sugars, vitamin C | He et al. (2022) | |
| WR, 2R:1B | 125 μmol·m−2 s−1 | Photoperiod 8 h for 30 days | WR ↑ plant hight, total leaf area, root lenght, root volume, root activity, photosynthetic parameters, starch and sucrose content; ↓ hypocotyl lenght. RB ↑ stem diameter, sucrose content; ↓ hypocotyl lenght | Zhang et al. (2022b) | |
| 3R:1B | 180 μmol·m−2 s−1 | Photoperiod 16 h for 60 days | ↑ lycopene and β-carotene after cold storage | Appolloni et al. (2023) | |
| Cucumis sativus (hybrid Larino) | 4R:1B | 220 μmol m−2 s−1 | Photoperiod 12 h for about 120 days | ↑ A, WUE and E, cucumber yield, precocity; ↓ fruit curve | de Freitas et al. (2021) |
| Ocimum basilicum (green-leafed basil) | B | 300 μmol m−2 s−1 | Photoperiod 16 h up to 48 days | B ↑ cichoric acid and quercetin rhamnoside | Taulavuori et al. (2016) |
| 4R:1B, 4R:1B + UVA, 2R:3B, 4R:1G | 120 μmol m−2 s−1 | Photoperiod 20 h for 28 days | 2R:3B ↑ Leaf Mass Area, leaf dry weight, abaxial stomatal density; ↓ Specific Leaf Area compared to 4R:1B. 4R:1G ↓ adaxial stomatal size, adaxial pore area, gs, ABA and ABA-GE | Jensen et al. (2018) | |
| W, B, R | 130 μmol m−2 s−1 | Photoperiod 16fh. for 45 days | B ↑ flavonol biosynthesis; ↓ stem length | Matysiak and Kowalski (2019) | |
| W (R:G:B, 1:1:1), G, B, R | 250 μmol m−2 s−1 | 5 h d−1 from 11 a.m. to 4 p.m. for 21 days | W ↑ n° stomata, gs, Ci, caftaric acid, rutin; ↓ WUE. G ↑ n° stomata, WUE, caffeic and caftaric acid; ↓ leaf area, gs, Ci, ΦPSII. B ↑ n° stomata, gs, Ci, ETR, ΦPSII, caffeic and caftaric acid, rutin; ↓ leaf area, WUE, ΦNPQ. R ↑ Ci, ↓ Pn, WUE | Lauria et al. (2023b) | |
| Ocimum basilicum (red-leafed basil) | W, B, R | 130 μmol m−2 s−1 | Photoperiod 16 h for 45 days | W ↑ plant height, leaf number and leaf size. B ↑ flavonol biosynthesis | Matysiak and Kowalski (2019) |
| 250 μmol m−2 s−1 | 5 h d−1 from 11 a.m. to 4 p.m. for 21 days | W ↓ WUE. G ↑ n° stomata, WUE, flavonol; ↓Pn, gs, Ci, caffeic acid, Cy-3-coum-Glc. B ↑ n° stomata, gs, flavonols, anthocyanins, caftaric acid; ↓ WUE, ΦPSII. R ↓ n° stomata, ΦPSII, caffeic acid | Lauria et al. (2023b) | ||
| Capsicum annuum | 4R:1B, 4R:1B + FR | 71 μmol m−2 s−1 (+ 55 μmol m−2 s−1 for FR) | Photoperiod 12 h for 30 days | 4R:1B ↑ sucrose, glucose, fructose, ascorbic acid, carotenoid; ↓ fruit weight, number of fruit. 4R:1B + FR ↑ yield, fruit lengths and widths, sucrose, glucose, fructose, ascorbic acid | Kim and Son (2022) |
| Fragaria × ananassa | W (R:G:B, 1:1:1), G, B, R | 250 μmol m−2 s−1 | 5 h d−1 from 11 a.m. to 4 p.m. for 17 days | After 1 day: W ↑ qN, DEPS, superoxide anion R ↑ chlorophylls, DEPS, superoxide anion. B ↑ qN, DEPS. G ↑ qN, VAZ, DEPS. After 17 days: W ↑ Pn, gs, gm, superoxide anion. R ↓ Pn, gm, Vcmax, J1300, TPU, qN, chlorophylls, ↑ VAZ, superoxide anion. B ↑ superoxide anion; ↓ Pn, gs, gm, Vcmax, J1300, TPU. G ↑ VAZ; ↓ qN | Lauria et al. (2021) |
| W (R:G:B, 1:1:1), G, B, R | 250 μmol m−2 s−1 | 5 h d−1 from 11 a.m. to 4 p.m. for about 90 days | G ↑ primary metabolites, phenolics. B ↑ primary metabolites, phenolics. R ↑ plant productivity, anthocyanins, expression of genes related to the cell wall defence; ↓ primary metabolites | Lauria et al. (2023a) | |
| W (R:G:B, 1:1:1), G, B, R | 250 μmol m−2 s−1 | 5 h d−1 from 11 a.m. to 4 p.m. for 60 days | R ↑ maximum trapped exciton flux per PSII, H2O2, MDA, SOD APX; ↓ petiole length, Pn, PIabs, ψ0, φEo, carotenoids, α-tocopherol. B ↑ MDA, SOD, APX; ↓petiole length, biomass, leaf area, carotenoid, α-tocopherol, H2O2. G ↓ petiole length, biomass, leaf area, PIabs, H2O2. W ↑ MDA; ↓biomass, leaf area. | Lauria et al. (2023c) | |
| Valerianella locusta | W, B, R | 130 μmol m−2 s−1 | Photoperiod 16 h for 30 days | W ↑ plant height, leaf number, leaf size. B ↑ flavonol biosynthesis | Matysiak and Kowalski (2019) |
| Eruca sativa | W, B, R | 130 μmol m−2 s−1 | Photoperiod 16 h for 30 days | W ↑ plant height, leaf number, leaf size. B ↑ flavonol biosynthesis | Matysiak and Kowalski (2019) |
| Lactuca sativa var. Lollo rossa | B | 300 μmol m−2 s−1 | Photoperiod 16 h up to 48 days | B ↑ quercetin-malonyl diglucoside, quercetin 3-malonylglucoside, chicoric acid; ↓ protocatechuic acid | Taulavuori et al. (2016) |
| Taxus baccata | B, R, 2R:1B + FR | 100 μmol·m−2 ·s−1 (R, B). 50, 100, 150 μmol m−2 s−2 (2R:1B + FR) | Photoperiod 14 h for 120 days | 2R:1B + FR ↑ plant height | Chiocchio et al. (2022) |
| Impatiens hybrida hort cv. ‘Royal Magenta’ and cv. ‘White’ | 83R:17B, 3R:1B, 67R:33B, 2R:1R1:1B | 150 μmol m−2 s−1 | Photoperiod 12 h for 210 days | White cv: R83:B17 ↑ trichomes, plugs compaction. 3R:1B ↑ cuttings. R67:B33 ↑ cuttings, quercetin, trichomes. Royal Magenta cv: R83:B17 ↓ oxidative damage; ↑ trichomes. 3R:1B ↑ photosynthesis; ↓ oxidative damage. R67:B33 ↑ photosynthesis, trichomes | Kobori et al. (2022) |
| Rosa × hybrida | R, B, W, RBW + FR (high R:FR ratio), RBW + FR (low R:FR ratio) | 200 μmol m−2 s−1 | Photoperiod 18 h for 42 days | R ↑ n° of shoots per plant. RBW + FR (both high and low R:FR ratio) ↑ Fv/Fm | Matysiak (2021) |
| Additional hours | |||||
| Fragaria × ananassa | B, R, 7R:3B | 75 μmolm−2 s−1 | 6 h for ≈ 170 days | R ↑ petioles, chlorophyll, oxalic and malic acid, total phenolic compounds, antioxidant activity. B ↑ length of leaflets, fruit production, anthocyanins. 7R:3B ↑ petioles, length of petioles, width of leaflets, fruit production, chlorophyll, fructose, anthocyanins | Choi et al. (2015) |
| Anoectochilus roxburghi | R, B, Y, G, W | 30 μmol·m−2 s−1 | 3 h for 40 days | R ↑ root length, qN; ↓ Chl a fluorescence yield. B ↑ leaf number, stem diameter, root length, fresh and dry weight, Chlorophyll a, total flavonoids, total polyphenol; ↓ soluble proteins. Y ↑ root length, Chlorophyll a fluorescence yield, soluble sugars, polysaccharides, stomatal density, root vitality. G ↑ qN; ↓ total polyphenols. W ↑ qN; ↓ soluble and reducing sugars | Wang et al. (2018) |
| Cucumis sativus | 7R:2B | Nd | 1, 2 or 3 h for 30 days | ↑ plant growth, root development and vigour, root dry matter accumulation, net photosynthetic rate, transpiration rate, stomatal conductance, chlorophylls and carotenoids, Fv/Fm, ΦPSII, qP, Fv’/Fm′, Calvin Cycle enzyme activity, phosphate synthase, acid invertase, neutral invertase, sucrose, fructose, and glucose; ↓ Ci | Wang et al. (2021) |
| Solanum lycopersicum | 7R:2B | 51 μmol m−2 s−1 | 3 h in the morning or in the evening for 30 days | In the morning ↑ dry matter accumulation, root growth, height, leaf area, gas exchange parameters, photosynthetic capacity, Rubisco enzyme activity, photosynthetic products, chlorophylls, carotenoids. In the evening ↑ stem diameter, height, leaf area, gas exchange parameters, photosynthetic capacity, Rubisco enzyme activity, photosynthetic products | Wang et al. (2022) |
| Eruca sativa | R, B, 75R:25B, 25R:75B, 50R:50B, W | 200 μmol m−2 s−1 | 6 h for 28 days | R ↑ GS activity; ↓ NH4+. B ↑ vitamin C, GS activity, NiR activity; ↓ nitrate content, NH4+. 3R:1B ↑ carbohydrates, flavonoids, tannins, GS activity, amino acids; ↓ nitrate content, NH4+. 1R:3B ↑ GS activity NiR activity; ↓ NH4+. 1R:1B↑ carbohydrates, phenols, flavonoids, tannins, GS activity, amino acids; ↓ nitrate content, NH4+. W ↓ NH4+ | Sarabi et al. (2022) |
| Night supplementation | |||||
| Fragaria × ananassa | B, G, R | 80 μmol m−2 s−1 | 16 h for 356 days | B ↑ number of florets and flower cluster per plant, fresh weight of flower cluster. R ↓ number of florets and flower cluster per plant | Magar et al. (2018) |
| Lactuca sativa cv. ‘Red Mist’ | W, 57R:43B, and B/UVA | 167 μmol m−2 s −1 | 12 h for 3 days | All treatments ↑ dry weight, carotenoids, total phenol content, trolox equivalent antioxidant capacity; ↓ specific leaf area. RB ↑ SPAD. W ↑ SPAD | Hooks et al. (2022) |
| Excluded light | Species | Effects | Reference |
|---|---|---|---|
| R | Cosmos bipinnatus | ↓ shoot dry weight | Cerny et al. (2003) |
| Dendranthema × grandiflorum | ↑ stem elongation in chrysanthemum, ↓ shoot dry weight | Cerny et al. (2003) | |
| ↑ height, internode length | Khattak and Pearson (2006) | ||
| Eustoma grandiflorum | ↑ height. No effects on dry weight. No effects on days to flower or bud number | Wilson and Rajapakse (2001a) | |
| Pachystachys lutea | ↑ height | Wilson and Rajapakse (2001b) | |
| Zinnia elegans | ↓ shoot dry weight | Cerny et al. (2003) | |
| FR | Antirrhinum majus | ↓ shoot dry weight. Delaying in anthesis | Cerny et al. (2003) |
| ↓ extension growth in all species both for continuative exposition and for a short time exposition. Delaying in flowering | Runkle and Heins (2002) | ||
| Capsicum annuum | ↓ height, total leaf area, leaf size. Number of leaves was not affected | Li et al. (2000) | |
| Cosmos bipinnatus | ↓ height, shoot dry weight | Cerny et al. (2003) | |
| Dendranthema × grandiflorum | ↓ height, total leaf area, leaf size. Number of leaves was not affected. | Li et al. (2000) | |
| ↓ height especially in the early vegetative period, flower dimension. Far-red filters did not affect anthesis | Li et al. (2003a) | ||
| ↓ height, shoot dry weight. Delaying in anthesis | Cerny et al. (2003) | ||
| Euphorbia pulcherrima | ↓ plant height. Delaying in flower induction | Clifford et al. (2004) | |
| Eustoma grandiflorum | ↓ height, stem dry weight. No effects on days to flower or bud number | Wilson and Rajapakse (2001a) | |
| Impatiens walleriana | ↓ height | Fletcher et al. (2005) | |
| ↓ extension growth in all species both for continuative exposition and for a short time exposition | Runkle and Heins (2002) | ||
| Lactuca sativa var. crispa | ↑ calcium content, ↓ dry matter, chlorophyll. The incidence of tip-burned leaves decreased. No effects on yield | Kleemann (2002) | |
| Orthosiphon stamineus | ↓ height, leaf area, leaf and stem dry weight | Wilson and Rajapakse (2001b) | |
| Pachystachys lutea | ↓ height, leaf and stem dry weight | Wilson and Rajapakse (2001b) | |
| Petunia × hybrida | ↓ shoot dry weight. Delaying in anthesis | Cerny et al. (2003) | |
| ↓ extension growth in all species both for continuative exposition and for a short time exposition. Delaying in flowering | Runkle and Heins (2002) | ||
| ↓ height. Flowering was delayed under lower PAR-transmission films | Fletcher et al. (2005) | ||
| Salvia x ‘Indigo Spires’, Salvia splendens ‘Van Houttei’, and Salvia leucantha | ↓ height, greenness (only in ‘Indigo Spires’), stem dry weight. ↑ greenness (only in ‘Van Houttei’) | Wilson and Rajapakse (2001c) | |
| Solanum lycopersicon | ↓ extension growth in all species both for continuative exposition and for a short time exposition | Runkle and Heins (2002) | |
| Strobilanthes dyerianus | ↓ leaf and stem dry weight | Wilson and Rajapakse (2001b) | |
| Viola × wittrockiana | ↓ extension growth in all species both for continuative exposition and for a short time exposition. Delaying in flowering | Runkle and Heins (2002) | |
| Zinnia elegans | ↓ height, shoot dry weight. Delaying in anthesis | Cerny et al. (2003) | |
| R + FR | Gardenia jasminoides | ↓ height, leaf area of cuttings and plants, number of nodes, shoot fresh and dry weight. No lateral shoot development | Lykas et al. (2005) |
| ↓ height, leaf area in cuttings, fresh and dry weight in plants rooted and grown under the film, lateral shoot if rooted under control and then moved under photo-selective film | Lykas et al. (2008) | ||
| B | Dendranthema × grandiflorum | ↓ height, internode length, time to flowering, leaf area, leaf size, fresh and dry weight | Khattak and Pearson (2006) |
| UV | Brassica oleracea | ↑ compactness | Stapel et al. (2011) |
| Cucumis sativus | ↑ compactness | Stapel et al. (2011) | |
| Gazania rigens | ↑ compactness | Stapel et al. (2011) | |
| Lactuca sativa cv Lollo Rosso | UV filters ↓ secondary metabolites, photosynthetic efficiency, ↑ growth. UV transparent films ↑ anthocyanin, flavonoid and phenolic content | Tsormpatsidis et al. (2008) | |
| Solanum lycopersicon | ↑ compactness | Stapel et al. (2011) | |
| No effects on fruit quality characteristics, nutritional value, and organoleptic properties | Papaioannou et al. (2012) | ||
| Solanum melongena | ↑ plant height, leaf length, leaf width, chroma parameter, production quantity and quality | Kittas et al. (2006) |
The supplementation of RL over sunlight increased plant productivity in strawberry (Lauria et al., 2023a) and mini cucumber (>200 μmol m−2 s−1; de Freitas et al., 2021) but decreased the yield in sweet pepper (Kim & Son 2022) and tomato (<100 μmol m−2 s−1; Kaiser et al., 2019). Similar results were observed when RL (<100 μmol m−2 s−1) was added before or after natural light exposure, resulting in increased strawberry fruit production (Choi et al., 2015) and plant development in cucumber and tomato (Wang et al., 2021, 2022, respectively). Despite the positive results observed in terms of productivity, supplemental RL often decreased net photosynthesis, reduced stomatal size, lowered Rubisco activity and decreased carbon export from the Calvin–Benson cycle in tomato (Kaiser et al., 2019), strawberry (Lauria et al., 2021, 2023c), and basil plants (Jensen et al., 2018; Lauria et al., 2023b). This reduction was associated with decreased PSII efficiency and PSII antenna's ability for energy conservation (Kaiser et al., 2019; Lauria et al., 2023c). Moreover, RL also led to a reduction in abscisic acid content in basil (Jensen et al., 2018). This behaviour could be partially explained by alterations in the expression of both PSII and PSI multiprotein complexes (Muneer et al., 2014). In contrast, when RL enrichment is applied in artificial lighting instead of over a natural background, even when RL is predominant, the observed effects are usually positive and consistent across different crops. In fact, it has been reported that a 2:1 to 4:1 red-to-blue (RL:BL) ratio, irrespectively of the fluence, enhances photosynthetic performances and increased net photosynthesis, transpiration rates and stomatal conductance in cucumber (Wang et al., 2021), mini cucumber (de Freitas et al., 2021), tomato (Wang et al., 2022; Zhang et al., 2022b) and Impatiens hybrida (Kobori et al., 2022).
Besides physiological effects, RL supplementation can also affect primary and secondary metabolism. Lower intensities of RL increased starch and sucrose (tomato; 125 μmol m−2 s−1; Zhang et al., 2022b) as well as glucose and fructose (sweet pepper; 71 μmol m−2 s−1; Kim & Son, 2022). These alterations could be related to the modulation of carbohydrate metabolism by phytochrome A and B (PHYA and PHYB), with PHYA promoting starch accumulation under a wide range of light conditions through a signalling cascade involving the plastidial nucleoside diphosphate kinase-2 (NDPK2) and glucose-1-phosphate adenylyltransferase small subunit (APS1) (Han et al., 2017). Conversely, Lauria et al. (2023a) observed that higher intensities (250 μmol m−2 s−1) of RL led to a reduction of sugars and organic acids content in strawberry fruits, suggesting that RL irradiation might accelerate glycolysis and tricarboxylic acid (TCA) cycle.
Regarding changes in secondary metabolism, supplemental RL has been shown to promote the accumulation of compounds belonging to different classes, including quercetin (I. hybrida cv. White; Kobori et al., 2022), ascorbic acid, carotenoids (sweet pepper; Kim & Son, 2022), anthocyanins (strawberry fruits; Lauria et al., 2023a), and xanthophylls (strawberry leaves; Lauria et al., 2021). After cold storage, tomato fruit treated with a pre-harvest ratio of 3:1 RL:BL showed increased lycopene and carotene contents (Appolloni et al., 2023). When RL was exclusively used to extend the photoperiod, an increase in chlorophyll content was reported in strawberry leaves and tomatoes (Choi et al., 2015, Wang et al., 2022, respectively), along with elevated carotenoid content in cucumber and tomatoes (Wang et al., 2021, 2022, respectively), higher carbohydrate levels in rocket salad (Sarabi et al., 2022), increased sugar content in strawberry fruit, cucumbers and tomatoes (Choi et al., 2015, Wang et al., 2021, 2022, respectively), and elevated flavonoid and tannin contents in rocket salad (Sarabi et al., 2022). Choi et al. (2015) also reported an increase in oxalic and malic acids, anthocyanins, and antioxidant activity in strawberry fruit, while Sarabi et al. (2022) noted a decrease in nitrate content and NH4+ in Eruca sativa due to increased glutamine synthetase activity, which catalysed the conversion of NH4+ into glutamine.
Although FR light is usually considered marginally in greenhouse cultivation, with a high R:FR ratio generally preferred (see section 3), some studies have reported impacts of supplemental FR light on the physiology, morpho-anatomy and both primary and secondary metabolisms of plants, for example, increased plant height (Taxus baccata; Chiocchio et al., 2022) and improved post-harvest cold tolerance in tomatoes (Affandi et al., 2020). When added to a background of supplemental wide-spectrum light, FR light increased the maximum quantum yield of PSII photochemistry, both in conditions of high and low R:FR ratio (Matysiak, 2021). Additionally, FR light supplementation promoted the accumulation of sucrose, glucose, fructose, and ascorbic acid in sweet pepper (Kim & Son, 2022).
A large body of experimental work explored the effect of supplemental BL on biomass, morpho-anatomical, and physiological responses, as well as its impact on both primary and secondary plant metabolism. The addition of 25–30% B light (approximately 100 μmol m−2 s−1) led to a reduction in triose phosphate use but correlates with an increase in photosynthetic capacity, quantum yield for CO2 assimilation, and rates of daytime respiration in tomato (Kaiser et al., 2019, Kalaitzoglou et al., 2021). In cucumber, the supplementation with 180 μmol m−2 s−1 of BL increased net photosynthesis and transpiration rates (Yan et al., 2022). These improvements were likely caused by the enhancement in the activity/expression of key components of the photosynthetic process, such as Rubisco, cytochrome f complexes, chlorophylls, and light harvesting complexes proteins of PSII (Matsuda et al., 2004). Conversely, the application of higher intensity of BL (250 μmol m−2 s−1) might exert a detrimental effect on photosynthetic activity (strawberry; Lauria et al., 2021). This effect likely stemmed from BL influences on leaf physiology, possibly through the impact on chloroplast movement, as well a reduction in carbonic anhydrase activity (Momayyezi &Guy, 2017), which might explain the decrease in plant biomass and leaf area observed in some species (tomato; Kaiser et al., 2019; Kalaitzoglou et al., 2021; basil; Matysiak &Kowalski, 2019; Lauria et al., 2023b). Of note, BL has been reported to be more effective at opening stomata than RL (Vialet-Chabrand et al., 2021), which involves the release of stored energy and osmolytes from starch degradation or lipid metabolism (Horrer et al., 2016). As a consequence, BL resulted in greater stomatal conductance and transpiration rates in both green- and red-leafed basil (Jensen et al., 2018; Lauria et al., 2023b). However, BL-induced stomatal opening does not necessarily rely on higher photosynthesis, suggesting that the guard cell mitochondria play a key role in powering the BL response and that species-specific BL-induced stomatal, in terms of both rapidity and magnitude, must be considered (Vialet-Chabrand et al., 2021).
Supplemental BL (250 μmol m−2 s−1) also increased primary metabolite content in strawberry fruit, mainly organic acids, sugars, and amino acids (Lauria et al., 2023a) and promoted changes to the secondary metabolism, such as increasing flavonoid content in a plethora of species including green basil (Taulavuori et al., 2016; Matysiak & Kowalski, 2019; Lauria et al., 2023b) red-leafed sweet basil (Matysiak & Kowalski, 2019; Lauria et al., 2023b), lamb's lettuce and garden rocket (Matysiak &Kowalski, 2019), red-leafed lettuce (Taulavuori et al., 2016), and tomato (He et al., 2022). BL also promoted the accumulation of phenolic content in red-leafed lettuce, tomato and strawberry (Taulavuori et al., 2016, He et al., 2022, Lauria et al., 2023a, respectively) as well as anthocyanins in red-leafed basil (Lauria et al., 2023b). Indeed, BL has been shown to overexpress the transcript levels of genes encoding PAL and flavonoid-3′-hydroxylase, with the former playing a key role in flavonoid biosynthesis by channelling primary metabolites into this secondary pathway (Thwe et al., 2014). In other cases, BL was also reported to increase antioxidative capacity, level of lycopene, vitamin C, free amino acids, and soluble sugars (e.g. tomato; He et al., 2022) and promote the decrease in nitrate and NH4+, likely due to the stimulated activity of nitrate metabolism enzymes (E. sativa; Sarabi et al., 2022).
To date, little research has explored the impact of supplementing GL in the greenhouse environment. Despite an increase in stomatal number, supplemental GL led to a decrease in stomatal conductance and, consequently, in internal CO2 concentration in both green- and red-leafed basil, thereby positively influencing water use efficiency (Lauria et al., 2023b). Regarding secondary metabolism changes, some studies have reported that GL increases xanthophyll content at high intensities after a single day of supplementation (strawberry leaves; Lauria et al., 2021), increases primary metabolites and phenolics in strawberry fruit (Lauria et al., 2023a), and enhances the flavonoid metabolism in red-leafed basil after long-term treatment (Lauria et al., 2023b). However, the few studies on the effect of supplemental GL on secondary metabolism make it challenging to draw general conclusions.
In addition to the aspects mentioned above, recent experiments have also explored the possibilities of supplementing additional hours of narrowband light during the night. Supplemental hours of both BL and yellow (Y) light during nighttime showed positive effects in terms of biomass, photosynthesis, and secondary metabolite production in Anoectochilus roxburghi, while RL, GL, and white light decreased secondary metabolite content (30 μmol m−2 s−1; Wang et al., 2018). Similarly, in strawberry plantlets, additional hours of BL during the night positively affect flowering and biomass, while R light revealed the opposite effect (Magar et al., 2018). The authors hypothesize that a BL increase in the cytokinin levels stimulates flowering, while the inhibition of this process was potentially connected with the RL activation of endogenous gibberellins. Moreover, Lin (2000) reported the control of flower induction genes (i.e., FLOWER LOCUS T and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1) by cryptochrome and phytochrome in Arabidopsis. Further investigations may shed light on whether night supplementation can represent a feasible greenhouse technique for improving the quality of horticultural crops. If it is proven to offer benefits in terms of yields or nutraceutical properties, it could potentially replace daytime light supplementation, thereby reducing lamp energy costs.
4 LIGHT MODULATION THROUGH COVERING MATERIALS
Among all the covering materials used in agriculture, those able to modulate the light spectrum and covered to some extent in literature are (1) films which can exclude specific undesirable wavebands, (2) photo-selective coloured shading nets and films which can considerably modify the full light spectrum, (3) photo-converting films with fluorescent dyes and the ability to induce a photonic shift toward more desirable wavebands, (4) and light-diffusing plastic films which can alter light distribution inside the greenhouses (Figure 3). Lastly, plants can also be influenced by light modification induced by coloured mulching films, which can alter the reflectance of the light spectrum.
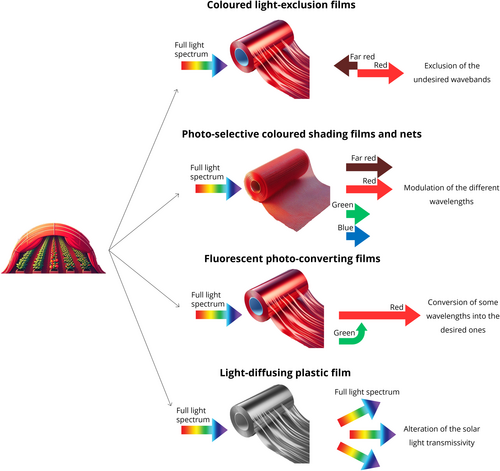
4.1 Light-exclusion films
The investigation into the possibility of modulating solar light spectra started mostly at the beginning of this century, and for this purpose, photo-selective plastic films emerged as a promising and widespread solution. They generated significant interest, especially within the floricultural sector, where the requirement to minimize the use of chemical products to control plant size, shape and development has become increasingly urgent.
Notably, light perception by photoreceptors resulted in a key process for the regulation of a plethora of physiological mechanisms. For example, chemical plant growth regulators (PGRs), commonly employed in the floricultural sector, act as anti-gibberellins, leading to a reduction in plant growth (Maki et al., 2002). However, hormones like gibberellic acid (GA) or ABA are originally mediated by phytochromes and can consequently be regulated through the manipulation of the light spectrum, particularly the R:FR ratio (Seo et al., 2006). Moreover, phytochromes regulate a wide variety of processes involving all stages of plant development, including seed germination, flowering time, fruit quality, root elongation and, partially, shade avoidance syndrome (Strasser et al., 2010; Casal et al., 2012; González et al., 2015).
Indeed, the use of R selective plastic films established a low R:FR ratio that may naturally occur following inter-plant canopy shading under high plant density conditions. This reduction in the R:FR ratio can lead to several undesirable consequences in ornamental plant development, including excessive stem elongation and internode extension in Dendranthema × grandiflorum (Cerny et al., 2003; Khattak & Pearson, 2006), Eustoma grandiflorum (Wilson & Rajapakse, 2001a), Euphorbia pulcherrima (Clifford et al., 2004) and Pachystachys lutea (Wilson & Rajapakse, 2001b). It also decreased plant dry weight in Cosmos bipinnatus, D. × grandiflorum, Zinnia elegans (Cerny et al., 2003), and Salvia spp. (Wilson & Rajapakse, 2001c) while decreasing axillary branching in E. pulcherrima (Clifford et al., 2004).
On the contrary, the depletion of FR light due to the employment of photo-selective plastic films leads to an increase in the R:FR ratio leading to more compact plant structures due to a reduced internode length and decrease in plant dry weight in many ornamental and horticultural species such as Antirrhinum majus (Runkle & Heins, 2002; Cerny et al., 2003), Capsicum annuum (Li et al., 2000), C. bipinnatus and Z. elegans (Cerny et al., 2003), D. grandiflorum (Li et al., 2000; Li et al., 2003a; Cerny et al., 2003), E. pulcherrima (Clifford et al., 2004), E. grandiflorum (Wilson & Rajapakse, 2001a), Gardenia jasminoides (Lykas et al., 2005, 2008), Gazania rigens, B. oleracea, Cucumis sativus and Solanum lycopersicum (Stapel et al., 2011), Impatiens walleriana and Viola × wittrockiana (Runkle & Heins, 2002), L. sativa (Kleemann, 2002), Orthosiphon stamineus, P. lutea and Stobilanthes dyerianus (Wilson & Rajapakse, 2001b), Petunia × hybrida (Cerny et al., 2003; Runkle & Heins, 2002; Fletcher et al., 2005), several Salvia species (Wilson & Rajapakse, 2001c). Furthermore, FR light can affect the timing of anthesis, generally delaying flowering in long-day plants as such Z. elegans and D. × grandiflorum (Cerny et al., 2003), A. majus (Runkle & Heins, 2002; Cerny et al., 2003), P. × hybrida (Runkle & Heins, 2002; Cerny et al., 2003; Fletcher et al., 2005) and E. pulcherrima (Clifford et al., 2004). This side effect can be avoided by exposing plants to an FR-deficient environment during the vegetative stage or ensuring high PAR transmission under the photo-selective film, thereby allowing for rapid anthesis (Runkle & Heins, 2002; Fletcher et al., 2005). Other effects induced by a FR-deficient environment include a decreased leaf area in cuttings and plants such as C. annuum and D. × grandiflorum (Li et al., 2000), and G. jasminoides, (Lykas et al., 2005, 2008), inhibition of lateral shoot development in G. jasminoides, (Lykas et al., 2008), reduced leaf size in D. × grandiflorum and C. annuum (Li et al., 2000), and a decrease in chlorophyll content in L. sativa (Kleeman et al., 2004).
On the other hand, B absorbing films did not alter phytochrome photo-equilibrium significantly and only slightly changed the R:FR ratio, which led to reduced plant height, shorter internode lengths, accelerated time to flowering, and reduced leaf area, and leaf size, as well as fresh and dry weight in D. grandiflorum (Khattak & Pearson, 2006). These effects observed in the absence of BL provide evidence for a connection between BL and phytochrome-mediated light perception. When phytochrome activity is lower, BL can promote plant elongation to a greater extent than R light (Kong et al., 2018).
Greenhouse covers can also differ in their UV-blocking properties, affecting crops differently. According to the Planck equation, UV photons (at 390 nm, minimum energy for UV) count for more than 80% more energy than R photons (at 700 nm), though high-energy photons are not fully exploited for photosynthesis due to inherent photochemical inefficiencies (Long et al., 2006). UV-transmitting films increased anthocyanin, flavonoid, and phenolic content in red L. sativa and Vitis vinifera (Tsormpatsidis et al., 2008; Marigliano et al., 2022, respectively). These films also led to increased compactness in different ornamental and horticultural plants such as G. rigens, cabbage, cucumber, and tomato (Stapel et al., 2011). In contrast, UV-blocking coverings decreased secondary metabolite accumulation and photosynthetic efficiency in red L. sativa but assured an increased growth and better quality in red L. sativa and Solanum melongena. This effect is particularly evident when utilizing materials with 0% UV transmission, which enhance the chroma parameter akin to colour intensity or saturation. This enhancement likely occurs through alterations in the flavonoid composition. (Kittas et al., 2006; Tsormpatsidis et al., 2008). Additionally, UV-blocking films serve as valid tools for pest management (Doukas & Payne, 2014; Papaioannou et al., 2012; Chi et al., 2019; Wang et al., 2023). However, it is worth noting that in tomato (cv. ‘Belladonna’) the lycopene and ascorbic acid content were similar when grown under low-density polyethylene and UV-absorbing polyethylene greenhouse covering films, despite differences in UV transmittance between the treatments (Papaioannou et al., 2012).
4.2 Photo-selective coloured shading films and nets
More recently, coloured shading nets and films have emerged on the market, offering enhanced shading properties. These coloured photo-selective nets are typically made of polypropylene or polyethylene, knitted with different mesh sizes to achieve the desired level of shading. They contain different chromophores that enable the nets to filter specific solar wavebands and scatter light (Zoratti et al., 2015; Manja & Aoun, 2019). Therefore, these nets not only enable light depletion but also bring about a complex alteration in the spectrum of light. This results in improvements in horticultural product quality, protection against unfavourable weather conditions, and a reduction in physiological disorders and flower abortion (Stagnari et al., 2018). The effects of coloured shading films and nets on horticultural species are summarised in Table 4.
| Covering net/film properties | Shade (%) | Species | Effects | Reference |
|---|---|---|---|---|
| R coloured nets/films: enhancement of R and FR light transmittance, absorbance of UV, B and G lights | 23 | Actinidia deliciosa | ↑ fruit dry matter, SSC, ↓ polyphenol concentration and antioxidant activity at fruit harvest | Basile et al. (2012) |
| 50 | Asparsgus spp. | ↑ height, spread, internodal lenght | Patil et al. (2020) | |
| 40 | Capsicum annuum | ↑ carotenoids, yield, titratable acidity, ↓ vitamin C | Ilić et al. (2017) | |
| 30 | Eustoma grandiflorum | ↑ chlorophyll b and total chlorophylls | Almeida et al. (2021) | |
| 30 | Fragaria × ananassa | Delaying of the flowering without reducing the number of the runner produced | Takeda et al. (2010) | |
| 40 | Malus domestica | ↓ photosynthetically active radiation | Bastías et al. (2012) | |
| 65 | Spinacia oleracea | ↑ yield, phenolic compounds | Lara et al. (2021) | |
| 50 | Thymus vulgaris | ↑ total chlorophylls, thymol, ↓ growth, carotenoids, EO content and yield, trichome density and diameter, ρ-cymene | da Cunha Honorato et al. (2023) | |
| 9 | Vaccinium spp. | ↑ berry size. | Zoratti et al. (2015) | |
| 50 | Vanilla planifolia | ↑ proportionally red and far-red light, total plant dry weight, leaf area, nocturnal CO2 fixation, level of antioxidant enzymes, compounds and osmolytes, anthocyanin index | Sanchez et al. (2022) | |
| B coloured nets/films: enhancement of B and G light transmittance, absorbance of UV, R, FR and Y lights | 27 | Actinidia deliciosa | ↓ flesh colour, chlorophylls, fruit dry matter %, SSC, polyphenol concentration and antioxidant activity at fruit harvest | Basile et al. (2012) |
| 30 | Allium fistulosum | ↑ height, fresh weight, quality, yield, photosynthetic pigment content, Pn, transpiration rate, gs, Fv/Fm, ΦPSII, qP, antioxidant enzymes, absorption and transformation of elements, ↓active oxygen content | Gao et al. (2021) | |
| 40 | Capsicum annuum | ↑ chlorophyll a, carotenoids, fruit mass | Ilić et al. (2017) | |
| 33 | Ficus carica | ↑ stomatal conductance, ↓ dry fruit diameter, total soluble solids | Jokar et al. (2021) | |
| 30 | Fragaria × ananassa | Delaying of the flowering without reducing the number of the runner produced | Takeda et al. (2010) | |
| 22 | Malus domestica | ↑ Fv/Fm, ΦPSII, photochemical reflectance index | Mupambi et al. (2018) | |
| 27 | ↑ maximal fruit growth rate, fruit weight, leaf photosynthesis, total leaf area | Bastías et al. (2012) | ||
| 50 | Melissa officinalis | ↑ height, leaf area, chlorophyll content, EO yield | Oliveira et al. (2016) | |
| 52 | Ocimum basilicum | ↑ plant height, leaf pairs on the main stem, number of axillary shoots, leaf area, ↓ leaf thickness, rosmarinic and caftaric acids, chlorophyll content | Stagnari et al. (2018) | |
| 65 | Spinacia oleracea | ↑ dry weight, antioxidant capacity. | Lara et al. (2021) | |
| 50 | Thymus vulgaris | ↑ total chlorophylls, thymol, ↓ growth, EO content and yield, trichome diameter, ρ-cymene, γ-terpinene | da Cunha Honorato et al. (2023) | |
| 15 | Vaccinium spp. | ↑ berry size, anthocyanin content | Zoratti et al. (2015) | |
| 50 | Vanilla planifolia | ↑ chlorophyll content, Fv/Fm, level of antioxidant enzymes, compounds and osmolytes, xanthopylls, ↓ nocturnal CO2 fixation | Sanchez et al. (2022) | |
| G coloured nets/films: enhancement of G light transmittance, absorbance of R and FR lights | 74 | Beta vulgaris | ↓ dry weight, total phenolic concentration, antioxidant activity, total pigment concentration, ↑ soluble and structural carbohydrates, K, Mg, Zn | Stagnari et al. (2014) |
| 43 | Ocimum basilicum | ↑ plant height, leaf pairs on the main stem, number of axillary shoots, leaf area, caffeic acid, ↓ biomass, leaf thickness, rosmarinic and caftaric acids | Stagnari et al. (2018) | |
| Y coloured nets/films: enhancement of G, Y and R light transmittance, absorbance of B-V lights | 30 | Allium fistulosum | ↑ intercellular CO2 concentration, NPQ, ↓ Fv/Fm, ΦPSII, qP | Gao et al. (2021) |
| 52 | Beta vulgaris | ↑ dry weight, leaf area, growth rate, water uptake | Casierra-Posada et al. (2014b) | |
| 27 | Capsicum anuum | ↑ water use efficiency | Casierra-Posada et al. (2014a) | |
| 36 | Ficus carica | ↑ chlorophylls, carotenoids, relative water content, antioxidant activity, anthocyanins, ↓ net photosynthesis, leaf temperature, ion leakage, titratable acidity, dry fruit diameter, total soluble solids | Jokar et al. (2021) | |
| 28 | Ocimum basilicum | ↑ plant height, leaf pairs on the main stem, number of axillary shoots, leaf area, biomass, ↓ leaf thickness, rosmarinic and caftaric acids | Stagnari et al. (2018) | |
| W coloured nets/films: | 20 | Actinidia deliciosa | ↑ fruit dry matter, ↓ flesh colour, chlorophylls, polyphenol concentration and antioxidant activity at fruit harvest | Basile et al. (2012) |
| 30 | Allium fistulosum | ↑ height, fresh weight | Gao et al. (2021) | |
| GR coloured nets/films | 27 | Actinidia deliciosa | ↓ fruit dry matter %, SSC, polyphenol concentration and antioxidant activity at fruit harvest | Basile et al. (2012) |
| 50 | Asparsgus spp. | ↑ leaf production, leaf area, number of cut cladophylls per plant and per square meter, vase life | Patil et al. (2020) | |
| 40 | Malus domestica | ↑ maximal fruit growth rate. | Bastías et al. (2012) | |
| B-GR coloured nets/films | 20 | Corylus avellana | ↓ vapour pressure deficit, stomata density, specific leaf weight | Salazar-Canales et al. (2021) |
| P-GR coloured nets/films | 20 | Corylus avellana | ↑ yield, FW, and SW, ↓ vapour pressure deficit. | Salazar-Canales et al. (2021) |
| P coloured nets/films | 40 | Capsicum annuum | ↑ yield, titratable acidity, ↓ palisade parechym | Ilić et al. (2017) |
| S coloured nets/films | 40 | Lactuca sativa | ↓ stomatal density, chlorophyll content, carotenoids | Rossi Pinheiro et al. (2020) |
R photo-selective films and nets enhance R and FR transmittance while absorbing UV, B, and G lights. Under these coverings, the R:FR ratio and phytochrome photo-equilibrium are generally unaffected. However, their influence on plant morphogenesis and specific physiological and biochemical parameters has been recorded. Under these nets, higher dry weights of both the plant and fruit of Vanilla planifolia (Sanchez et al., 2022) and Actinidia deliciosa (Basile et al., 2012) were reported. In the case of kiwifruit, this increase was associated with an elevated soluble solid content, likely a result of altered source-sink relationships induced by the nets (Basile et al., 2012). Furthermore, kiwifruit exposed to 23% shading with R nets showed a decrease in polyphenol concentration and antioxidant activity at fruit harvest, probably due to a decreased UV component in both total and scattered light under the nets (Basile et al., 2012). In contrast, vitamin C content decreased in the fruit of C. anuum under 40% shading. However, as vitamin C development is often related to glucose metabolism, R light exposure may affect fruit ripening in a species-specific way (Ilić et al., 2017). Sanchez et al. (2022) reported an increase in leaf area, photosynthetic efficiency, levels of antioxidant enzymes, compounds, osmolytes, and anthocyanin index in V. planifolia when grown under 50% R shading condition. In E. grandiflorum, total chlorophyll content increased under a 30% R shading environment (Almeida et al., 2021). For Vaccinium spp., a 25% R shading level resulted in favourable for increasing fruit size (Zoratti et al., 2015), while a higher shading percentage (i.e., 80%) delayed the harvest time without detrimental effects on return bloom, yield, or fruit quality in Vaccinium corymbosum (Lobos et al., 2013). The application of R-coloured photo-selective nets also resulted in increased yields and phenolic compounds in Spinacia oleracea (Lara et al., 2021) and increased yield in C. anuum (Ilić et al., 2017), as well as a higher cumulative number of cut cladophylls per plant and per square meter better performance in vegetative parameters in Asparagus spp. (Patil et al., 2020), potentially due to a deficiency in BL.
R shading nets, together with B ones, induce a delay in flowering without reducing the production of strawberries (Takeda et al., 2010). B photo-selective films transmit more B and G light while absorbing UV, R, FR, and Y light. This improvement in high-energy wavebands results in increased PSII efficiency and higher photosynthetic performances in different species such as Allium fistulosum (Gao et al., 2021), Ficus carica (Jokar et al., 2021), Malus domestica (Bastías et al., 2012; Mupambi et al., 2018), and V. planifolia (Sanchez et al., 2022). It also led to increased levels of photosynthetic pigments in A. fistulosum, C. anuum, S. oleracea and V. planifolia (Gao et al., 2021; Ilić et al., 2017; Lara et al., 2021; Sanchez et al., 2022, respectively). Thus, biometric features such as plant height, fresh weight, quality, and yield of green onion, fruit mass in bell pepper, dry weight in spinach, height and leaf area in lemon balm, and total leaf area of apple trees have improved with B photo-selective films (Gao et al., 2021; Ilić et al., 2017; Lara et al., 2021; Oliveira et al., 2016; Bastías et al., 2012, respectively). B nets also influence fruit characteristics, increasing berry size in Vaccinium spp. and the apple fruit growth rate (Zoratti et al., 2015; Bastías et al., 2012, respectively), as well as increasing essential oil content in lemon balm (Oliveira et al., 2016). However, there are exceptions reported in the literature regarding the positive vegetative effects of B nets, including, for example, decreased chlorophyll content in basil (Stagnari et al., 2018) and loss of kiwifruit quality, dependent on the reduction in soluble solid content (Basile et al., 2012).
Modifying light quality using G photo-selective coloured shading films, which absorb R and FR lights, decreases the R:FR ratio, phytochrome photo-equilibrium, and solar radiation transmittance, increasing instead the B:FR ratio. In Beta vulgaris, G coverings concentrated sugars and minerals in the roots, leading to a decrease in dry weight, TPC, pigment concentration, and antioxidant activity. In basil, this treatment resulted in decreased biomass but increased caffeic acid content in leaves (Stagnari et al., 2014, 2018).
Y photo-selective plastic films, which absorb in the blue-violet region, increased biomass in basil by 28% (compared to G and B films; Stagnari et al., 2018), and improved B. vulgaris plant growth (Casierra-Posada et al., 2014a). Moreover, Jokar et al. (2021) reported that fig fruit under Y nets exhibited reduced total soluble solids and titratable acidity but increased antioxidant activity and anthocyanin content. An impairment in photosynthetic performances was recorded in both F. carica and A. fistulosum (30–36% light shading; Jokar et al., 2021; Gao et al., 2021, respectively). In fig trees, the decreased net photosynthesis and increased photosynthetic pigment content were probably due to the prevalence of G wavelengths under this treatment, which strongly influenced stomatal conductance (Folta & Carvalho, 2015).
Other coloured photo-selective nets that can be used are white, grey, or silver. White nets effectively increased plant height and fresh weight in green onion (Gao et al., 2021), while in kiwifruit, despite the higher dry matter, white shade nets induced slight negative effects on flesh colour and chlorophyll concentration (Basile et al., 2012). Grey nets negatively affected fruit dry matter and soluble solids content in A. deliciosa fruit due to the higher shading percentage in these nets compared to others (Basile et al., 2012). Pearl-gray nets significantly increased accumulated yield, fruit weight, and seed weight (12, 13, and 6%, respectively, compared to the control – i.e. uncovered trees) in Corylus avellana. In the same experiment, B-grey shade nets resulted in a decline in net photosynthesis, which was attributed to non-stomatal limitations and changes in leaf morphology (Salazar-Canales et al., 2021). Grey nets also had a positive effect on apple fruit growth rate, Asparagus spp. leaf production and number in cladophylls, as well as pepper fruit yield (Bastías et al., 2012; Patil et al., 2020; Ilić et al., 2017, respectively). Finally, silver coverings, reflecting a large amount of incident solar radiation, may strongly alter the microclimate under the net, thereby leading to changes in leaf transpiration as well as photosynthetic pigments, as observed in lettuce (Rossi Pinheiro et al., 2020). However, the concentration of chlorophylls and carotenoids showed variations dependent upon the cultivar, demonstrating the genotype-dependent responses to these silver nets.
4.3 Fluorescent photo-converting films
Technology in polymer materials used in agricultural films has continuously improved. Currently, the trend is to employ light conversion agents in the polymer film manufacturing process to introduce wavelength conversion capabilities to greenhouse materials. These agents include fluorescent dyes, organic rare-earth complexes, and inorganic rare-earth complexes, which are designed to convert sunlight into specific wavelength ranges suitable for plant growth and, more specifically, plant photochemical processes (Figure 1; Table 5) (Liu et al., 2022). Notwithstanding, a critical factor in selecting this technology is the photophysical properties, particularly the photostability of the material under extreme weather conditions, including intense irradiation. Such conditions can cause a strong decrease in the light absorption and, consequently, loss of photo-conversion properties (Ooyama et al., 2012).
| Light shift | Species | Effects | Reference |
|---|---|---|---|
| Fluorescent orange; Fluorescent magenta: UV absorption and conversion of B and G radiation into R light. Alteration of R:FR relation | Cucumis sativus | ↑ vegetative growth; orange film, ↓ yield; magenta film, ↓fruit weight | González et al. (2003) |
| Fragaria × ananassa | ↑ yield | ||
| Fluorescent blue: UV radiation conversion into B light | Fragaria × ananassa | ↑ yield, fruit number, fruit size | Hemming et al. (2006) |
| Ethylene tetrafluoroethylene spectrum conversion film: G radiation conversion into R light | Cucumis sativus | ↑ fruit yield, growth, fruit dry matter, P concentration in leaf and fruit, other mineral element concentration | Nishimura et al. (2012) |
| Gold nanoparticles fluoropolymer:UV and V radiation conversion into B and R light. G radiation partial conversion into heat | Capsicum annum; Cucumis sativus; Solanum lycopersicum; Solanum melongena | ↑ growth rate, assimilation rate, chlorophyll content | Gudkov et al. (2020) |
| Fluorescent red: G radiation conversion into R light | Lactuca sativa | ↑ production, plant diameter, leaf area, leaf number, leaf length | Shen et al. (2021) |
| Light conversion film: Y-G radiation conversion into R-O light | Lactuca sativa | ↑ photosynthetic rate, yield, total soluble sugars, reduction-type Vitamin C | Li et al. (2022b) |
| Rare-earth light conversion film: UV, V, and G radiation conversion into B, R-O, and FR light | Capsicum anuum | ↑ plant height, stem diameter, internode length, Gibberellic acid 3, Indole-3-acetic acid, Zeatine Riboside, fruit yield, ascorbic acid, soluble protein, soluble sugars, net photosynthesis, Rubisco activity and Rubisco small subunit transcription | Gao et al. (2022) |
Thanks to their photophysical and chemical properties, the incorporation of nanosized inorganic particles into polymer matrices absorbs the green-yellow range of visible solar spectra and fluoresce or re-emit it as either RL (El-Bashir et al., 2016) or conversion of UV to BL (Kang et al., 2018). At present, nanoparticles with plasmon or exciton emission are used; the most popular pairs are cadmium-selenium (Fitzmorris et al., 2013) or gold-sulfur (Pu et al., 2018). However, there are significant difficulties with the inclusion of these pigments in polymer matrices, as well as a problem with their susceptibility to reactive oxygen species, which are always formed in vapour-permeable materials. Other agents, such as the rare-earth materials for light conversion films, increased transmittance and photosynthetic range of B, R-orange, and FR light, whereas UV, purple, and GL decreased inside the greenhouse.
Examining the effect of a rare-earth-complex light conversion film on leaf structure, Gao et al. (2022) observed that sweet pepper leaves from plants grown under these films exhibited increased thickness and longer stomata than those grown with a polyolefin film (used as control). Furthermore, endogenous hormone content in sweet pepper fruit, specifically gibberellic acid 3 (GA3) and/or indole-3-acetic acid (IAA), was significantly higher in plants grown under the rare-earth material light conversion film. These results underscore the ability of R light to activate PHYA and PHYB coupled with GA3 biosynthesis (Gao et al., 2022). Hemming et al. (2006) investigated the optimal concentration of blue fluorescent pigments for converting UV radiation into BL and evaluated the increment level of light intensity inside the greenhouse. Fluorescence effects of the plastic film prototypes were rising with increasing pigment concentration, and though fluorescent effects by blue pigments increased total PAR transmission by only 1–3%, the fluorescent film with high blue pigment concentration resulted in an increase in strawberry fruit number, thereby suggesting this film has good potentials to improve strawberry production (Hemming et al., 2006).
The challenge of designing a durable and resistant spectrum conversion film urged Nishimura et al. (2012) to investigate its effects on cucumber plants development and nutrient absorption. Natural light-type film was compared with a spectrum conversion film that converted part of the G wavelength range to R wavelengths (Nishimura et al., 2012). The results showed that cucumber fruit and leaves from plants grown with the spectrum conversion film were richer in P in comparison with those grown under a neutral light film (control), with no significant differences in macronutrients such as K, Mg and Ca (Nishimura et al., 2012). Phosphorous plays a crucial role in plant physiology, serving as an essential element for ATP energy production and contributing significantly to carbon metabolism during both photosynthesis and respiration. Phosphorous deficiency is known to inhibit photosynthetic rates by depressing the Calvin cycle activity and reducing stomatal conductance, ultimately limiting nitrate uptake and, thus, chlorophyll biosynthesis (Naureen et al., 2018). The higher level of P may have allowed the activation of photosynthesis in both cucumber fruit and leaves under the spectrum conversion films, resulting in faster plant growth. This was evident in the substantial increase in total leaf fresh weight (+49% under the conversion film compared to natural light film) and total fresh weight (+41% under the conversion film compared to natural light film) observed in both cases (Nishimura et al., 2012).
In another study, R-shifting films increased yield, photosynthetic rates, total soluble sugars, and vitamin C content in lettuce, proving to be even more convenient under low-light environmental conditions (Shen et al., 2021; Li et al., 2022). González et al. (2003) observed an increased vegetative growth in cucumber plants under fluorescent covers. However, this did not correlate with fruit production, as a decrease was observed in fruit yield using an orange fluorescent film and a decrease in fruit weight under a magenta fluorescent film.
In regions with distinct climatic conditions, photoconversion films have proven to be suitable for areas frequently characterized by low temperatures and low photosynthetic light, such as high latitudes. Apart from the UV conversion into B and R wavelengths, the photoconverting films tested by Gudkov et al. (2020) demonstrated the capacity to convert the G sunlight waveband into heat and R light acknowledged to be in line with high latitudes cultivated crops needs. From the physiological point of view, tomato plants grown in greenhouses covered with polymer film packed with fluorophores for photoconversion technology exhibited a remarkable increase in chlorophyll content (+55%) and a significant improvement in CO2 assimilation (30–40%) compared to plants under standard polymer film (Gudkov et al., 2020).
In conclusion, the reaction of certain crops to light alterations, ΦPSII such as UV radiation exclusion, the enhancement of the light distribution or the selection of certain wavelengths from the spectrum of light absorbed by the plant inside the structure may yield neutral or dynamic reactions. Thereafter, the choice between employing plastic films with single or multiple functionalities to address plant metabolite concentrations should be guided by economic feasibility and, most importantly, by the results desired by farmers (Papaioannou et al., 2012; Zheng et al., 2020; Gudkov et al., 2020).
4.4 Light-diffusing plastic film for canopy species
For crops with significant canopy volumes, the solar radiation distribution inside the greenhouse/tunnel is not always at its optimum due to the canopy shading with limited incident light reaching bottom leaves compared to the upper part of the canopy. This issue can be solved by using light-diffusing plastic films designed to distribute solar radiation, reducing the shade effect within the canopy (Zheng et al., 2020; Ávalos-Sánchez et al., 2022). These films increase solar energy availability and distribute direct light to different leaf levels (Holcman et al., 2015). Plastic film with 29% haze, resulting in more uniform PPFD directions to lower and middle canopies and higher leaf photosynthesis (Zheng et al., 2020; Moreno-Teruel et al. 2021). The functions of diffusing plastic films and their agronomic use are described in Table 6.
| Film characteristics | Species | Effects | Reference |
|---|---|---|---|
| 55% of diffusive capacity | Solanum lycopersicum | ↑ productivity | Holcman et al. (2015) |
| Two polyethylene films, one simple and one double, with low diffusion fraction and an external 50% transmission to infrared radiation and an internal 18% transmission to infrared radiation; 90% transmission to solar radiation | ↑ tocopherol, carotenoids, and chlorophyll contents. ↓sugar content | Petropoulos et al. (2019) | |
| Two ethylene-vinyl acetate films with 20 and 29% haze respectively 89% transmission | ↑ yield and photosynthetic activity | Zheng et al. (2020) | |
| Transmission of photosynthetically active radiation of 0.90 55% light diffusion 90% thermal efficiency | ↑ marketable yield and photosynthetic activity | Moreno-Teruel et al. (2021) | |
| >90% light diffusion ≥88% light transmission | Lactuca sativa | ↑ microelement, nitrate and soluble solid contents, acidity. ↓ biomass, leaf number, leaf area, photosynthetic activity, pigment content and leaf pH | Riga and Benedicto (2017) |
| light diffusing polyethylene-based film | Valerianella locusta | ↑ SPAD index, yield, ascorbic acid, and nitrate content | Cozzolino et al. (2020) |
Plants take advantage of diffuse light, leading to improved productivity. For example, in cucumber cultivation, an experimental film with 55% light diffusion improved marketable yield by 5% in the first growth cycle and 15% in the second cycle (Ávalos-Sánchez et al., 2022). Diffusive plastic films have been shown to allow greater incidence of solar radiation inside glasshouses without causing major changes in air temperature and relative humidity, ensuring higher productivity (Holcman et al., 2015).
The unique structure of these films increased the fraction of diffuse light, improving the uniformity of the light environment and guaranteeing higher performance not only in terms of photosynthesis but also in terms of leaf metabolite composition. For example, an increase in tocopherol, carotenoid and chlorophyll content was found in tomato grown with a diffusive film compared to a conventional polyethylene-based one (Petropoulos et al., 2019). However, light-diffusive films have also been reported to increase nitrate content in L. sativa and Valerianella locusta (Riga & Benedicto, 2017; Cozzolino et al., 2020), which need to be considered in view of the Commission Regulation (EC) No 1881/2006 which fixes maximum nitrate levels in some leafy vegetables, including lettuce.
4.5 Coloured mulching films
In the case of deploying coloured films exclusively on the below-ground area, R plastic film has shown positive results in the form of mulch. R plastic film is able to reflect more FR and R lights and has a lower R:FR ratio compared to black plastic mulch. However, in some cases, the use of B plastic film alternated with R films (Zhou et al., 2023) may exert positive results. The functions of coloured films used as mulching are described in Table 7.
| Covering film classification | Film characteristics | Species | Effects | Reference |
|---|---|---|---|---|
| Coloured film | R film: ↓ R:FR ratio of reflected light | Fragaria × ananassa | ↑ fruit weight, size, yield, anthocyanin content | Shiukhy et al. (2015) |
| W, S and GR film: W ↓ root-zone temperature. S ↑ light reflectance | Solanum lycopersicum | Spring season: GR and S ↑ vegetative FW. W ↓ fruit FW, fruit number per plant, fruit yield. Fall season: GR and S ↑ vegetative and fruit FW, fruit number per plant, fruit yield | Díaz-Pérez and Batal (2002) | |
| R, B and R ± B: increase the proportion of R, B and R + B light respectively below plant canopy | Vitis vinifera | R ↑ chl, RS, S:A ratio; ↓ acid and neutral invertase. B ↑ acid and neutral invertase; ↓ TSS, sucrose, S:A ratio. R + B ↑ Pn, chl, sucrose and sucrose phosphate synthase, TSS, RS, S:A ratio; ↓ TA. | Zhou et al. (2023) | |
| Photo-selective film | Y polyethilene film:↓soil temperature | Capsicum annuum; Cucumis melo | ↑ root mycorrhizal (%) | Bonanomi et al. (2017) |
| Lycopersicon esculentum; Citrullus lanatus; Brassica oleracea var. gongylodes | ↑ yields |
Under R plastic mulch, Shiukhy et al. (2015) reported a phytochrome-mediated gene expression leading to enhanced aroma compounds and increased fruit production (34%) in strawberry plants cv. Camarosa. Although no significant differences were observed between treatments for the content of fruit phenolic compounds, the concentration of anthocyanins and flavonoids was higher in fruit from plants grown over an R plastic mulch (Shiukhy et al., 2015). Another experiment by Zhou et al. (2023) demonstrated that in grape the use of R, B, R and B mulching films strongly influenced the photosynthetic rate, leaf photosynthetic pigments, as well as the activities of both sucrose synthase and invertase in leaves. In particular, the alternation of R with B mulching films exerted the best effects, which resulted in higher total soluble solids in fruits. All these studies suggest that coloured mulching films are a valuable tool to modulate light quality below the plant canopy, thereby improving fruit carbohydrate contents by regulating leaf photosynthetic pigments and sucrose metabolism enzyme activities.
Of note, the effect of mulch colour on plant growth and yield may vary according to the geographic location and season (Csizinszky et al., 1995), suggesting that plants grown on coloured mulches respond to other factors in addition to the light reflected from the mulch, such as the mulch-related cooling factor. Indeed, besides the use of photo-selective films for the positive effect on photosynthesis and fruit phytochemical quality, their application on the root zone areas is worth investigation due to their ability to maintain cooler soil temperatures compared to black mulch (the most used) that occasionally impairs plant growth due to high root-zone temperatures (Díaz-Pérez & Batal, 2002). For example, tomato plants exposed to a range of root-zone temperatures, resulting from growing the plants in different seasons and by using coloured mulches that differed in reflectance, showed a + 15% increase in tomato fruit yield (coloured versus black mulch) due to a low root-zone temperature provided by the coloured film (−0.5°C) (Díaz-Pérez & Batal, 2002). Similarly, photo-selective mulching films applied at the root zone showed an increase in crop irrigation water productivity compared with black film by 25% in sweet pepper, tomato, lettuce, melon, and kohlrabi with the most positive results observed in winter crops (Bonanomi et al., 2017). Indeed, though mulching films showed similar water-saving prerogatives to those of a black film, soil temperature and plant evapotranspiration under photo-selective mulching films were consistently lower than that under B mulch.
5 LIGHT DISTURBANCE: THE CASE OF STREETLAMP ILLUMINATION ON TREES
Although there are benefits to supplemental lighting in greenhouse cultivation, artificial illumination may negatively affect plant physiology. This is particularly evident in urban areas, where streetlamps extend the “day length” throughout the night. In our cities, light pollution has constantly risen by around 6% per year over the last decades to facilitate human activities (Hölker et al., 2010), altering animal behaviour, like herbivores and pollinators, and affecting plant phenology, growth, and resource allocation (Lian et al., 2021; Liu et al., 2021; Lo Piccolo et al., 2023). The first observations of this phenomenon on plants occurred in the first half of the 20th century when Matzke (1936) observed a delay in leaf senescence in trees growing in close proximity to streetlamps (e.g., in Populus canadensis and Platanus × acerifolia individuals). Subsequently, Chaney (2002), compiled a comprehensive table detailing the susceptibility of woody plants to the influence of illumination from streetlamps. However, since then, only a handful of studies have investigated the repercussions of light pollution on trees, and even fewer have explored the physio-chemical traits of plants illuminated by streetlamps at night. Furthermore, no one has ever studied the long-term effect of night illumination on tree health and ecosystem services. Consequently, much of the real amplitude of this phenomenon remains to be thoroughly understood and elucidated.
In plants, photoreceptors (i.e., phytochromes, cryptochromes, and phototropins) are pivotal for regulating circadian rhythms based on external light stimuli, perceiving seasonal changes, controlling the repair and recovery of plant physiological functions, and inducing the onset of flowering and dormancy (Björn, 2015; Kong & Zheng, 2020). In this framework, it seems obvious that night streetlamp illumination could influence plant circadian rhythms by interfering/stimulating photoreceptors (Bennie et al., 2016; Bennie et al., 2017). The light emission spectrum of streetlamp illumination can vary greatly depending on the technology (Bennie et al., 2016). In the urban environment, the most common streetlamp solutions used in the past were High-Pressure Sodium (HPS) lamps, with high irradiance peaks in the R spectrum, which are highly likely to interfere with plant phytochromes. However, more recently, HPS lamps have been gradually replaced by LED lamps, causing different problems for tree metabolism, as these lamps have an additional peak in the B light emission spectrum (Lo Piccolo et al., 2023), which interferes with plant cryptochromes. These photoreceptors regulate stem growth, flowering time, stomatal opening, circadian clock, and other UV/BL-driven responses (Wang & Lin, 2020). Among others, one of the main reactions triggered by cryptochrome is the BL-induced stomatal response, which occurs at low light intensities and is often considered independent of photosynthesis (Vialet-Chabrand et al., 2021). However, some studies demonstrated that the intensity of RL over BL influences the magnitude of the stomatal response (Shimazaki et al., 2007), considering that this ratio varies during the day and plants experience a high BL:RL at dawn and before dusk (Matthews et al., 2020). Therefore RL:BL, as well as the addition of GL on the streetlamp spectra (which is opposite the effect of BL on cryptochromes; Johkan et al., 2012; Landi et al., 2020) should be considered to develop less impacting streetlamps on tree species.
To date, information on the effects of specific streetlamp technologies on plant physiology is scarce, largely due to the lack of investigation into different streetlamp technologies and the species-specific nature of these effects. Therefore, the following text will discuss the general impacts of nocturnal illumination by streetlamps on trees. Table 8 outlines the multiple effects on plant phenology and physiology due to streetlamp illumination on trees.
| Plant species | Lamps | Treatment | Effects | Reference |
|---|---|---|---|---|
| Betula pendula | HPM lamps | PAR 0.16 μmol m−2 s−1, at sampling point, ranging from 6 to 10.5 h | No effects on chlorophyll and carotenoid contents | Sarala et al. (2013) |
| Rhus typhina | FLS lamps | - | Delay of the autumn phenological phase of leaf colouring in some crown parts of several trees | Tuhárska et al. (2014) |
Acer pseudoplatanus Fagus sylvatica Quercus robur Fraxinus excelsior |
- | - | Advance in budburst (up to 7.5 d) in deciduous trees, especially in later-budding species | Ffrench-Constant et al. (2016) |
| Liriodendron tulipifera | HPS lamps | PAR 0, 1, 3, 50 μmol m−2 s−1, for 11 h | ↓ stomatal aperture and stomatal size ↓ stacked grana thylakoid membranesDawn: ↑ ABA content, ↓ degradation of ABADusk: ↓ starch and ABA contents Night: ↓ starch degradation |
Kwak et al. |
Acer pseudoplatanus Rhus typhina |
HPS and LED lamps | Light pollution of 2.9, 6.5 or 9.6 lx depending on the area and lamp technology. Measures were taken at 1.3 m under the light source | Delay of 13 to 22 days on the onset of the autumn phenophase. Duration of leaf fall was prolonged by 6 to 7 d | Škvareninová et al. (2017) |
| Liriodendron tulipifera | HPS lamps | PAR 0, 1, 3, 50 μmol m−2 s−1, for 11 h | ↓ plant biomass, ↓ chlorophyll contents ↓ PIABS ↑ H2O2 and O2− contents Premature leaf senescence and leaf tissue necrosis |
Kwak et al. (2018) |
| Platanus × acerifolia | HPS lamps | Between 7.9 and 12.8 W m−2 | Delay on the onset of the autumn phenophase | Massetti (2018) |
Calotropis procera Butea monosperma Delbergia sissoo Azadirachta indica Plumeria rubra Eucalyptus globulus Bauhinia purpurea |
- | 340–360 lx at sampling point | ↓ Fv/Fm, ΦPSII and Φ(NPQ) ↑Φ (NO) |
Meravi and Prajapati (2018) |
| Cinnamomum camphora | - | - | ↑ leaf chlorophyll content ↑ diameter at beast height ↑ branch growth |
Li et al. (2019) |
Aesculus hippocastanum Alnus glutinosa Betula pendula Fagus sylvatica Fraxinus excelsior Quercus robur Tilia cordata |
- | Dataset of measures from 1992 to 2015 | Plant phenological phases are substantially delayed | Lian et al. (2021) |
Acer truncatum Quercus mongolica |
LED lamps | PAR 80 μmol m−2 s−1, for 5 h | ↑ shoot biomass ↑ carbohydrate production N diluition |
Liu et al. (2021) |
Tilia × platyphyllos Platanus × acerifolia |
LED lamps | PAR 200 μmol m−2 s−1 at sampling point | Nocturnal CO2 assimilation ↑ leaf chlorophyll content Circadian rhythmicity of chlorophylls was not affected |
Lo Piccolo et al. (2021) |
Tilia tomentosa Betula pendula Fagus sylvatica ‘Purpurea’ Acer campestre Cornus alba Lonicera pileata Kerria japonica ‘Pleniflora’ Spiraea × cinerea ‘Grefsheim’ |
LED lamps | Experiment conducted in light chambers. PAR 100 μmol m−2 s−1 during the day and PAR 0, 1 or 30 μmol m−2 s−1 during the night | ↑ bud developing (up to 20 days, depending on the species, with the highest acceleration observed in K. japonica) ↑ soluble sugars content in branches of L. pileata and C. alba ↓ soluble sugars content measured in apical twigs of T. tomentosa, S. × cinerea, K. japonica and A. campestre |
Czaja and Kołton (2022) |
Tilia × platyphyllos Platanus × acerifolia |
LED lamps | PAR 700 μmol m−2 s−1 and of 300 μmol m−2 s−1 measured at the canopy level | ↓ Pn at sunrise ↑ chlorophyll contents at leaf mature stage ↓ starch accumulation ↑ starch degradation rate in young and mature leaves of T. × platyphyllos, and in mature and senescet leaves of P. × acerifolia Delay of winter dormancy by two months in P. × acerifolia |
Lo Piccolo et al. (2023) |
In general, nocturnal illumination by streetlamps leads to a delay in the autumn leaf fall and the entry into winter dormancy, as was observed in P. canadensis and P. occidentalis (Matzke 1936), P. × acerifolia (Massetti, 2018; Lo Piccolo et al., 2023), Rhus typhina (Tuhárska et al., 2014; Škvareninová et al., 2017) and Acer pseudoplatanus (Škvareninová et al., 2017) (Figure 4). These delays could potentially have adverse consequences for deciduous trees, especially in the presence of frosts, which may damage leaves and buds.

On the contrary, no alterations in the tree phenology were observed in Betula pendula and Tilia × platyphyllos (Sarala et al., 2013, Lo Piccolo et al., 2023, respectively). These differential responses among tree species could be due to the species-specific sensitivity to external stimuli, as winter dormancy can be influenced by factors such as photoperiod and temperature, or the combination of both (Zhang et al., 2020). Lo Piccolo et al. (2023) observed that the entry into winter dormancy of T. × platiphyllos was less influenced by the altered photoperiod, suggesting that external environmental temperatures played a more significant role in regulating dormancy induction than the photoperiod. On the other hand, in the case of B. pendula, both photoperiod and temperature can influence the onset of winter dormancy (Junttila et al., 2003, Li et al., 2003b). Therefore, the absence of an influence in tree phenology observed by Sarala et al. (2013) could be due to the low light intensity tested (0.16 μmol m−2 s−1 at the sampling point).
A 13-year-long investigation conducted in the UK on spatially referenced budburst data from four deciduous tree species, matched with satellite imagery of night-time, unveils that streetlamp night illumination anticipate bud burst by 7.5 days on brighter areas affecting particularly later-budding species (Ffrench-Constant et al., 2016). Similarly, an experiment with four tree and four shrub species found that all the species investigated were influenced by artificial light at night and developed buds faster than the control group (Czaja & Kołton, 2022). Moreover, it was reported that night light pollution could lead to an anticipated start of the growing season in the climatically temperate regions of the USA, though in colder or hotter regions, environmental temperatures have a predominant role in the induction of the spring phenology (Zheng et al., 2021). On the contrary, Lian et al. (2021), observed a delay in leaf burst in Alnus glutinosa, B. pendula and F. excelsior grown in light-polluted areas in Europe between 1991–2015. The apparent contradictions in these findings may be due to the fact that little research has been performed on the interactive effects of urban environmental temperatures (e.g., heat islands) and streetlamp illumination (e.g., if it heats, its light spectrum, its brightness) on urban tree phenology. Lian et al. (2021) suggested that while temperature plays a dominant role in shifts in plant phenological phases, the delaying effect of artificial light pollution is evident when considering these interactions.
In the literature, there are conflicting reports regarding the effect of streetlamp illumination on leaf pigments of urban trees, which, again, suggests the species-specificity of this response. Kwak et al. (2018) reported that leaves of plants exposed to streetlamp illumination rapidly turned yellow and fell. In contrast, Sarala et al. (2023) found no significant differences in leaf chlorophyll content in B. pendula trees exposed to streetlamps, whilst Li et al. (2019) reported higher values in leaf chlorophyll content in Cinnamomum camphora. Interactions between external light signals and the endogenous plant circadian clock regulate chlorophyll biosynthesis and breakdown. For instance, the conversion from protochlorophyllide to chlorophyllide is light-dependent, thus, this control mechanism leads to decreased chlorophyll synthesis at night and increased synthesis during the day (Kobayashi & Masuda, 2019; Kumar et al., 2020; Suzuki & Bauer, 1995). Lo Piccolo et al. (2023) argued that nighttime lighting provided by streetlamps hampered the natural decrease in chlorophyll biosynthesis during the night, thus explaining the higher chlorophyll content in the leaves of trees grown under streetlamp illumination and possibly increasing the demand for N assimilation by the leaves. This assumption agrees with the observations of Liu et al. (2021), who reported low N concentrations in the roots and wood of trees illuminated by streetlamps, probably due to an increased demand for N assimilation by leaves and, subsequently, more chlorophyll biosynthesis. The contrasting results reported by Kwak et al. (2018) were probably linked to a premature leaf senescence phenomenon stemming from oxidative damage produced by high levels of ROS, such as O2− and H2O2, which led to increased lipid peroxidation and membrane permeability under artificial night illumination.
Nighttime illumination, if of sufficient intensity, can induce plants to photosynthesise during nocturnal hours (Lo Piccolo et al., 2021, 2023; Kwak et al., 2018), although the observed values of net photosynthesis (Pn) were lower than those at sunrise. Kwak et al. (2017) suggested that artificial nighttime light may unbalance the endogenous circadian levels of ABA, resulting in stomatal opening. The reduced Pn values at dawn may also be related to impairment of the photosynthetic machinery. Indeed, as observed in Populus alba, the greatest reductions in PSII performances were observed at dawn, where the lack of starch degradation during the night and the relative impairment in the sinking capacity, which may induce a feedback limitation, thus impairing linear electron transport flow, also evidenced by the downregulation of two ferredoxin nitrite/sulphite reductase domains (Lo Piccolo et al., 2024). Furthermore, the abundance of light-harvesting complex (LCHII) of PSII also exhibited a reduction in L. tulipifera (Kwak et al., 2017). Nocturnal alterations in photosynthesis due to streetlamp lighting can alter the primary metabolism of illuminated leaves, consequently impairing the source/sink balance. Kwak et al. (2017) observed a carbon starvation phenomenon in the leaves of L. tulipifera, resulting from reduced daytime accumulation and decreased nighttime starch degradation. Liu et al. (2021) observed an increase in starch and total soluble sugar concentrations in Acer truncatum and Quercus mongolica in streetlamp-lighted trees due to an accelerated translocation of carbon to sinks (i.e., new shoots that remained active even during the winter; Lo Piccolo et al., 2023).
The few observations obtained from the literature regarding leaf primary metabolism and carbon content suggest that tree species may adopt different strategies in response to nighttime streetlamp illumination, emphasising differences in susceptibility to artificial night lighting and highlighting the fact that this topic still needs to be fully elucidated.
All of the aforementioned reports highlight the deep influence of streetlamp lighting on urban trees. However, it is important to note that streetlamps do not only affect trees but also grass species, inducing vegetation changes in biomass and plant cover over time (i.e., Agrostis tenuis, Anthoxanthum odoratum and Holcus lanatus; Bennie et al., 2017).
To strike a balance between citizen safety and the well-being of urban vegetation, more research is required to fully understand both the annual and long-term impacts that urban illumination has on ecosystem functioning. It is necessary to investigate the effects of different streetlamp technologies, test innovative light spectra enriched with green and orange wavelengths and provide a comprehensive list of tree species susceptibility to artificial nocturnal lighting. Such research could help urban planners select the proper tree species for roadside avenues and lessen the impact of lighting solutions in our cities without compromising the fundamental need for citizen safety.
6 CONCLUSION AND PERSPECTIVES
The capabilities of new light technologies and cutting-edge materials for covering films to improve plant growth as well as metabolic performance have been increasingly exploited. The trade-off between costs and benefits (which goes beyond the aim of the present review) has to be considered and contextualized for each species. However, it is undeniable that “photomodulation” is a powerful tool to shape plant morpho-anatomical and metabolic features, though a lot of aspects still need to be investigated. For example, improving light use efficiency by the plant, even in condition of fluctuating light, is a frontier topic of investigation to feed a constantly growing population. On the other hand, artificial lighting may exert negative side effects, i.e. light pollution in urban environments, posing the urgent need for less-impacting streetlamp spectra on tree physiology. Besides the methodologies for light management proposed herein, some others for which the research and application are in their infancy are still worth mentioning. For example, root illumination in hydroponic cultivation (Cabrera et al., 2022) and fruit bagging (i.e. the use of coloured plastic films; Ali et al., 2021) could be proficiently used as an unconventional method to promote the quality and development of horticultural species. To date, few reports are available on these topics, and few details are provided regarding the spectra supplied to plants, which makes the result unreliable for drawing any solid and consistent conclusions. Thus, scientists and growers have nowadays a unique opportunity to contribute to generating knowledge on existing products for light management, while companies have the possibility to extend the portfolio of available products through cutting-edge innovation on light technologies and photomodulating materials.




