Different metabolic adaptation strategies after overwintering in Eutrema sp. and Arabidopsis accessions under field conditions in Germany
Abstract
Successful overwintering is a prerequisite for high fitness in temperate perennials and winter annuals and is highly dependent on increased freezing tolerance and timely balancing of deacclimation with growth resumption in spring. To assess fitness costs associated with overwintering and elucidate metabolic mechanisms underlying winter survival and the switch from acclimated freezing tolerance to growth resumption, we performed a comparative field study using 14 Eutrema salsugineum accessions, E. halophilum, E. botschantzevii and 11 Arabidopsis thaliana accessions differing in freezing tolerance. Winter survival and reproductive fitness parameters were recorded and correlated with phenological stage and metabolite status during growth resumption in spring. The results revealed considerable intraspecific variation in winter survival, but survival rates of the extremophyte Eutrema were not inherently better. In both Eutrema and A. thaliana, improved winter survival was associated with reduced reproductive fitness. Metabolic analysis by GC–MS revealed intrinsic differences in the primary metabolism of the two genera during deacclimation. Eutrema contained higher levels of several amino and chlorogenic acids, while Arabidopsis had higher levels of several sugars and sugar conjugates. In both genera, increased levels of several soluble sugars were associated with increased winter survival, whereas myo-inositol has different roles in overwintering of Eutrema and A. thaliana. In addition, differences in amino acid metabolism and polyhydroxy acids levels after winter survival were found. The results provide strong evidence for a trade-off between increased winter survival and reproductive fitness in both Eutrema and Arabidopsis and document inherent differences in their metabolic strategies to survive winter.
1 INTRODUCTION
Successful overwintering is a prerequisite for high fitness in temperate perennial plants and winter annuals. Plant winter survival is affected by a multitude of factors associated with genotype, plant vigour and the occurrence of biotic and abiotic stress factors (Bergjord Olsen et al., 2018; Wagner et al., 2021). Low temperatures represent a crucial abiotic factor determining winter survival as well as the geographical distribution, growth and reproductive fitness of plants (Boinot et al., 2022; Kreyling et al., 2015; Li et al., 2015; Weiser, 1970). To overcome the constraints of low temperatures, plants native to temperate climates show natural low-temperature acclimation during autumn in preparation for winter frost. This process is termed cold acclimation. Maximum freezing tolerance is reached midwinter. Upon exposure to warmer temperatures in spring, plants lose the freezing tolerance acquired during acclimation by deacclimation while they resume growth and development (Xin & Browse, 2000). Cold acclimated freezing tolerance has been shown to vary greatly among genotypes with different geographical origins (Bahrani et al., 2021; Hannah et al., 2006; Zuther et al., 2012;), and freezing tolerance is a highly adaptive trait contributing to geographic differentiation, especially in colder climates (Ågren & Schemske, 2012). However, increased freezing tolerance and winter survival may entail a fitness cost for the plants. A recent study monitoring fitness parameters and winter survival in field experiments or freezing tolerance in controlled chamber experiments of 11 Arabidopsis thaliana accessions found lower fitness, expressed as 1000-seed mass, in more freezing-tolerant accessions than freezing-sensitive accessions (Boinot et al., 2022).
While climate warming reduces the occurrence of frost events and the date of the last spring frost is shifting earlier in many regions (IPCC 2021), the warming-induced advancement of the growing season of plants in the Northern Hemisphere may induce more frequent frost days during deacclimation, when organs and tissues are becoming increasingly vulnerable to freezing temperatures (Liu et al., 2018; Zohner et al., 2020). Hence, balancing the deacclimation rate in spring with the initiation of growth and development is likely becoming increasingly important for winter survival and fitness (Zuther et al., 2015). In addition, as the climate warms, the frequence and severity of unseasonable warm spells in late winter and spring might increase, leading to repeated deacclimation and acclimation cycles and impaired winter survival (Pagter et al., 2023; Pagter & Arora, 2013; Vitasse et al., 2014; Vyse et al., 2019).
Plants in natural environments are exposed to a greater range of day lengths and greater variation in temperature, humidity and light quality across all timescales than those typically encountered under controlled conditions (Bergelson & Roux, 2010). Consequently, phenotypes observed in controlled environments do not necessarily correlate to the phenotypes observed under natural conditions with regard to fitness assessments (Köhl & Laitinen, 2015) and the leaf metabolome observed under recurring environmental conditions in climate chambers may be remarkably different from the metabolome observed under natural conditions (Annunziata et al., 2018; Buckley et al., 2019). Also, phenotypic analyses under natural conditions reveal strong genotype–environment interactions for numerous traits (Alonso-Blanco et al., 2021). Field or common garden experiments are therefore highly needed in order to capture metabolic responses that are important under ecologically realistic conditions.
The low-temperature acclimation response is a multigenic, quantitative trait involving massive re-programming of the metabolome (see Fürtauer et al., 2019; Guy et al., 2007; Hincha et al., 2012 for reviews). Similarly, deacclimation involves extensive metabolic regulation of the developmental switch, resulting in reduced freezing tolerance and the resumption of growth (Pagter et al., 2023; Pagter et al., 2017; Rathore et al., 2021; Vyse et al., 2019). In particular, carbohydrate metabolism plays an exceptionally important role in overwintering and growth resumption of perennials and winter annuals (Pagter et al., 2017; Rathore et al., 2021). The two processes additionally involve changes in the metabolism of amino acids, storage lipids and cell wall components (Kjær et al., 2018; Pagter et al., 2017).
Arabidopsis thaliana is widely distributed in the Northern Hemisphere, and several studies have shown significant natural variation in the response of Arabidopsis accessions to low temperatures (Hannah et al., 2006; Korn et al., 2008; Zuther et al., 2012). In addition, specific deacclimation patterns were shown for 12 Arabidopsis accessions under controlled conditions (Juszczak et al., 2016; Zuther et al., 2015). For 11 Arabidopsis accessions, winter survival was strongly correlated with their respective freezing tolerance after cold acclimation (Boinot et al., 2022), indicating high importance of cold acclimation capacity for winter survival of A. thaliana. Eutrema (tribe Eutremeae) is an extremophyte emerging as a model plant for stress resistance (Bressan et al., 2001). It is a close relative to Arabidopsis but with a closer relation to agronomically important Brassica species (Bailey et al., 2006; Wong et al., 2005). Although some accessions of Eutrema are not extremophile with regard to freezing tolerance, others show significantly higher freezing tolerance than any Arabidopsis accession under controlled conditions (Lee et al., 2012). Additionally, E. salsugineum shows a remarkable long-term acclimation capacity, with a significant increase in freezing tolerance after three weeks of cold acclimation compared to two weeks. Such an increase was not observed in A. thaliana (Khanal et al., 2015). Metabolite data indicate different metabolic adaptation strategies to freezing between Eutrema and Arabidopsis (Lee et al., 2012). However, an understanding of metabolic mechanisms underlying deacclimation and growth resumption of Eutrema is completely lacking. Differences between the model species A. thaliana, with limited stress tolerance traits, and its more stress tolerant relative Eutrema provide possibilities for the discovery of novel metabolic mechanisms underlying winter survival and the switch from acclimated freezing tolerance to growth resumption in the spring (Zuther et al., 2018).
Thus far, most studies investigating metabolism during deacclimation and growth resumption used individual genotypes, and results could therefore not be correlated quantitatively with differences in phenology or winter survival. Here, we performed a comparative study using 14 E. salsugineum accessions, the two closely related species E. halophilum and E. botschantzevii and 11 accessions of A. thaliana for a complete analysis of phenotypic variation in winter survival, developmental stage, reproductive fitness and metabolite status, and a correlation analysis of differences at the metabolic and whole-plant level. We hypothesize that Eutrema accessions generally have a higher winter survival rate than A. thaliana accessions but that improved winter survival entails a fitness cost in both species. We further expect that the two species share some general metabolomic responses to successfully survive winter, but also accumulate divergent metabolites that may partly explain differences in winter survival and/or spring phenology. In addition to interspecific variation in metabolic profiles, it is hypothesized that there is intraspecific variation in the regulation of primary metabolism during deacclimation and growth resumption between accessions originating from different climates.
2 MATERIALS AND METHODS
2.1 Plant material and experimental set-up
The experiment included E. halophilum, E. botschantzevii and 14 natural accessions of E. salsugineum, spanning a large geographical range from the USA and Canada through China and Russia, and 11 natural accessions of Arabidopsis thaliana originating from Canada, Cape Verde, Portugal, Spain, Poland, Russia or India. The accessions of both Eutrema and A. thaliana have previously been shown to vary in cold-acclimated freezing tolerance, ranging from very tolerant to very sensitive (Lee et al. 2012; Zuther et al. 2012). Seeds of the Eutrema salsugineum (Pall.) Al-Shehbaz & Warwick, accessions Colorado, Cracker Creek, Dillibrough, Hebei, Henan, Jiangsu, Shandong, Xinjiang and Yukon were kindly provided in 2011 by Prof. Ray A. Bressan (Purdue University, West Lafayette, IN). Seeds of further E. salsugineum accessions (Altai 1, Altai 2, Buriatia, Tuva and Yakutsk), E. halophilum (C.A.Mey.) Al-Shehbaz & Warwick (Bayanual) and E. botschantzevii (D.A. German), Al-Shehbaz & Warwick (Saratov) were collected in Russia and Kazakhstan and kindly provided in 2011 by Alexei Babakov (All-Russia Research Institute of Agricultural Biotechnology RAAS, Moscow, Russia). The geographical origins (latitude and longitude) of the E. salsugineum accessions, E. halophilum and E. botschantzevii and mean minimum habitat temperature recorded during the coldest month of the growing season at the climate station nearest to the collection site were previously published by Lee et al. (2012). Despite being different species, E. halophilum and E. botschantzevii will hereafter be referred to as accessions along with the E. salsugineum accessions. Seeds of the A. thaliana accessions were originally obtained from Nottingham Arabidopsis Stock Center (NASC; University of Nottingham, Loughborough, UK). The geographical origins (latitude and longitude) of the A. thaliana accessions were previously published by Zuther et al. (2012). Seeds of both Eutrema and A. thaliana were further propagated at the Max Planck Institute of Molecular Plant Physiology.
For the experiment, 100 seeds per accession were sown on October 25, 2013 in plastic boxes with holes (50 x 40 x 15 cm; W x L x H) with sandy substrate “Haufen B” supplemented with 1 g Osmocote Start (ICL Group Ltd.)/1 L substrate that was sterilized prior to use. Five replicate boxes were prepared for each accession. Two weeks after sowing on November 7, 2013, boxes were transferred from the polytunnel to the field in Potsdam - Golm (52° 24′ N 13° 04′ E) following a random design and were aligned with a water spirit level to avoid uneven distribution of rain (Köhl & Laitinen, 2015). Plants were grown under natural weather conditions except for some additional watering when necessary. Weather conditions during the experimental period (November 7, 2013 to May 12, 2014) were previously described in detail (Boinot et al., 2022). In brief, the mean air temperature 5 cm above ground ± SE was 0.6 ± 0.3°C, while the absolute minimum temperature was −16.5°C. The number of days with snow cover was 17. In May of the following year, boxes were transferred back to the polytunnel and inflorescences bagged in groups for seed harvest. After 2 months, ripe seeds were collected and weighed for determination of total seed yield per plant.
2.2 Visual phenotyping
On March 28, 2014 field grown plants were visually scored for absolute number (winter survival) and on April 4 for phenological developmental stage. The developmental stage was determined according to the BBCH scale (Meier 1997) adopted for A. thaliana (Schwachtje et al., 2011). For Arabidopsis, the results of the visual phenotyping have previously been published by Boinot et al. (2022). The non-numeric BBCH scores for each species were ranked in Excel with the function Rank.avg and further statistical analysis was done in RStudio.
2.3 Seed harvest and weighing
After harvesting, seeds were weighed to obtain the total seed yield per box for each accession. Seed mass per plant was obtained by dividing the total seed mass by the number of survivors per box. For A. thaliana, 1000 seeds of each accession and replicate were counted at the Institute of Applied Genetics at the Free University of Berlin with an Elmor C3 seed-counting machine (Elmor AH). 1000-seed mass was weighed with a Sartorius LE244S precision balance (240 g x 0.0001 g). For Arabidopsis, the results concerning seed yield per plant and 1000-seed mass have previously been published by Boinot et al. (2022).
2.4 GC–MS metabolite profiling
Pools of one to nine plants from five boxes per accession were harvested for metabolite profiling on April 3 between 12:30 and 13:30. Polar metabolites were extracted from 80 mg (fresh weight) with a tolerance of ~5–10% and processed as described previously (Erban et al. 2020) by dual, sequential injections using splitless-mode and split-modes at a split flow ratio of at least 1:30 optimized to the linear quantification range of abundant metabolites. Gas chromatography coupled to electron impact ionization-time of flight-mass spectrometry (GC/EI-TOF-MS) was performed and metabolites were identified as described previously (Erban et al. 2020). Metabolite intensities were normalized to sample fresh weight and the internal standard 13C6-sorbitol. In total, 129 primary metabolites were annotated, including known and yet unknown compounds that are archived by the Golm Metabolome Database, http://gmd.mpimp-golm.mpg.de/ (Kopka et al. 2005). Acidic metabolites were named as ‘acids’. We consider this naming as synonymous to their respective metabolic anion, according to the naming and list of synonyms used by the KEGG and BioCyc databases. Metabolites with more than 50% missing values over all samples within a species and known contaminations, annotated reagents and internal standards were removed from further analysis, resulting in 102 and 101 metabolites for Eutrema and A. thaliana, respectively. Missing values of the remaining metabolites were imputed by half of the minimum value per metabolite over all samples within a species. A complete list of normalized metabolite levels can be found in Table S1.
2.5 Statistical data analysis and visualization
Data analysis and figure generation were performed in R v4.1.0 and RStudio. To examine whether winter survival and seed yield per plant differed between Eutrema and A. thaliana and between accessions within a species data were analyzed using two-way ANOVA in R. Heterogeneities of variance were tested using Bartlett's test and differences between individual means were identified using Tukey's Studentized Range Test at the 5% significance level. A non-parametric Kruskall–Wallis test was also used to test differences in winter survival between Eutrema and A. thaliana and between accessions. This test was done to supplement the results from the ANOVA, as the assumption concerning homogeneity of variance was not fulfilled.
Outliers in the metabolite dataset were identified using the Rosner Test (rosnerTest) included in R-package EnvStats (Millard, 2014) and replaced by ‘NA’, if values much higher or lower than the rest of the data were not related to genotype. In E. salsugineum, the compound classified ‘similar to Glycerolaldopyranosid’ was identified as an outlier in four out of five samples of Altai 2 and all replicate samples of Saratov. However, the metabolite remained in the dataset, as this was interpreted as a genotype-specific high concentration. Melibiose and putrescine were identified as outliers in two and three samples of Saratov and Bayanaul, respectively. The concentrations of these metabolites were, however, also high in the other replicates of Saratov and Bayanaul, and therefore remained in the dataset. In A. thaliana, xylose and ribonic acid were identified as outliers in four out of five samples of N13, but both metabolites remained in the dataset as these were interpreted as genotype-specific high concentrations. Normalized metabolite levels were compared between Eutrema and A. thaliana using a t-test in R with correction for multiple testing using the Benjamini-Hochberg method (Benjamini & Hochberg, 1995). Metabolic differences between Eutrema and A. thaliana were also investigated by Principal Components Analysis (PCA) applied to the normalized metabolite levels using the R-package pcaMethods (Stacklies et al., 2007). The PCA indicated different metabolic compositions of the two species and the metabolite data were subsequently analyzed separately for Eutrema and A. thaliana. For statistical analysis only, the remaining metabolite intensities were divided by the median intensity across all measurements and resulting fold changes were log2-transformed to approximate normal distribution, i.e. log2-median transformation. To detect metabolites whose levels differed significantly between accessions, the log2-transformed metabolites levels were analyzed using a one-way ANOVA with correction for multiple testing using the Benjamini-Hochberg method.
Spearman rank order correlation was performed with log2-median transformed data and the survival percentage, ranked BBCH scores or total seed yield per plant utilizing the function rcorr in the Hmisc package and a significance threshold of P < 0.05 following correction for multiple testing using the Benjamini-Hochberg method. Heatmaps were generated using the package pheatmap (https://cran.r-project.org/package=pheatmap). Heatmap rows and columns were hierarchically clustered using the pheatmap function and an Euclidian distance measure.
3 RESULTS
3.1 Winter survival and phenological development
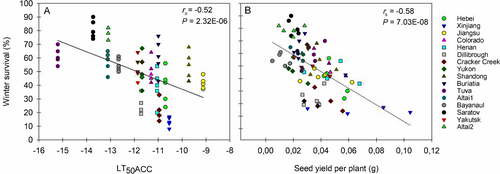
Winter survival, analyzed in late March after the cold in winter, did not vary significantly between Eutrema and A. thaliana, indicating that winter survival of Eutrema is not consistently greater than that of A. thaliana. In contrast, winter survival varied strongly between accessions (P = 6.029e-11). The average winter survival of Eutrema accessions ranged from 13% (Xinjiang) to 80% (E. botschantzevii Saratov) (Figure 1A). In A. thaliana, the average winter survival rate varied less, ranging from 33% (Sah-0 and C24) to 61% (N13) (Figure 1B). The winter survival of the A. thaliana accessions in the current field trial has previously been shown to correlate with the cold-acclimated freezing tolerance (LT50ACC) determined for plants grown under controlled conditions (Boinot et al. 2022). An analogous correlation analysis for Eutrema between the winter survival of the accessions in the current field trial and cold-acclimated freezing tolerance (LT50ACC) previously determined for plants grown under controlled conditions (Lee et al. 2012) confirmed that cold acclimation capacity is also of high importance for field winter survival of Eutrema (Figure 2A).
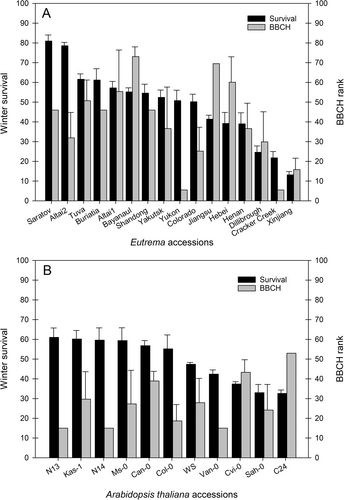
The plants' phenological development was recorded in early April, concurrent with sampling for metabolite profiling, using the BBCH scale for plant development. In Arabidopsis, some accessions are rapid-cycling summer annuals, whereas others are vernalization responsive and thus behave as winter annuals (Michaels et al., 2003). In the current study, all Arabidopsis accessions performed as winter annuals, which germinate in autumn, overwinter and then bolt and flower the following spring. Compared to A. thaliana, a longer vernalization period is required for flowering in Eutrema (Batelli et al., 2014). Accordingly, the Eutrema accessions in the current study also performed as winter annuals. Spring phenology was advanced in A. thaliana compared to Eutrema. Among the Eutrema accessions, E. halophilum Bayanaul had reached BBCH growth stage 58 (first flower petals visible), while the two latest developing accessions Cracker Creek and Yukon had reached BBCH stage 50 (vegetative plant parts have reached final size). In A. thaliana, average BBCH growth stage ranged from 63 (30% of flowers open) for N13, N14 and Van-0 to 73 (30% of fruits have reached final size) for C24. For the Eutrema accessions, the ranked BBCH index (Figure 1A) was weakly positively correlated with winter survival (rs = 0.31, P = 0.009, Table 1). For the A. thaliana accessions, no significant correlation was found between the ranked BBCH index (Figure 1B) and winter survival (rs = −0.22, P = 0.18, Table 2).
| Parameter | Cluster | Survival | Seed yield | BBCH |
|---|---|---|---|---|
| Survival | NA | -0.5855*** | 0.3082** | |
| Seed yield | -0.5855*** | NA | -0.1487 | |
| BBCH | 0.3082** | -0.1487 | NA | |
| NA213001 | III | 0.7664*** | -0.4793*** | 0.4083*** |
| similar to glycerolaldopyranosid | II | 0.7447*** | -0.4459*** | 0.1568 |
| Glucose | II | 0.6682*** | -0.3162** | 0.4558*** |
| NA211001 | III | 0.6626*** | -0.3938*** | 0.4138*** |
| Inositol, myo- | II | 0.6604*** | -0.2080 | 0.2544* |
| A250001-101 | III | 0.6568*** | -0.3744** | 0.4643*** |
| Sucrose | II | 0.6558*** | -0.2024 | 0.3321** |
| A214004-101 | III | 0.6346*** | -0.3800** | 0.3659** |
| Phosphate | III | 0.6257*** | -0.3879** | 0.1965 |
| A214003-101 | III | 0.6205*** | -0.3146** | 0.3795** |
| A185003-101 | II | 0.6160*** | -0.2731* | 0.4554*** |
| Glycerophosphoglycerol | III | 0.5910*** | -0.1734 | 0.4142*** |
| Raffinose | II | 0.5875*** | -0.1927 | 0.1808 |
| Galactose | II | 0.5862*** | -0.2318 | 0.2394* |
| myo-Inositol-1-phosphate | III | 0.5588*** | -0.2361 | 0.3720** |
| A311002-101 | II | 0.5432*** | -0.1500 | 0.2419* |
| A196004-101 | II | 0.5382*** | -0.3325** | 0.2969* |
| A217004-101 | III | 0.5310*** | -0.1458 | 0.3355** |
| Fructose | II | 0.5126*** | -0.1721 | 0.5429*** |
| Glucose-6-phosphate | III | 0.4988*** | -0.2450* | 0.2765* |
| Glutaric acid, 2-hydroxy- | II | 0.4952*** | -0.2077 | 0.3541** |
| Trehalose, alpha,alpha'- | II | 0.4873*** | -0.1024 | 0.2437* |
| Isoleucine | III | 0.4848*** | -0.1534 | 0.2690* |
| Maltose | III | 0.4819*** | -0.1439 | 0.2203 |
| Ribose | III | 0.3890*** | -0.2820* | 0.3331** |
| NA176001 | III | 0.3656 | -0.0518 | 0.3461** |
| Dehydroascorbic acid dimer | III | 0.3469 | -0.1506 | 0.2775* |
| Valine | I | 0.2919* | -0.4023*** | 0.1805 |
| Ribitol | I | 0.2623* | -0.1947 | -0.0950 |
| A228001-101 | IV | 0.1521 | -0.1055 | 0.2791* |
| Citric acid | I | 0.0785 | -0.0841 | -0.2651* |
| Erythronic acid | IV | 0.0135 | 0.1940 | 0.2098 |
| Threonic acid | IV | -0.0064 | 0.2872* | 0.2135 |
| NA298001 | IV | -0.0290 | 0.2382* | 0.1629 |
| Glutaric acid, 2-oxo- | IV | -0.0981 | 0.0544 | 0.0462 |
| Quinic acid, 3-caffeoyl-, trans- | IV | -0.1976 | 0.2853* | 0.1624 |
| A304001-101 | V | -0.3967*** | 0.2735* | -0.1044 |
| Glutamic acid | V | -0.4130*** | 0.0980 | -0.0949 |
| Serine | V | -0.4165*** | 0.0440 | -0.3271** |
| Glyceric acid | V | -0.4273*** | 0.1468 | 0.0261 |
| Succinic acid | V | -0.4327*** | 0.2648* | -0.1863 |
| Aspartic acid | V | -0.4376*** | 0.2121 | -0.2662* |
| Pyroglutamic acid | V | -0.4486*** | 0.0775 | -0.2544* |
| A240004-101 | IV | -0.4595*** | 0.3150** | 0.0443 |
| Quinic acid | IV | -0.5414*** | 0.4000*** | 0.0182 |
| Alanine | V | -0.5791*** | 0.1080 | -0.1790 |
| Glycine | V | -0.6588*** | 0.2463* | -0.3517** |
| Parameter | Cluster | Survival | Seed yield | BBCH | 1000 seed mass |
|---|---|---|---|---|---|
| Survival | 1.0000 | -0.2848 | -0.2239 | -0.5442*** | |
| Seed yield | -0.2848 | 1.0000 | -0.1087 | -0.0259 | |
| BBCH | -0.2239 | -0.1087 | 1.0000 | 0.4507** | |
| 1000 seed mass | -0.5442*** | -0.0259 | 0.4507** | 1.0000 | |
| Galactose | II | 0.5260*** | -0.2953 | -0.4279** | -0.5240*** |
| Fructose | II | 0.4763** | -0.3248* | -0.2890 | -0.4099** |
| Glucose | II | 0.4653** | -0.1675 | -0.2790 | -0.4179** |
| Maltose | I | 0.4592** | -0.4473** | -0.2008 | -0.2730 |
| Galactinol | IV | 0.4446** | -0.2831 | -0.5757*** | -0.4514** |
| Raffinose | II | 0.4404** | -0.2474 | -0.5383*** | -0.4670** |
| Melibiose | II | 0.4366** | -0.1038 | -0.4906*** | -0.5053*** |
| Sucrose | II | 0.4348** | -0.3971* | -0.2231 | -0.3531* |
| Ascorbic acid | II | 0.4215** | -0.0147 | -0.3359* | -0.4716** |
| similar to Glycerolaldopyranosid | II | 0.4097** | -0.4293** | -0.2902 | -0.3596* |
| Xylose | IV | 0.3989** | -0.3395* | -0.4790** | -0.6126*** |
| Threonic acid | II | 0.3857* | 0.0081 | -0.2936 | -0.3725* |
| A185003-101 | II | 0.3694* | -0.2784 | -0.2977 | -0.3645* |
| NA213001 | II | 0.3592* | -0.1750 | -0.2623 | -0.2686 |
| Erythronic acid | II | 0.3580* | 0.0351 | -0.3833* | -0.5719*** |
| NA298001 | IV | 0.3556* | -0.2178 | -0.5128*** | -0.3556* |
| Galactonic acid | II | 0.3277* | -0.3270* | -0.3529 | -0.3370* |
| A214004-101 | IV | 0.3118 | -0.3934* | -0.1661 | -0.3730* |
| Arabinose | II | 0.2935 | -0.1877 | -0.4809** | -0.3905* |
| A228001-101 | II | 0.2932 | -0.3230 | -0.3365 | -0.2656 |
| Glucose-1-phosphate | III | 0.2806 | -0.4071** | -0.2875 | -0.1168 |
| A311002-101 | IV | 0.2738 | -0.2312 | -0.4958*** | -0.4832** |
| Glutaric acid, 2-hydroxy- | I | 0.2563 | -0.2474 | -0.0484 | -0.1410 |
| A250001-101 | IV | 0.2456 | 0.0790 | -0.2155 | -0.5916*** |
| Trehalose, alpha,alpha'- | I | 0.2267 | -0.2307 | -0.3041 | -0.0956 |
| Glucose-6-phosphate | I | 0.1971 | -0.4764** | -0.2483 | -0.0496 |
| Fructose-6-phosphate | I | 0.1757 | -0.4822** | -0.1705 | -0.0807 |
| A251003-101 | IV | 0.1698 | 0.2298 | -0.4227** | -0.4425** |
| A203003-101 | I | 0.1510 | 0.0585 | 0.0195 | -0.0057 |
| A236005-101 | III | 0.1322 | -0.3355* | -0.2934 | -0.2884 |
| A148006-101 | II | 0.1252 | 0.0669 | -0.3983* | -0.1106 |
| Ribonic acid | IV | 0.1181 | -0.2701 | -0.2466 | -0.3346* |
| Pyroglutamic acid | III | 0.1133 | -0.0990 | -0.2691 | 0.0507 |
| A199004-101 | III | 0.0903 | -0.6079*** | -0.2777 | 0.0647 |
| A300001-101 | III | 0.0875 | -0.2179 | -0.3872* | -0.1096 |
| Ethanolaminephosphate | III | 0.0493 | -0.1560 | -0.1154 | 0.0611 |
| Gluconic acid | III | 0.0276 | -0.4044** | -0.2500 | 0.2245 |
| A240004-101 | III | -0.0006 | -0.3436* | -0.1668 | -0.0972 |
| Citric acid | IV | -0.0396 | -0.0762 | 0.0035 | 0.0061 |
| A304001-101 | III | -0.0602 | -0.3573* | -0.2177 | 0.1357 |
| NA176001 | IV | -0.0750 | 0.0612 | -0.2268 | -0.0280 |
| D155405 | III | -0.0878 | -0.1865 | -0.2578 | 0.0824 |
| Glutamic acid | III | -0.1104 | -0.3244* | -0.2063 | 0.1385 |
| Valine | III | -0.1370 | -0.2008 | 0.0754 | 0.4291** |
| Glycerophosphoglycerol | III | -0.1507 | -0.2302 | -0.3371* | 0.0821 |
| Threonine | IV | -0.1649 | 0.2194 | -0.1775 | 0.1450 |
| A175008-101 | IV | -0.1897 | -0.0668 | 0.1736 | 0.0544 |
| Malic acid | IV | -0.2188 | 0.3433* | 0.4404** | 0.1073 |
| Inositol, myo- | IV | -0.2330 | -0.0493 | -0.1964 | 0.1671 |
| Serine | IV | -0.2693 | 0.0573 | 0.0560 | 0.0978 |
| Fumaric acid | IV | -0.3517* | 0.1054 | 0.0574 | 0.1099 |
| Succinic acid | IV | -0.3875* | 0.1317 | -0.0037 | 0.2257 |
3.2 Reproductive output
The average seed yield per plant differed significantly between the two species (P < 2e-16), with the seed yield per plant over all accessions being on average six times greater in A. thaliana than in Eutrema. The average seed yield per plant also differed between accessions within a species (P = 4.55e-11 for Eutrema and P = 0.00608 for A. thaliana). In Eutrema, it ranged from 0.01147 g per plant for E. halophilum Bayanaul to 0.0558 g per plant for Xinjiang, while in A. thaliana it ranged from 0.1184 g per plant for Cvi-0 to 0.3033 g per plant for Van-0. To investigate if increased winter survival entailed a fitness cost for the plants, correlation analyses of the seed yield per plant, and for A. thaliana also 1000-seed mass, with the winter survival rate of the respective accessions were done. In Eutrema, a significant negative correlation between seed yield per plant and winter survival was obtained (Figure 2B), representing a lower fitness for accessions with higher winter survival. No significant correlation was found between winter survival and seed yield per plant in A. thaliana (rs = −0.28, P = 0.08, Figure S1A); however, winter survival was negatively correlated with the 1000-seed mass (rs = −0.54, P = 0.0001, Figure S1B), revealing lower seed mass with increasing winter survival. In addition, 1000-seed mass was positively correlated with the BBCH index (rs = 0.45, P = 0.0024, Table 2), indicating higher seed mass with earlier phenological development.
According to Boinot et al. (2022), there was no correlation between the seed yield of the A. thaliana accessions in the current field trial and their acclimated freezing tolerance (LT50ACC), whereas the 1000-seed mass was positively correlated with LT50ACC, revealing higher seed mass for accessions with lower freezing tolerance. In comparison, we found a significant positive correlation between the seed yield of the Eutrema accessions in the current field trial and LT50ACC previously determined for plants grown under controlled conditions (Lee et al., 2012) (rs = 0.43, P = 0.00015, data not shown), representing a higher seed yield for accessions with lower freezing tolerance (higher LT50ACC).
3.3 Metabolite profiling
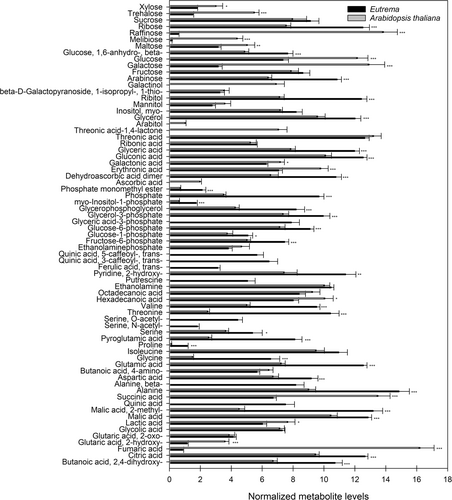
The measurement of the relative metabolite levels in the close-to-simultaneously sampled (within a 1-h-period) Eutrema accessions and A. thaliana accessions showed substantial differences between the two species, as shown by PCA (Figure S2). The normalized metabolite levels in Eutrema and A. thaliana, averaged across all accessions, are compared in Figure 3. The relative levels of 67 out of 94 common metabolites differed between the two species. Eutrema had significantly higher levels of 2-methylmalic acid, quinic acid and several amino acids, including alanine, glutamic acid, glycine, proline, pyroglutamic acid, threonine, valine, β-alanine and O-acetylserine (OAS) and its derivative N-acetylserine. The latter three non-proteinogenic amino acids were not detected in A. thaliana under field sampling conditions. Eutrema also contained putrescine, trans-ferulic acid and the chlorogenic acids 3-O-caffeoylquinic acid (3-CQA) and 5-O-caffeoylquinic acid (5-CQA), which were not detected in A. thaliana. Another observation was the significantly higher levels of several phosphates in Eutrema, including glyceric acid-3-phosphate, myo-inositol-1-phosphate, phosphate and phosphate acid monomethyl ester. Arabidopsis, on the other hand, had significantly higher levels of fumaric acid, threonic acid-1,4-lactone, arabitol and the sugar and sugar conjugates galactinol, galactose, glucose, maltose, melibiose, raffinose and trehalose. Threonic acid-1,4-lactone, arabitol and galactinol were not detected in Eutrema. Levels of several unknown compounds also varied significantly between Eutrema and A. thaliana accessions (unknown compounds shown in Figures 5 and 6).
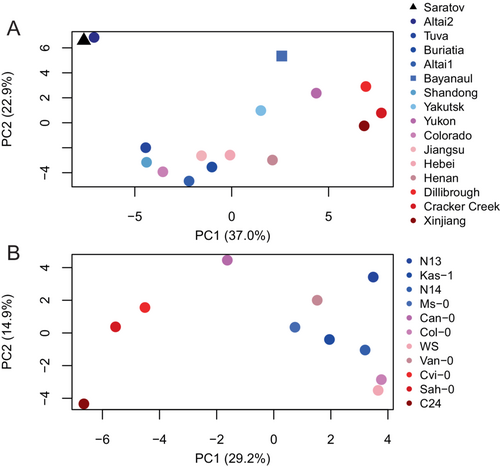
In order to detect metabolites or pathways of importance for winter survival and/or growth resumption within A. thaliana, with limited stress tolerance traits, and its more stress-tolerant relative Eutrema, we then analyzed the metabolite data for each species separately. According to PCA, winter survival was the dominant source of variance underlying PC1 for both Eutrema and A. thaliana, explaining 37.0% and 29.2% of the variance for Eutrema and A. thaliana, respectively (Figure 4). For Eutrema, the profiles of accessions with lowest winter survival (Cracker Creek, Dillibrough and Xinjiang) and accessions with highest winter survival (E. botschantzevii Saratov and Altai2) were distributed furthest apart, while profiles of accessions with intermediate winter survival were located in between. PC2 explained an additional 22.9% variability and tended to separate the species E. botschantzevii Saratov, E. halophila Bayanaul and E. salsugineum Altai2 with high winter survival from the other E. salsugineum accessions (Figure 4A). For A. thaliana, PC1 separated the profiles of C24, Sah-0 and Cvi-0 with low winter survival (33–37%) from profiles of seven accessions with intermediate-high winter survival (42–61%); the profile of Can-0, having an early phenological development and a survival rate of 57%, was located in between (Figure 4B). Neither PC2 nor PC3, which explained 14.9% and 13.8% of the total variance, respectively, yielded any clear separation with respect to winter survival, phenological development or seed yield.
A total of 47 metabolites in Eutrema showed significantly different pool sizes between accessions. In accordance with the PCA, hierarchical clustering of these metabolites separated the accessions into four clusters according to winter survival, highlighting the importance of metabolic reprogramming in the winter survival of Eutrema (Figure 5). Cluster A included E. botschantzevii Saratov and Altai2 with the highest winter survival rates (79–81%). Cluster B contained accessions with high-to-intermediate winter survival rates (50–62%, Altai1, Buriatia, Tuva, Colorado, Shandong). Cluster C contained accessions with intermediate-to-low survival rates (39–55%, E. halophilum Bayanaul, Henan, Hebei, Jiangsu, Yakutsk, Yukon), while cluster D contained Xinjiang, Cracker Creek and Dillibrough with the lowest winter survival rates (13–24%). Clustering further grouped the metabolites into five clusters of varying sizes, corresponding to different metabolite levels (Table S2). (I) Metabolites with quite varied accumulation patterns. This cluster was small, containing only valine, citric acid and ribitol. (II) and (III) Metabolites decreasing in abundance with decreasing winter survival. Cluster II contained six sugars, four unknown compounds, one acid and one polyol. Cluster III was dominated by four phosphates and 6 unknown compounds. Pool sizes of metabolites in (IV) were highest in accessions with intermediate-high winter survival and lowest in the accessions with lowest and highest winter survival. This cluster included two polyhydroxy acids, two acids, one phenylpropanoid and three unknown compounds. Metabolites in cluster (V) were found in the highest amounts in accessions with the lowest winter survival and in the lowest amounts in the accessions with the highest winter survival. This cluster was dominated by six amino acids.
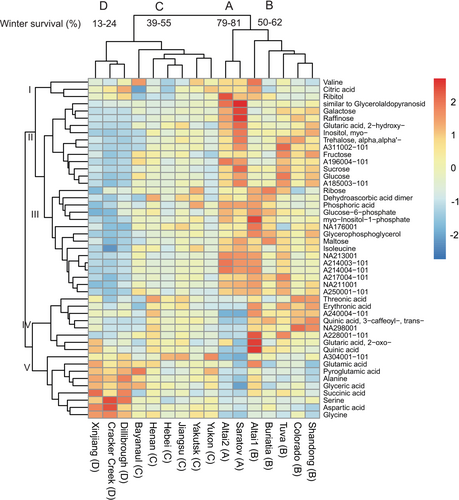
To identify individual metabolites that may contribute to winter survival, growth resumption in spring or reproductive fitness of Eutrema, we correlated metabolite levels with winter survival, ranked BBCH index and seed yield per plant. All metabolites in clusters II and III showed moderate or strong positive correlations with the winter survival of all replicates of the 16 Eutrema accessions (Table 1). Most metabolites in clusters II and III were additionally weakly or moderately positively significantly correlated with the ranked BBCH index. In contrast, levels of all metabolites in cluster V were negatively significantly correlated with the winter survival of field grown Eutrema accessions, and four of them were also negatively, although weakly, correlated with the ranked BBCH index. Few correlations were found between levels of metabolites in clusters I or IV and winter survival or ranked BBCH index. Seed yield of the Eutrema accessions was significantly positively correlated with levels of five and three metabolites in clusters IV and V, respectively, and significantly negatively correlated with altogether 12 metabolites in clusters II or III. However, most correlations between individual metabolites and seed yield were weak (rs <0.4).
In A. thaliana, a total of 52 metabolites showed significantly different pool sizes between accessions. Between the accessions, it was clear that C24, with low winter survival and advanced phenological development, was most unlike the other 10 accessions as it formed its own cluster following hierarchical clustering of significant metabolites (Figure 6 cluster A). Cluster B corresponded to N13, Van-0, Col-0 and N14, which were characterized by intermediate winter survival (42–61%) and less advanced phenological development (BBCH 63) than the four accessions in cluster C (Ms-0, Kas-1, WS, Can-0), which also had intermediate winter survival rates (47–60%) but more advanced spring phenology (BBCH 65–66). Cluster D consisted of Sah-0 and Cvi-0 characterized by low winter survival rates (33–37%). Clustering of the metabolites revealed four major metabolite accumulation patterns (Table S3). The major clusters included (I) metabolites whose pool sizes were highest in cluster C accessions with intermediate winter survival rates and relatively advanced phenological development. This cluster included two phosphates, two sugars, one unknown compound and one acid. (II) Metabolites found in the lowest amounts in the accessions with lowest winter survival. This cluster was dominated by seven sugars and four polyhydroxy acids. Metabolites in cluster (III) were found in the highest pool sizes in Sah-0 and Cvi-0 and the lowest pool sizes in C24, despite all three accessions being characterized by low winter survival rates. This cluster contained three phosphates, three amino acids, one polyhydroxy acid and six unknown compounds. (IV) Metabolites present in the highest amounts in C24 and accessions with intermediate-high winter survival and relatively late phenological development (cluster B). This was a diverse cluster including, among others, four acids and two amino acids.
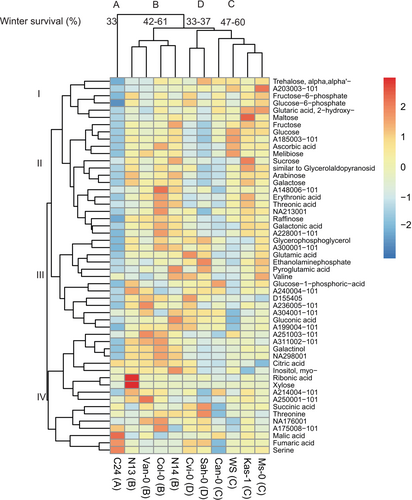
The potential significance of the metabolites in winter survival, phenological development and reproductive output of A. thaliana were explored by correlation analysis (Table 2). In cluster I, the levels of fructose-6-phosphate, glucose-6-phosphate and maltose were significantly negatively correlated with seed yield, and maltose was additionally positively correlated with winter survival. None of the metabolites in cluster I were correlated with the ranked BBCH index. Except for arabinose and one unknown compound, levels of all metabolites in cluster II were positively significantly correlated with the winter survival of all replicates of the 11 A. thaliana accessions. Most of these metabolites were additionally negatively significantly correlated with the 1000-seed mass and ranked BBCH index and less frequently with seed yield per plant. Metabolites in cluster III appeared functionally most related to seed yield as seven metabolites, including four unknows, were negatively significantly correlated with seed yield per plant. Only a few significant correlations were found between winter survival or ranked BBCH index and metabolites in cluster III. In cluster IV, five metabolites showed a significant negative correlation with the ranked BBCH index. Few significant but generally weak correlations were found between metabolites in cluster IV and winter survival or seed yield per plant.
4 DISCUSSION
4.1 Natural variation in winter survival and phenological development of Eutrema and A. thaliana accessions
Our survey of 16 and 11 geographically diverse Eutrema and A. thaliana accessions identified considerable natural inter- and intraspecific variation in winter survival. Winter survival varied more among Eutrema than A. thaliana accessions, with some of the Eutrema accessions having higher (Saratov, Altai2) or lower (Xinjiang, Cracker Creek and Dillibrough) average winter survival than any of the investigated A. thaliana accessions. Low winter survival rates of some of the Eutrema accessions disagree with our first hypothesis as the Eutrema accessions have previously been shown to have overlapping or greater cold acclimated freezing tolerance than 54 A. thaliana accessions when cold acclimated for two weeks under controlled conditions (Lee et al., 2012), and Eutrema remarkably outperforms Arabidopsis in long-term (three weeks) acclimation capacity (Khanal et al., 2015). Nonetheless, it has previously been shown that cold acclimation capacity is of high importance for successful overwintering of Arabidopsis (Boinot et al., 2022). Following that, we could show that the winter survival of the Eutrema accessions was strongly correlated with their respective cold acclimated freezing tolerance, determined as LT50 in electrolyte leakage assays under controlled conditions (Lee et al., 2012). Hence, cold acclimation capacity is also very important for winter survival of Eutrema. Large variation in winter survival rates highlights the importance of other factors than acclimated freezing tolerance for successful winter survival. Other factors influencing overwintering of winter annuals include, for instance, deacclimation resistance in response to intermittent warm periods, snow cover depth and duration and soil water content (Bergjord Olsen et al., 2018; Rapacz et al., 2017). Variation in winter survival rates may additionally be due to accessions being adapted to their local environment, resulting in a fitness trade-off when grown in a different environment (Oakley et al., 2014). Nevertheless, all the Eutrema accessions were from rather distant origins, and in A. thaliana, accessions from more distant origins (N13, Kas-1) showed similar or higher winter survival than e.g. Col-0 from Poland.
The timing of flowering affects the environmental conditions in which fertilization and seed maturation occur. Consequently, flowering time influences fecundity rate and plant fitness. Flowering time is controlled by various environmental signals experienced at both the seed and rosette stages (Debieu et al., 2013). Advanced spring phenology in A. thaliana compared to Eutrema is in accordance with previous observations of later flowering in Eutrema than in Arabidopsis (Amtmann, 2009) and may be due to a greater vernalization requirement of Eutrema (Guo et al., 2012). Natural variation in bolting and flowering time in A. thaliana is well documented and likely reflects adaptation to different environmental conditions depending on geographical site of origin. In some studies, latitudinal clines in flowering time across accessions have been demonstrated (Stinchcombe et al., 2004; Shindo et al., 2006). In accordance, we found a significant negative relationship between latitude of origin and BBCH index recorded in early April (rs = −0.66, P = 0.026, data not shown), as determined by Spearman rank order correlation, indicating that accessions from southern latitudes showed more advanced spring phenology than accessions from northern latitudes. Little is known about variation in bolting and flowering time within Eutrema. According to our results, Eutrema also show genetic variation in early-season phenology; however, we found no empirical evidence in support of a latitudinal cline in the spring phenology of Eutrema (rs = −0.35, P = 0.2, data not shown). Similarly, Lee et al. (2012) found no significant correlation between the latitude of the geographical origin of the accessions and their freezing tolerance before or after cold acclimation.
4.2 Improved winter survival reduces reproductive fitness in both Eutrema and A. thaliana
There was a large divergence in seed yield per plant between Eutrema and Arabidopsis, but also considerable variation in seed yield within A. thaliana and even more within Eutrema. In accordance with Boinot et al. (2022), the lower reproductive fitness in Arabidopsis accessions was associated with a higher winter survival rate and a higher freezing tolerance, as evidenced by the lower 1000-seed mass in accessions with higher winter survival rates compared to accessions with lower winter survival rates. Plants can increase their fitness by producing larger and/or heavier seeds, which support the embryo and the seedling with more resources. Heavier seeds in more southern accessions may be a result of earlier flowering, which allows for a longer development time of seeds and larger seeds (Bolmgren and Cowan, 2008). Hence, the 1000-seed mass was positively correlated with the BBCH index, indicating higher seed mass with earlier phenological development. In contrast, earlier flowering onset can also imply fewer resources allocated for maternal plant growth, smaller size at the time of reproduction, and thus fewer resources available for seed production (Bolmgren and Cowan, 2008).
In Eutrema, increased winter survival and higher acclimated freezing tolerance were also associated with reduced reproductive fitness, as evidenced by a negative correlation between seed yield per plant and winter survival and a positive correlation between seed yield per plant and LT50ACC previously determined for plants grown under controlled conditions. This is in accordance with the allocation cost theory, stating that the attribution of energy and metabolites into defense and acclimation limits growth and reproduction (Wan et al., 2017). Increased tolerance to abiotic stressors has previously been associated with reduced fitness parameters in other plant species; i.e. more frost-tolerant A. lyrata populations from higher latitudes depicted smaller plant size (Wos & Willi, 2015) and transgenic A. thaliana plants constitutively expressing cold tolerance genes had lower fitness, as determined by fruit number, than the wildtype (Jackson et al., 2004).
4.3 Eutrema and A. thaliana have distinct metabolic profiles under field conditions
The two species have very distinct metabolic profiles, suggesting different basal metabolic compositions and/or different metabolic adaptation strategies for overwintering. This is in accordance with previous studies investigating metabolic differences between the two species both in the absence of stress and in response to different abiotic stressors under controlled conditions (Benina et al., 2013; Lee et al., 2012; Pinheiro et al., 2019; Zuther et al., 2018), but has not previously been shown under natural conditions. Interestingly, several of the metabolites found at different levels in the two species were also among the most important metabolites distinguishing the E. salsugineum accessions Tuva and Yukon from 10 A. thaliana accessions under controlled non-acclimating and cold acclimating conditions (Zuther et al., 2018). Unique features of the Eutrema metabolome under field conditions, averaged across all accessions, were higher levels of amino acids, in particular glycine, proline, pyroglutamic acid, serine, threonine, β-alanine, O-acetylserine (OAS) and its derivative N-acetylserine. Proline is a well-documented osmoprotectant with a prominent role in freezing tolerance, which is known to accumulate to a much larger extent in Eutrema than in Arabidopsis in response to cold (Lee et al., 2012; Benina et al., 2013). Pyroglutamic acid and, therefore, glutamic acid can serve as precursors for proline synthesis (Mazelis & Pratt, 1976). Accordingly, glutamic acid was also found in higher levels in Eutrema than in A. thaliana. Increased levels of serine and glycine may be a sign of high photorespiratory activity during deacclimation and growth resumption in Eutrema. Photorespiration can act as an electron sink, especially under stress conditions such as high light and cold, by consuming reducing equivalents during the refixation of released ammonia and by exporting reduced components from the chloroplast to the mitochondrion (Wingler et al., 2000; Peterhansel et al., 2010). However, these free amino acids may also contribute to osmoregulation in response to osmotic stress (Di Martino et al., 2003). OAS is a signaling molecule regulating the expression of a common set of six genes in response to environmental factors (Apodiakou & Hoefgen, 2023), while beta-alanine is involved in multiple stress responses in plants and may additionally function in lignin biosynthesis (Parthasarathy et al., 2019). Benina et al. (2013) also found higher levels of β-alanine in Eutrema than in A. thaliana.
The chlorogenic acids 3-O-caffeoylquinic acid (3-CQA) and 5-O-caffeoylquinic acid (5-CQA) and their precursors quinic acid and ferulic acid were solely found in Eutrema after overwintering in the field. CQAs are specialized secondary metabolites derived from the phenylpropanoid biosynthesis pathway and are often induced by stress. They serve specific roles in plant protection, i.e. chemical defense against herbivores and pathogens, ultraviolet screening, or structural components of the cell wall (Soviguidi et al., 2022). In addition, 3-CQA and 5-CQA may function as antioxidants serving to mitigate the effects of oxidative stress (Grace & Logan, 2000; Yamasaki & Grace, 1998), further pointing to increased photoprotection in Eutrema.
Sugar metabolism, in particular, plays a major role in several types of abiotic stress, especially in tolerance against osmotic stressors such as drought, salt and freezing temperatures. Arabidopsis was revealed to possess unique sugar metabolism with specific sugars and sugar conjugates at much higher levels than Eutrema. Particularly, much higher levels of galactinol, galactose, raffinose and melibiose suggest that the raffinose family oligosaccharides (RFOs) play a central role in overwintering of A. thaliana. The biosynthesis of RFOs begins with the galactosylation of myo-inositol to produce galactinol. Raffinose is synthesized from sucrose by the subsequent addition of activated galactose moieties donated by galactinol (Sengupta et al., 2015). RFOs have been shown to accumulate during cold acclimation of several plant species and function as compatible solutes (Hincha et al., 2006; Yan et al., 2022). The much higher level of trehalose in A. thaliana than in Eutrema implies a different role of this disaccharide in the overwintering of the two species. Trehalose is implicated in responses to cold and salinity (Lunn et al., 2014) and can serve as an osmoprotectant of biological membranes and can stabilize macromolecular structures (Crowe et al., 1998). Higher levels of raffinose and trehalose in A. thaliana than in Eutrema are consistent with findings by Zuther et al. (2018).
4.4 Common metabolic strategies of winter survival within Eutrema and A. thaliana
Despite substantial differences in the levels of a range of metabolites, common metabolic adaptation strategies to survive winter were also observed when correlating metabolite pool sizes with winter survival rates or phenological development within Eutrema or A. thaliana. Sampling for metabolite profiling was carried out in early April, at which time all accessions had initiated spring growth and presumably lost some or all of their acclimated freezing tolerance, although nights were still cold, with the average minimum temperature 5 cm above ground being 0.6°C 7 days before harvesting. Considering the advanced developmental state of the plants, it is somewhat surprising that we, in both Eutrema and A. thaliana, mostly identified metabolites that appear functionally related to winter survival. This may reflect the high metabolic interconnection between deacclimation and ontogenetic development (Pagter et al., 2017) or may be an indication that the extent of primary metabolism response to changes in growth temperature is connected to the geographical site of origin, similarly to the winter survival rate. Thus, the metabolic response of A. thaliana to a change in growth temperature differs significantly between accessions (Weiszmann et al., 2023). Also, there is intraspecific variation in the metabolic reprogramming underlying cold acclimation and deacclimation of A. thaliana (Korn et al., 2008; Zuther et al., 2015) and cold acclimation of Eutrema (Lee et al., 2012).
The most obvious common response was the positive correlations between winter survival rates and pool sizes of fructose, galactose, glucose, raffinose and sucrose in both species. The contents of glucose, fructose, sucrose and raffinose in leaves have previously been shown to be linearly correlated with leaf freezing tolerance in a larger collection of A. thaliana accessions under controlled conditions (Lee et al., 2012; Zuther et al., 2012), and these sugars were also identified as important predictors of freezing tolerance in A. thaliana (Korn et al., 2008). In Eutrema, on the other hand, biochemical data for raffinose, sucrose, glucose and fructose only found a significant positive correlation between leaf sucrose and freezing tolerance under controlled conditions (Lee et al., 2012). However, carbohydrate reserves may increase winter survival in other ways than via increased freezing tolerance, i.e. as substrates for respiration and regrowth (Bertrand, 2003). Accordingly, contents of fructose and glucose were also positively correlated with the ranked BBCH index in Eutrema, but not in A. thaliana. In both species, maltose, a degradation product of starch, was also positively correlated with the winter survival rate.
4.5 Myo-inositol has different functional roles in overwintering of Eutrema and A. thaliana
Increased winter survival of accessions with increased contents of myo-inositol and its precursors glucose-6-phosphate and myo-inositol-1-phosphate suggests an important role of the myo-inositol biosynthetic pathway in overwintering of Eutrema and highlights the inherent differences in the metabolomes and/or strategies to survive winter in the two species. Myo-inositol has been proposed to enhance cold stress tolerance in several plant species (Li et al., 2021; Zhuo et al., 2013) and myo-inositol phosphate synthase, the rate-limiting enzyme for myo-inositol biosynthesis, is upregulated when plants encounter cold, drought and salt stress (Li et al., 2021). Myo-inositol is a versatile compound that can act as a compatible solute to balance the cell turgor and as a regulator of ROS-induced programmed cell death (Loewus & Murthy. 2000; Meng et al., 2009). In A. thaliana, no correlation between winter survival and myo-inositol was observed. Instead, myo-inositol was likely conjugated with UDP-D-galactose to form galactinol, a precursor for RFO biosynthesis, as indicated by the much higher levels of galactinol, galactose, raffinose and melibiose (a raffinose degradation product) in A. thaliana, as discussed above. In accordance, winter survival of A. thaliana was also positively correlated with galactinol, galactose and melibiose. Conversely, galactinol was not detected in Eutrema and levels of galactose and melibiose did not differ between Eutrema accessions. Interestingly, both galactinol, raffinose and melibiose were negatively correlated with the ranked BBCH index in A. thaliana, suggesting that the persistence of the acclimated state may compete for these RFOs with growth resumption in the spring.
4.6 Species-specific metabolic responses of Eutrema
In addition to the sugars discussed above, the winter survival rate of Eutrema was positively correlated with ribose. Similarly, in flower buds of Ribes nigrum, seasonal changes in levels of ribose correlated with various sugars and transcript levels of several genes putatively associated with freezing tolerance (Andersen et al., 2017).
The functional significance of negative correlations between winter survival rates and several amino acids (cluster V) in the Eutrema accessions is unclear. As has been mentioned before, pyroglutamic acid is a reservoir of glutamate, which is the predominant amino donor for transamination reactions in the cell. In addition, glutamic acid is the precursor for the important osmoprotectants proline and gamma-aminobutyric acid (GABA) and chlorophyll synthesis in developing leaves (Liao et al., 2022). The α-amino group of glutamate may be transferred to oxaloacetate to form aspartate, which is a precursor of asparagine and the aspartate family of amino acids, but also associated with multiple metabolic pathways, such as protein synthesis, nucleotide metabolism, TCA cycle, glycolysis and hormone biosynthesis (Han et al., 2021). The α-amino group of glutamate may also be transferred to pyruvate to form alanine. Deacclimation and growth resumption involve major changes in protein and amino acid metabolism, including degradation of freezing tolerance-related proteins (Pagter et al., 2014), increased respiratory energy production and carbon depletion for the biosynthesis of the pyruvate, the aspartate and glutamate families of amino acids and increased protein biosynthesis (Pagter et al., 2017). Depending on the time of sampling, these changes may be reflected in higher or lower levels of specific amino acids. Alternatively, both glutamic acid, aspartic acid and alanine are glucogenic; thus, their synthesis is dependent on the carbohydrate scaffolds and higher levels in accessions with the lowest winter survival rates and generally lowest accumulation of carbohydrates could be involved in the regulation of photosynthesis intensity. Using the example of Arabidopsis, it was proved that, during low-temperature treatment, most of the carbon assimilated in photosynthesis was inserted into amino acids, preventing the accumulation of carbohydrates, which, on the way of feedback, could stop photosynthesis (Bocian et al., 2015).
4.7 Species-specific metabolic responses of A. thaliana
In A. thaliana, four polyhydroxy acids (ascorbic acid, erythronic acid, galactonic acid and threonic acid) were positively correlated with winter survival rates and negatively correlated with phenological development. Ascorbic acid is a universal antioxidant involved in the ascorbate-glutathione cycle but may also be implicated in regulating processes such as photosynthesis, floral induction and senescence (Akram et al., 2017). Exposure of A. thaliana to cold acclimating conditions has previously been shown to induce increasing levels of polyhydroxy acids in leaves (Vyse et al., 2022) and exogenous application of ascorbic acid can increase plant freezing tolerance (Min et al., 2020). Galactonic acid is an intermediate compound in one proposed biosynthetic pathway of ascorbic acid (Agius et al., 2003), while threonic acid is an ascorbic acid degradation product (Loewus, 1999).
In Arabidopsis, arabinose and xylose were negatively correlated with the phenological development of the accessions (ranked BBCH index), suggesting that modification of cell wall properties may be involved in growth resumption. Both xylose and arabinose are associated with hemicellulose component of the cell wall and their contents have previously been shown to change during deacclimation of Arabidopsis (Pagter et al., 2017). Changes in cell wall composition are known to be a component of deacclimation and growth resumption (Kutsuno et al., 2023).
The TCA cycle intermediates citric acid, succinic acid, fumaric acid and malic acid, which were all found in cluster IV, were not quantitatively related to winter survival rates, phenological development or reproductive fitness in a consistent way, suggesting that their accumulation patterns mostly reflected intraspecific variation in TCA cycle activity at the time of sampling.
5 CONCLUSIONS
In conclusion, freezing tolerance is an important, but not the sole, component of winter survival of Eutrema, which is not an extremophile with regard to winter survival when tested under temperate conditions. In both Eutrema and A. thaliana, increased winter survival entails a fitness cost, expressed either as reduced seed yield or smaller seed size. In A. thaliana accessions, flowering time and seed mass co-vary with the latitude of origin, whereas in Eutrema, intraspecific variation in reproductive fitness traits is not related to the latitudinal site of origin. The first metabolite profiling of Eutrema and A. thaliana after overwintering under field conditions verifies previous findings from controlled chamber experiments showing that the two species have distinct metabolic profiles, including differences in metabolism of amino acids, chlorogenic acids and sugars. In both species, however, increased winter survival is associated with increased pool sizes of several soluble carbohydrates. The myo-inositol biosynthetic pathway appears to play an important role in Eutrema's overwintering, whereas myo-inositol likely predominantly contributes to RFO biosynthesis in A. thaliana. Other unique metabolic responses during deacclimation include amino acid metabolism in Eutrema and levels of polyhydroxy acids and cell wall constituents in A. thaliana. Altogether, the results enable a better understanding of different inter- and intraspecific metabolic mechanisms in determining the ability to survive winter, which aids our understanding of the complex genetic and environmental factors underlying plant metabolic adaptations.
AUTHOR CONTRIBUTIONS
Ellen Zuther and Majken Pagter designed and performed the experiment. Alexander Erban and Joachim Kopka measured and annotated the metabolites. Majken Pagter analyzed the data and Ellen Zuther and Majken Pagter wrote the manuscript with contributions of Alexander Erban and Joachim Kopka.
ACKNOWLEDGMENTS
We would like to thank the plant cultivation team of the MPI-MP, especially Dirk Zerning, for help during the preparation of the field experiment, Ines Fehrle for excellent technical assistance with the GC–MS measurements and Ulrike Seider for help during the bagging of plants.
Open Research
DATA AVAILABILITY STATEMENT
All data generated during this study are included in this published article and its supplementary information files.




