Is the application of bioactive anti-stress substances with a seaweed-derived biostimulant effective under adequate growth conditions?
Abstract
The use of biostimulants in agriculture is currently emerging as a resource to increase crop productivity and quality. Additionally, the concurrent application of other bioactive materials or bioregulators alongside biostimulants can prove valuable in mitigating oxidative stress induced by various abiotic stresses. However, in some cases, these compounds applied at inadequate doses produce toxic effects under non-stressful conditions. Therefore, the objective of this study was to analyze the effects of applying various doses of a seaweed-derived biostimulant containing additional bioactive compounds (Cytolan® Stress) under optimal growth conditions. Lettuce plants were grown under control conditions without biostimulants (control plants) and with two biostimulants Cytolan® (seaweed-derived biostimulant with proline) and Cytolan® Stress applied foliarly at three doses (150, 300, and 500 mL/hL). Growth and quality parameters and physiological processes within primary and secondary metabolism were analyzed. The results identified the 300 mL/hL dose of Cytolan® Stress as the best treatment. Thus, it improved biomass production, induced photosynthetic activity, increased phytohormone, amino acids (AAs), and mineral nutrient profiles, and stimulated N assimilation. Besides, it substantially enhanced the antioxidant capacity and the concentration of various antioxidant compounds, leading to increased lettuce quality and potential stress tolerance. However, the 500 mL/hL Cytolan® Stress caused phytotoxic effects, underscoring the importance of applying the biostimulant at the proper dose.
1 INTRODUCTION
The regulation of plant growth and the mitigation of the negative effects of environmental stresses during ontogenesis are important factors determining the productivity of agricultural crops. Abiotic stress can be prevented by optimizing plant growth conditions by providing water, nutrients, and growth regulators. In addition to these conventional approaches, biostimulants are increasingly being incorporated into production systems to enhance productivity and bolster tolerance to the growing prevalence of stresses in the current climate change scenario (Jardin, 2012; Brown and Saa, 2015; Zulfiqar et al. 2020; Ma et al. 2022; Rakkammal et al. 2023).
Biostimulants represent a novel category of agricultural products distinct from fertilizers because their function and role in plant growth are independent of their nutrient content. They have recently been included in the new EU regulation on fertilizer products (Soppelsa et al. 2020). The EU officially recognizes plant biostimulants as a separate class of agricultural inputs (Carmody et al. 2020) and defines them as any product that stimulates plant growth and plant nutritional processes regardless of nutrient content to improve one or more of the following characteristics of the plant or plant rhizosphere: nutrient use efficiency, abiotic stress tolerance, quality characteristics, and nutrient availability (Caradonia et al. 2020; Poberezny et al. 2020). According to different studies, the global market is projected to exceed four billion dollars in turnover by 2025, and significant growth is anticipated on a global scale in the coming years (Carmody et al. 2020).
At present, the characteristics of biostimulants' bioactive components, the elucidation of their action mechanisms, and the plant responses at different levels, including morphological, biochemical, and metabolic levels, are the focal points of attention and research among scientists worldwide (Kocira et al. 2020). Biostimulants exhibit diverse action mechanisms, encompassing the activation of nitrogen (N) metabolism and the release of phosphorus (P) in the soil, stimulation of microbial activity, promotion of root growth, and enhancement of physiological processes such as germination, photosynthesis, nutrient absorption, growth hormone synthesis, and reduction of senescence. Furthermore, biostimulants have the capacity to mitigate the negative effects of abiotic stress factors in plants by inducing defense responses, including the activation of the antioxidant system or the synthesis of osmoprotective compounds in plants (Francesca et al. 2020; Zulfiqar et al. 2020; Franzoni et al. 2022; Ma et al. 2022; Ziaei and Pazoki, 2022).
Among the different types of existing biostimulants, those based on algae extracts are currently among the most used. Algae are generally considered an attractive biological material that provides a large number of direct and indirect benefits to the development of most crops (Ma et al. 2022). Algae extracts have gained interest as biostimulants because of their biochemical profile as they usually contain a wide variety of bioactive compounds and signaling molecules such as cytokinins, auxins, gibberellins (GAs), abscisic acid (ABA), vitamins, amino acids (AAs), polysaccharides, and mineral nutrients (Mosa et al. 2022; El Khattabi et al. 2023).
In addition, the concurrent application of other bioactive materials or bioregulators alongside biostimulants can prove valuable in mitigating the oxidative stress induced by various abiotic stresses. Consequently, the use of phytohormones, nitrogenous compounds, sugars, and polyols serves to diminish the generation of reactive oxygen species (ROS), regenerate oxidized molecules, detoxify ROS, and function as osmoprotectors. Moreover, these compounds positively regulate the expression of genes associated with stress resistance responses (Zulfiqar and Ashraf, 2021; Rodrigues de Queiroz et al. 2023). The advantageous effects of these bioactive materials have been observed in plants exposed to stress conditions. However, under optimal growth conditions, these effects may not be as conspicuous and, in some cases, negative responses that hinder plant growth have been observed. For example, in several species, the application of compounds such as ascorbate (AsA), proline, melatonin, and tocopherol during normal growth conditions has been shown to inhibit plant growth (Rodrigues de Queiroz et al. 2023).
The primary objective of this study was to analyze the effects of applying the biostimulant Cytolan® Stress, developed by Promisol S.A., under optimal growth conditions. This biostimulant not only includes an extract of Ascophyllum nodosum algae but also comprises various bioactive compounds. This study assesses the impact of different doses of two biostimulants (Cytolan®, seaweed-derived biostimulant with proline, and Cytolan® Stress) by examining growth and quality parameters and investigating fundamental physiological processes within primary and secondary metabolism. These processes encompass assessing photosynthetic efficiency, hormone, total phenols, flavonoids and anthocyanin concentrations, and nutritional status.
2 MATERIALS AND METHODS
2.1 Plant material and growing conditions
Lettuce plants (Lactuca sativa cv. Maravilla de Verano) were used. The seeds of these plants germinated and grew for 45 days in a tray with cells (cell size, 3 cm x 3 cm x 10 cm) at Saliplant SL (Carchuna, Granada, Spain). Subsequently, the seedlings were transferred to a growth chamber of the Department of Plant Physiology of the University of Granada under controlled conditions with temperatures of 25°C/15°C (day/night), relative humidity 60–80%, and a 16 h/8 h photoperiod with a PPFD (photosynthetic photon-flux density) of 350 μmol−2 s−1 (measured with an SB quantum 190 sensor, LI – COR Inc.).
Under these conditions, the plants grew in individual pots (13 cm upper diameter, 10 cm lower diameter, 12.5 cm high, and a volume of 2 L) filled with a perlite:vermiculite mixture. Fertilization consisted of a complete Hoagland-type nutrient solution, with small modifications for lettuce cultivation, composed of 4 mM KNO3, 2 mM Ca(NO3)2, 2 mM MgSO4, 1 mM KH2PO4, 1 mM NaH2PO4, 125 μM Fe-EDDHA, 50 μM H3BO3, 2 μM MnCl2, 1 μM ZnSO4, 0.25 μM CuSO4, and 0.1 μM Na2MoO4 with a pH of 5.8. The amount of nutrient solution applied each day will be approximately 50 mL per pot, never exceeding the drainage volume by 10%.
2.2 Treatments description and experimental design
The effect of foliar application of Cytolan® and Cytolan® stress products from Promisol S.A. was analyzed. The doses applied were 150, 300, and 500 mL/hL. The biostimulant Cytolan® is composed of an alkaline extract of the seaweed Ascophyllum nodosum and the amino acid proline, whereas Cytolan® Stress is composed of an alkaline extract of the seaweed Ascophyllum nodosum and proline, to which AsA, α-tocopherol, salicylic acid (SA), and mannitol have been added. These treatments were foliarly applied using a sprayer. A control treatment without the application of the biostimulant was arranged.
The application of the different treatments started 7 days after transplanting the plants in the growth chamber, and the products were applied twice with a periodicity of 15 days between each application. The experimental design consisted of a complete randomized block with 8 plants per treatment arranged in individual pots with the treatments distributed randomly in the growth chamber.
2.3 Plant sampling and biomass measurement
Sampling of the aerial part was performed 7 days after the last application of the treatments. All plants from each treatment were processed instantly for subsequent analysis. Sampling was performed 29 days after transplantation.
The plant material was rinsed and subsequently dried on filter paper to obtain the fresh weight (FW). Half of the fresh or frozen samples at −40°C were used for the analysis of the following parameters: leaf area, chlorophyll (Chl) a fluorescence (Fv/Fm, RC/ABS, PI(Abs), pigment concentration, photosynthetic efficiency (Infra-Red Gas Analyzer, IRGA-LiCOR 6400), hormonal and AA profiles, proteins and soluble sugar concentrations, FRAP and TEAC antioxidant tests, and concentration of antioxidant compounds, ascorbate, and nitrates. The remaining half of the plant materia was freeze-dried and used to determine the dry weight (DW) and the concentration of mineral nutrients. To obtain plant biomass, fresh and dry plant leaves were weighed on a laboratory scale, and their weight was expressed as g.
2.4 Plant analysis
2.4.1 Leaf area
The leaves of all plants were separated and leaf area was measured using a LI-COR optical reader, model LI-3000A. Leaf area was expressed as cm2.
2.4.2 Analysis of Chl a fluorescence
Plants were dark-acclimated for 30 min before conducting measurements. During this process, a specialized leaf clip was affixed to each leaf. Chl a fluorescence kinetics were assessed using a Handy PEA Chlorophyll Fluorimeter (Hansatech Ltd.). The OJIP phases were initiated by exposure to red light at 650 nm, with a light intensity of 3000 μmol photons m−2 s−1. Subsequently, the OJIP fluorescence phases were evaluated using the JIP test, as detailed by Strasser et al. (2000). These measurements were performed on fully mature leaves located in the central part of the plant. To examine energy flow and photosynthetic activity, we relied on the parameters obtained from the JIP test: initial fluorescence (Fo), maximum fluorescence (Fm), variable fluorescence (Fv = Fm-Fo), maximum quantum product of primary photochemistry (ΦPo = Fv/Fm), performance index (PIabs), and proportion of active reaction centers (RC) (RC/ABS) (Strasser et al. 2000).
2.4.3 Concentration of photosynthetic pigments
- Chl a = 15.65 x A666nm – 7.34 x A653nm
- Chl b = 27.05 x A653nm – 11.21 x A666nm
- Carotenoids = (1000 x A470nm – 2.86 x Chl a – 129.2 X Chl b) / 221
Chl a and Chl b concentrations were expressed as mg g−1 FW and carotenoids concentration was expressed as μg g−1 FW.
2.4.4 Analysis of gas exchange parameters
We used a LICOR 6800 Portable Photosynthesis System Infrared Gas Analyzer to collect measurements. The middle leaves were positioned within the measurement chamber to ensure that they were exposed to the best growth conditions. To ensure accuracy, the instrument was prepped for 30 min and underwent calibration before use. Measurements were performed using standard optimal cuvette conditions at 400 μmol mol−1 CO2 concentration, 500 μmol m2 s−1 photosynthetically active radiation (PAR), 60% relative humidity, and leaf temperature at 30°C. The net photosynthetic rate (A), intercellular CO2 (Ci) transpiration rate (E), and stomatal resistance (r) were recorded simultaneously. Data were stored on the LICOR device and analyzed using “Photosyn Assistant” software. The units for gas exchange parameters were as follows: A (μmol m−2 s−1), Ci (μmol mol−1), r (s cm−1), E (mmol m−2 s−1), and WUE (μmol mmol−1).
2.4.5 Non-structural carbohydrates concentration
To determine the concentration of non-structural carbohydrates (glucose + fructose + sucrose), 0.5 g of plant material was homogenized in 5 mL of 96% ethanol. The insoluble fraction of the extract was washed with 5 mL of 70% ethanol. The extract was centrifuged at 3500 g for 10 min and the supernatant was stored at 4°C for the determination of sugars according to the method described by Irigoyen et al. (1992).
2.4.6 Hormonal profile analysis
Indoleacetic acid (IAA), gibberellins (GA, GA1 + GA3 + GA4), cytokinins [CKs: trans-zeatin (tZ) + isopentenyl adenine (iP)], ABA, aminocyclopropane carboxylic acid (ACC, as precursor of ethylene), SA, and jasmonic acid (JA) were analyzed as described by Navarro-Morillo et al. (2023) using a U-HPLC-MS system. The concentration of phytohomones was expressed as ng g−1 DW.
2.4.7 NO3− determination
To determine the concentration of soluble NO3−, an aqueous extraction was performed following the method of Cataldo et al. (1975). The determination of NO3− was based on the colorimetric reaction formed by the union of NO3− with salicylic acid in a basic medium (Cataldo et al. 1975). NO3− was expressed as mg g−1 FW.
2.4.8 Nutrient concentration, N content, and nutrient utilization efficiency (NUtE)
The determination of the concentration of mineral nutrients was performed using ICP-OES. The leaf samples were subjected to a mineralization process following the Wolf (1982) method. Dry leaves (0.2 g) were taken and subjected to digestion with 30% HNO3 and H2O2 at 300°C and the mineralized product obtained was used for the analysis of ionic elements. For the determination of N concentration, 0.2 g of dry leaves and roots were ground and mineralized with 98% H2SO4 and 30% H2O2 at a temperature of 300°C, and the mineralized product was used for N analysis. The total N concentration was determined by colorimetry based on the Berthelot reaction, according to the method described by Krom (1980). The N content was calculated by multiplying the N concentration by the dry weight. To calculate nitrogen utilization efficiency (NUtE), the equation described by Xu et al. (2012) was used: NUtE = Dry biomass (g) / mg N. NUtE was expressed as g DW mg−1 N.
2.4.9 Determination of aminogram and soluble proteins
AAs were extracted and analyzed using a UHPLC system equipped with a fluorescence detector following the method described by Navarro-Morillo et al. (2023). AAs concentrations were expressed as μg g−1 DW.
To determine soluble proteins, approximately 0.5 g of plant material was homogenized with 5 mL of 50 mM phosphate buffer, pH 7.0. The homogenate was filtered with 4 layers of gauze and subsequently centrifuged at 12360 g for 15 min. The supernatant was used for the quantification of the soluble proteins. To a 0.1 mL aliquot of the supernatant, 0.9 mL of phosphate buffer was added to 50 mM pH 7.0 and 5 mL of Coomassie blue. After 20 min, the samples were measured at a wavelength of 595 nm against an albumin standard curve (Navarro-León et al. 2016). Protein concentration was expressed as mg g−1 FW.
2.4.10 Phenols, flavonoids, anthocyanins, and AsA concentration
The phenols present in the plant samples were obtained based on Rivero et al. (2001). A total of 0.1 g of the plant material was macerated, to which 0.5 mL of methanol, 0.5 mL of chloroform, and 0.25 mL of 1% NaCl were added. Subsequently, it was centrifuged at 2000 g for 10 min, obtaining the methanolic phase to which 180 μL of H2O, 240 μL of Na2CO3 and 90 μL of the 50% Folin–Ciocalteu reagent were added. After the addition of all the compounds, the mixture was shaken and incubated for 1 h at room temperature, after which the absorbance of its content was measured at 725 nm.
The total flavonoid concentration was determined according to the colorimetric method described by Kim et al. (2003) with some modifications. The same extraction process used in the determination of phenols was carried out for the plant samples. 85 μL of the methanolic phase was extracted, to which 340 μL of H2O and 26 μL of NaNO2 were added, stirred, and allowed to rest for 5 min in the dark. Afterwards, 26 μL of AlCl3 was added, turning the samples yellowish, and 170 μL of NaOH, turning them pink, stirred again, and kept in the dark for 15 min. The absorbance of the samples was measured at 415 nm.
The method of Law et al. (1983) was used to quantify the AsA concentration.
Phenols, flavonoids, anthocyanins, and AsA concentrations were expressed as mg g−1 FW.
2.4.11 Antioxidant capacity: FRAP and TEAC tests
The ferric-reducing antioxidant power (FRAP) assay was performed using the FRAP reagent. A 100 μL extract obtained by homogenizing leaves in 10 mL of methanol was added to 2 mL of FRAP reagent. Finally, absorbance was measured at 593 nm (Benzie and Strain, 1996). FRAP was expressed as mg g−1 FW.
The trolox equivalent antioxidant activity (TEAC) test was performed using the Cai et al. (2004) method. The previous extracted sample for the FRAP test was mixed with 2, 2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) reagent (Sigma-Aldrich), and then the absorbance of the mixture was recorded at 734 nm. TEAC was expressed as mM g−1 FW.
2.5 Statistical analysis
We conducted the analyses three times, and the results were subjected to statistical analysis of variance (ANOVA) with a 95% confidence interval. To compare the means of different treatments, we employed Fisher's least significant differences (LSD) test at a 95% probability level. The significance levels were denoted as follows: * for P < 0.05, ** for P < 0.01, *** for P < 0.001, and “NS” indicating no significance. Statgraphics Centurion XVI software was used to perform statistical analysis.
3 RESULTS AND DISCUSSION
3.1 Plant growth
One of the primary criteria that must define a biostimulant is the absence of phytotoxic effects and, conversely, the promotion of plant growth and productivity (Jardin, 2012). In this study, to verify the biostimulant effect of Cytolan® and Cytolan Stress®, we analyzed parameters such as the production of fresh and dry biomass of the shoot and leaf area, which are indicative of plant growth (Brown and Saa, 2015; Rakkammal et al. 2023).
Foliar application of all doses of Cytolan® produced positive effects, increasing both fresh and dry biomass compared with control plants. Regarding the application of Cytolan® Stress, we observed an improvement in the growth of lettuce plants with the application of 150 and 300 mL/hL doses. More specifically, the optimal dose for plant growth was 300 mL/hL, and Cytolan® was better than Cytolan® Stress for biomass enhancement, with increases of 35% for fresh biomass and 62% for dry biomass with respect to the data obtained in control plants (Table 1; Figure S1). Other studies also found an increase in biomass after the application of seaweed-derived biostimulants in soybean, apple, tomato, and other species (Tandon and Dubey 2015; Ma et al. 2022; Mosa et al. 2022; El Khattabi et al. 2023).
| Treatment | Fresh weight (g) | Dry weight (g) | Leaf area (cm2) |
|---|---|---|---|
| Control | 36.63 ± 1.13e | 1.77 ± 0.04e | 405.25 ± 12.58de |
| Cytolan® 150 mL/hL | 38.67 ± 1.63d | 2.08 ± 0.06d | 518.66 ± 40.76c |
| Cytolan® 300 mL/hL | 49.26 ± 1.02a | 2.86 ± 0.06a | 559.81 ± 31.49a |
| Cytolan® 500 mL/hL | 41.76 ± 1.89c | 2.25 ± 0.09c | 391.04 ± 5.29e |
| Cytolan® Stress 150 mL/hL | 41.43 ± 2.02c | 2.14 ± 0.04c | 433.31 ± 35.78d |
| Cytolan® Stress 300 mL/hL | 46.85 ± 0.73b | 2.52 ± 0.16b | 512.08 ± 19.44b |
| Cytolan® Stress 500 mL/hL | 32.48 ± 0.93f | 1.36 ± 0.15f | 335.37 ± 14.13f |
| p-value | *** | *** | *** |
- All data values represent means ± standard error (n = 8 data). The level of significance was represented as.
- *** (p < 0.001). Values with different letters indicate significant differences according to Fisher's least significant differences test.
On the other hand, the highest dose of Cytolan® Stress (500 mL/hL) resulted in a significant decrease in the shoot growth of lettuce plants (Table 1; Figure S1). Cytolan® Stress contains bioactive compounds such as AsA, α-tocopherol, proline, SA, and mannitol. However, most of the beneficial effects resulting from the application of these bioactive compounds have been observed in plants subjected to stress conditions. In plants grown under optimal conditions, these effects have not been very evident, and even negative responses have been observed, reducing plant growth. For instance, in Arabidopsis thaliana, Pistacia vera, Malus domestica, Prunus avium, and Beta vulgaris plants, the application of these compounds under normal growth conditions decreased plant growth (Rodrigues de Queiroz et al. 2023). Our results confirm these observations, suggesting that under adequate growth conditions without the presence of any type of stress, the application of Cytolan® Stress at high doses would eliminate the biostimulant effect of this product; therefore, its use at this dose would not be recommended.
Regarding the leaf area values, the 150 and 300 mL/hL doses of Cytolan® and the 300 mL/hL dose of Cytolan® Stress increased the leaf area compared with the control plants. Therefore, the application of Cytolan® and Cytolan® Stress, both at doses of 300 mL/hL, could significantly increase the photosynthetic capacity of plants by increasing the light capture area. Again, the phytotoxic effect of applying Cytolan® Stress at 500 mL/hL was observed in this parameter as a significant reduction in leaf area was observed (Table 1).
3.2 Photosynthesis-related parameters
Other studies have reported positive effects of biostimulant application on the photosynthetic process and pigments (Francesca et al. 2020; Ali et al. 2021; Franzoni et al. 2022). To establish a connection between enhanced plant growth and the induction/activation of various fundamental physiological processes in plants, we examined carbon metabolism by analyzing photochemical activity and photosynthesis efficiency. Thus, it has been demonstrated that Chl a fluorescence mirrors plant photochemical conditions and photosynthetic alterations resulting from stress factors. When metabolic disruptions occur, the plant generates fluorescence to dissipate excess energy and safeguard against stress-induced damage (Strasser et al. 2000).
Table 2 shows the Chl a fluorescence results. The Fv/Fm index in plants supplied with Cytolan® at all doses and Cytolan® Stress at low and intermediate doses presented values around 0.87, which indicates that the application of these treatments did not give rise to phytotoxicity (Abdeshahian et al. 2010). In general, the application of the biostimulant did not affect the Chl a fluorescence parameters. However, the application of Cytolan® Stress applied at the highest dose (500 mL/hL) produced a significant reduction in the Fv/Fm, RC/ABS, and PI(Abs) parameters (Table 2). The reduction in these parameters indicates that the use of this treatment produces an imbalance in the photochemical activity, increasing the emission of fluorescence by lettuce plants and reducing the energy available for the photosynthetic process (Strasser et al. 2000). These results, in turn, could explain the reduction in growth that occurs in plants receiving this treatment (Table 1).
| Treatment | Fv/Fm | RC/ABS | PI(Abs) |
|---|---|---|---|
| Control | 0.865 ± 0.001a | 0.32 ± 0.002ab | 1.92 ± 0.10a |
| Cytolan® 150 mL/hL | 0.868 ± 0.005a | 0.30 ± 0.01b | 1.91 ± 0.28a |
| Cytolan® 300 mL/hL | 0.875 ± 0.002a | 0.31 ± 0.01ab | 1.96 ± 0.16a |
| Cytolan® 500 mL/hL | 0.871 ± 0.004a | 0.32 ± 0.01a | 1.96 ± 0.28a |
| Cytolan® Stress 150 mL/hL | 0.868 ± 0.005a | 0.32 ± 0.02ab | 2.16 ± 0.18a |
| Cytolan® Stress 300 mL/hL | 0.865 ± 0.002a | 0.30 ± 0.005b | 1.97 ± 0.17a |
| Cytolan® Stress 500 mL/hL | 0.831 ± 0.005b | 0.21 ± 0.004c | 1.45 ± 0.29b |
| p-value | * | ** | * |
- Variable fluorescence/maximum fluorescence ratio (Fv/Fm), proportion of active reaction centers (RC/ABS), performance index (PIabs). Values are means ± standard deviation (n = 9 data). The levels of significance were represented as.
- * (p < 0.05) and.
- ** (p < 0.01). Values with different letters indicate significant differences according to Fisher's least significant differences test.
The concentration of Chl a, b, and carotenoids, as well as the parameters of Chl a fluorescence, are indicative of photosynthetic activity and photochemical efficiency (Li et al. 2010). In the present study, the application of Cytolan® and Cytolan® Stress at the highest doses resulted in a decrease in the foliar concentration of these pigments, which was confirmed especially in the case of Cytolan® Stress at a dose of 500 mL/hL (Table 1). Concerning the rest of the treatments, the application of Cytolan® and Cytolan® Stress at low and intermediate doses (150 and 300 mL/hL) produced a positive effect by increasing the foliar concentration of Chls, although it did not affect the carotenoids concentration with respect to the values obtained in control plants (Table 3). The increase in Chls concentration suggests a beneficial action of these biostimulants applied at these doses, improving light capture and thus the transfer of light energy to chemical energy (Brotosudarmo et al. 2018).
| Treatment | Chl a | Chl b | Carotenoids (μg g−1 FW) |
|---|---|---|---|
| (mg g−1 FW) | (mg g−1 FW) | ||
| Control | 0.20 ± 0.00d | 0.11 ± 0.002b | 24.56 ± 5.19ab |
| Cytolan® 150 mL/hL | 0.24 ± 0.01a | 0.14 ± 0.004a | 23.79 ± 1.01b |
| Cytolan® 300 mL/hL | 0.25 ± 0.01a | 0.13 ± 0.004a | 23.90 ± 1.13b |
| Cytolan® 500 mL/hL | 0.18 ± 0.01c | 0.11 ± 0.002b | 18.86 ± 1.12c |
| Cytolan® Stress 150 mL/hL | 0.23 ± 0.01b | 0.13 ± 0.004a | 28.04 ± 4.61a |
| Cytolan® Stress 300 mL/hL | 0.22 ± 0.00b | 0.12 ± 0.001b | 23.89 ± 0.93b |
| Cytolan® Stress 500 mL/hL | 0.16 ± 0.00e | 0.10 ± 0.002c | 13.49 ± 0.55d |
| p-value | *** | *** | *** |
- Values are means ± standard deviation (n = 9 data). The levels of significance were represented as.
- *** (p < 0.001). Values with different letters indicate significant differences according to Fisher's least significant differences test.
The analysis of gas exchange parameters related to photosynthesis showed that all the treatments applied, except Cytolan® Stress at a dose of 500 mL/hL, increased A and Ci levels, presenting the maximum values in plants supplied with Cytolan® and Cytolan® Stress at 300 mL/hL, respectively. However, the highest dose of Cytolan® Stress reduced the values of these parameters (Table 4), which could contribute to the lowest biomass production of plants that received this treatment (Table 1). Regarding the parameters indicative of plant transpiration, Cytolan® applied at all doses increased r and, thereby, reduced E, whereas the effect of Cytolan® Stress depended on the dose. Thus, the 300 mL/hL dose reduced r and increased E, whereas the 500 mL/hL dose produced the opposite effect with plants having the lowest E (Table 4). These results lead to a higher WUE in plants supplied with the biostimulants compared with control plants, which could be useful under water deficit conditions by allowing greater CO2 assimilation with less water loss through the stomata (Hatfield and Dold 2019). Nevertheless, the excessive closure of stomata by Cytolan® Stress 500 mL/hL could be a rapid mechanism of adaptation to stress that reduces intracellular CO2 influx, leading to a reduction in photosynthesis and, thus, to a lack of the endogenous electron acceptor NADP−, which ultimately results in ROS formation (Nxele et al. 2017).
| Treatment | A (μmol m−2 s−1) | Ci (μmol mol−1) | r (s cm−1) | E (mmol m−2 s−1) | WUE |
|---|---|---|---|---|---|
| Control | 5.68 ± 0.01f | 240.00 ± 1.67e | 7.63 ± 0.02d | 2.72 ± 0.05b | 2.12 ± 0.09f |
| Cytolan® 150 mL/hL | 6.45 ± 0.03d | 293.71 ± 0.17b | 8.23 ± 0.04c | 2.65 ± 0.01c | 2.43 ± 0.01d |
| Cytolan® 300 mL/hL | 7.21 ± 0.01a | 289.81 ± 0.16c | 8.19 ± 0.02c | 2.61 ± 0.02c | 2.76 ± 0.02c |
| Cytolan® 500 mL/hL | 6.32 ± 0.02e | 273.15 ± 0.28d | 11.00 ± 0.18b | 2.13 ± 0.00d | 2.96 ± 0.01b |
| Cytolan® Stress 150 mL/hL | 6.52 ± 0.02c | 295.05 ± 0.19b | 7.61 ± 0.003d | 2.72 ± 0.01b | 2.40 ± 0.01d |
| Cytolan® Stress 300 mL/hL | 6.94 ± 0.04b | 299.05 ± 0.46a | 7.10 ± 0.01e | 3.03 ± 0.00a | 2.29 ± 0.01e |
| Cytolan® Stress 500 mL/hL | 4.77 ± 0.01 g | 232.03 ± 1956f | 19.83 ± 0.18a | 1.41 ± 0.01e | 3.37 ± 0.02a |
| p-value | *** | *** | *** | *** | *** |
- Net photosynthetic rate (A), intercellular CO2 (Ci) transpiration rate (E), stomatal resistance (r), water use efficiency (WUE). Values are means ± standard deviation (n = 9 data). The level of significance was represented as.
- *** (p < 0.001). Values with different letters indicate significant differences according to Fisher's least significant differences test.
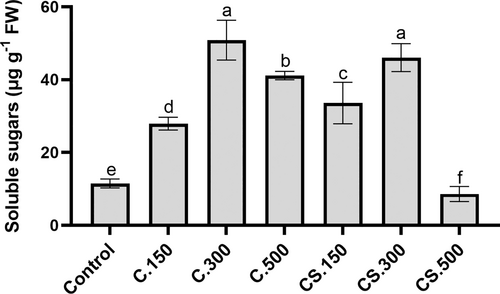
The induction of photosynthetic activity in plants usually induces carbon metabolism and the synthesis of sugars for plant growth (Cho et al. 2015). In general, the treatments of this study produced a significant increase in the concentration of soluble sugars in lettuce plants, with the highest values found in plants sprayed with 300 mL/hL dose (Figure 1). This result is directly proportional to the highest A (Table 4), and the highest biomass production observed in these plants (Table 1). In contrast, the minimum values of soluble sugars were presented in plants supplied with Cytolan® Stress (500 mL/hL dose) (Figure 1), which also supports the phytotoxic effect of this treatment by reducing photosynthesis and carbon metabolism.
3.3 Phytohormone profile
One of the beneficial effects of the application of algae extracts as a biostimulant is the exogenous supply of hormones that usually act by stimulating plant growth (Mosa et al. 2022; El Khattabi et al. 2023). The analysis of the hormonal profile is useful to explain the biostimulant effect of any compound or prototype applied to plants (Blázquez et al. 2020). In the present study, this analysis showed that both Cytolan® and Cytolan® Stress increased the concentration of the different growth hormones studied (IAA, tZ, iP, GA1, GA3, and GA4) compared with control plants, but no differences between the different applied doses were observed (Table 5).
| Treatment | Control | Cytolan 150 mL/hL | Cytolan 300 mL/hL | Cytolan 500 mL/hL | Cytolan Stress 150 mL/hL | Cytolan Stress 300 mL/hL | Cytolan Stress 500 mL/hL | p-value |
|---|---|---|---|---|---|---|---|---|
| IAA | 1.50 ± 0.10b | 1.99 ± 0.23a | 1.96 ± 0.12a | 1.87 ± 0.22a | 1.82 ± 0.22a | 1.89 ± 0.20a | 1.86 ± 0.26a | * |
| tZ | 686.19 ± 13.60b | 824.42 ± 19.86a | 817.40 ± 18.88a | 806.71 ± 16.32a | 809.02 ± 77.30a | 809.11 ± 33.58a | 815.01 ± 62.34a | * |
| IP | 0.53 ± 0.07b | 0.62 ± 0.03a | 0.60 ± 0.04a | 0.62 ± 0.05a | 0.60 ± 0.06a | 0.63 ± 0.07a | 0.61 ± 0.07a | * |
| GA1 | 0.30 ± 0.02b | 0.49 ± 0.07a | 0.51 ± 0.06a | 0.47 ± 0.03a | 0.48 ± 0.07a | 0.43 ± 0.03a | 0.44 ± 0.03a | * |
| GA3 | 0.13 ± 0.02b | 0.18 ± 0.03a | 0.21 ± 0.02a | 0.18 ± 0.02a | 0.18 ± 0.03a | 0.19 ± 0.03a | 0.20 ± 0.03a | * |
| GA4 | 0.27 ± 0.03b | 0.46 ± 0.06a | 0.44 ± 0.04a | 0.40 ± 0.04a | 0.42 ± 0.02a | 0.41 ± 0.04a | 0.42 ± 0.04a | * |
| ABA | 58.84 ± 1.59b | 59.57 ± 1.73b | 59.84 ± 1.74b | 61.79 ± 1.79b | 62.02 ± 2.65b | 60.71 ± 0.81b | 81.83 ± 5.27a | * |
| ACC | 15.13 ± 0.48b | 14.71 ± 0.12b | 14.89 ± 0.56b | 15.17 ± 0.22b | 15.25 ± 0.84b | 15.41 ± 0.65b | 22.39 ± 2.06a | * |
| JA | 407.85 ± 10.68 | 404.71 ± 7.07 | 396.85 ± 8.81 | 400.10 ± 4.40 | 402.53 ± 11.68 | 408.98 ± 7.89 | 409.44 ± 6.47 | N.S. |
| SA | 3023 ± 105b | 3118 ± 81b | 3114 ± 65b | 3133 ± 213b | 3014 ± 141b | 3078 ± 158b | 4082 ± 231a | * |
- Indoleacetic acid (IAA), gibberellin (GA), trans-zeatin (tZ), isopentenyl adenine (iP), abscisic acid (ABA), aminocyclopropane carboxylic acid (ACC), jasmonic acid (JA), salicylic acid (SA). Values are means ± standard deviation (n = 9 data). The levels of significance were represented as NS (p > 0.05) and * (p < 0.05). Values with different letters indicate significant differences according to Fisher's least significant differences test.
Regarding the stress-related hormones (ABA, ACC as a precursor of ethylene, and SA), there were no significant differences between treatments except for an increase in plants supplied with the highest dose of Cytolan® Stress. In contrast, we did not observe changes in JA concentration between the different treatments (Table 5). Cytolan® Stress contains SA as one of its bioactive compounds, which in this experiment only increased in plants supplied with the 500 mL/hL dose (Table 5). SA accumulation regulates the level of other hormones by inducing the accumulation of ABA and ethylene (Blázquez et al. 2020). The higher accumulation of ABA in the Cytolan® Stress treatment at 500 mL/hL could contribute to the lower E (Table 4), and the increase in ACC as ethylene could favor ROS accumulation (Sharma et al. 2019), which supports the phytotoxic effect of this treatment (Table 1).
3.4 Nitrogen parameters, AAs, and protein concentrations
Some studies have shown that applying biostimulants can improve the growth and productivity of plants by influencing N metabolism (Di Mola et al. 2020; Navarro-León et al. 2022). In the present study, the application of both Cytolan® and Cytolan® Stress at all doses reduced foliar NO3− concentrations but there were no differences between the different applied doses of both products (Table 6). NO3− is the predominant source of N for plants, and its decrease is usually indicative of an increase in N assimilation processes (Maathuis, 2009; Vidal et al. 2020). Alternatively, given that the biostimulants contain proline, the degradation of this AA may produce glutamate and aspartate that could act by reducing the assimilation processes and NO3− absorption due to an accumulation of organic N compounds (Iqbal et al. 2020; The et al. 2021). Indeed, this result agrees with the higher concentration of these two AAs in our experiment (Table 7). Furthermore, high NO3− levels in lettuce leaves increase the risk of certain diseases after human consumption (Mensinga et al. 2003). Therefore, the use of Cytolan® and Cytolan® Stress would improve the nutritional quality of lettuce plants intended for human consumption.
| Treatment | NO3− | N content | NUtE | Soluble proteins |
|---|---|---|---|---|
| (mg g−1 FW) | (mg N) | (g DW mg−1 N) | (mg g−1 FW) | |
| Control | 10.05 ± 0.90a | 66.86 ± 1.20e | 0.047 ± 0.001e | 0.48 ± 0.06c |
| Cytolan® 150 mL/hL | 6.07 ± 0.40b | 86.62 ± 1.29c | 0.050 ± 0.001d | 0.89 ± 0.03b |
| Cytolan® 300 mL/hL | 6.89 ± 0.30b | 114.45 ± 3.37a | 0.072 ± 0.001a | 0.97 ± 0.01a |
| Cytolan® 500 mL/hL | 6.84 ± 0.17b | 91.59 ± 3.77c | 0.056 ± 0.001c | 0.92 ± 0.04ab |
| Cytolan® Stress 150 mL/hL | 6.83 ± 0.44b | 81.49 ± 1.56d | 0.056 ± 0.002c | 0.95 ± 0.05a |
| Cytolan® Stress 300 mL/hL | 7.05 ± 0.17b | 98.67 ± 5.20b | 0.065 ± 0.002b | 0.98 ± 0.12a |
| Cytolan® Stress 500 mL/hL | 6.00 ± 0.39b | 52.46 ± 4.14f | 0.035 ± 0.002f | 0.48 ± 0.04c |
| p-value | *** | *** | *** | *** |
- Nitrogen utilization efficiency (NUtE). Values are means ± standard deviation (n = 9 data). The level of significance was represented as.
- *** (p < 0.001). Values with different letters indicate significant differences according to Fisher's least significant differences test.
| Treatment | Control | Cytolan 150 mL/hL | Cytolan 300 mL/hL | Cytolan 500 mL/hL | Cytolan Stress 150 mL/hL | Cytolan Stress 300 mL/hL | Cytolan Stress 500 mL/hL | p-value |
|---|---|---|---|---|---|---|---|---|
| Alanine | 8.90 ± 0.40c | 11.53 ± 1.12b | 15.20 ± 0.43a | 15.09 ± 0.84a | 11.91 ± 0.60b | 14.51 ± 0.65a | 14.74 ± 1.36a | *** |
| Arginine | 2.53 ± 0.16c | 3.20 ± 0.21b | 4.34 ± 0.12a | 4.72 ± 0.33a | 3.38 ± 0.36b | 4.20 ± 0.24a | 4.68 ± 0.57a | *** |
| Aspartate | 34.37 ± 2.23d | 38.10 ± 1.50c | 42.03 ± 1.70b | 41.61 ± 2.08b | 41.12 ± 0.76b | 45.00 ± 0.14a | 45.78 ± 1.82a | *** |
| Glutamate | 40.31 ± 0.85d | 45.22 ± 1.80c | 50.97 ± 0.68b | 51.79 ± 1.58b | 51.05 ± 0.68b | 56.99 ± 1.00a | 56.36 ± 1.78a | *** |
| Histidine | 3.16 ± 0.14 | 3.43 ± 0.41 | 3.39 ± 0.33 | 3.27 ± 0.30 | 3.22 ± 0.19 | 3.49 ± 0.43 | 3.36 ± 0.15 | N.S. |
| Isoleucine | 618.43 ± 5.22 | 621.50 ± 8.55 | 627.23 ± 4.36 | 626.06 ± 8.76 | 625.95 ± 5.90 | 623.75 ± 9.70 | 617.28 ± 5.11 | N.S. |
| Leucine | 418.05 ± 5.06 | 426.89 ± 4.57 | 416.56 ± 5.44 | 427.01 ± 4.21 | 415.05 ± 4.09 | 425.46 ± 3.90 | 424.67 ± 8.48 | N.S. |
| Lysine | 3.59 ± 0.13 | 3.63 ± 0.06 | 3.59 ± 0.41 | 3.63 ± 0.28 | 3.41 ± 0.13 | 3.22 ± 0.26 | 3.38 ± 0.33 | N.S. |
| Phenylalanine | 322.30 ± 4.13c | 360.18 ± 3.00b | 398.01 ± 7.82a | 395.45 ± 4.60a | 356.06 ± 7.88b | 404.47 ± 5.59a | 400.09 ± 11.16a | *** |
| Proline | 56.58 ± 2.22d | 65.97 ± 3.09c | 82.97 ± 3.39b | 83.53 ± 2.96b | 78.81 ± 1.15b | 96.98 ± 2.17a | 96.24 ± 3.46a | *** |
| Serine | 15.62 ± 1.22c | 19.86 ± 0.53b | 25.90 ± 0.82a | 26.84 ± 0.30a | 20.67 ± 1.39b | 26.31 ± 0.46a | 24.67 ± 0.58a | *** |
| Threonine | 10.33 ± 0.86 | 10.57 ± 0.40 | 10.88 ± 0.60 | 10.40 ± 0.35 | 10.83 ± 0.30 | 10.44 ± 0.95 | 10.91 ± 1.58 | N.S. |
| Tryptophan | 81.60 ± 2.11c | 91.06 ± 0.67b | 103.10 ± 3.13a | 103.55 ± 3.00a | 93.68 ± 1.75b | 106.95 ± 1.70a | 106.76 ± 2.17a | *** |
| Tyrosine | 94.25 ± 3.62 | 92.01 ± 1.08 | 96.94 ± 2.33 | 95.65 ± 1.92 | 94.42 ± 0.99 | 93.75 ± 0.70 | 94.65 ± 2.77 | N.S. |
| Cysteine | 6.21 ± 0.24 | 6.53 ± 0.37 | 5.90 ± 0.24 | 5.86 ± 0.64 | 5.78 ± 0.53 | 6.62 ± 0.18 | 5.98 ± 0.07 | N.S. |
| TOTAL | 1716 ± 7d | 1799 ± 18c | 1887 ± 10b | 1894 ± 19b | 1815 ± 13b | 1922 ± 15a | 1909 ± 25a | *** |
- Values are means ± standard deviation (n = 9 data). The levels of significance were represented as NS (p > 0.05) and.
- *** (p < 0.001). Values with different letters indicate significant differences according to Fisher's least significant differences test.
The application of biostimulants also has the objective of increasing NUtE, improving production with lower N fertilization (Laurent et al. 2020; Navarro-León et al. 2022). In our experiment, the application of Cytolan® and Cytolan® Stress increased the total N concentration and content and NUtE compared with the control plants, except for the 500 mL/hL dose of Cytolan® Stress, which reduced these parameters with respect to the control (Table 6 and S1). The maximum values for N content and NUtE occurred with the application of 300 mL/hL of Cytolan® (Table 6). These results, together with the induction of photosynthetic efficiency (Table 2) and the increase in the concentration of growth-promoting hormones (Table 5), could explain the positive effect of Cytolan® and Cytolan® Stress applied at 300 mL/hL in increasing plant growth (Table 1). Furthermore, the use of these biostimulants could reduce the application of N fertilizers without impairing crop yields, which would reduce costs and environmental impact.
As we delve further into the examination of various nitrogenous compounds, the analysis of AAs profile becomes crucial, not only because of their significance in plant growth but also because of their regulatory influence on N absorption and assimilation processes (Iqbal et al. 2020; The et al. 2021). The application of Cytolan® product and Cytolan® Stress at all dosage levels increased the total concentration of soluble AAs, with the highest values occurring in plants supplied with the Cytolan® Stress (300 and 500 mL/hL doses) (Table 7). The greater presence of glutamate and aspartate in plants supplied with the biostimulants could have a positive effect on plant growth since these AAs are nitrogenous compounds that transport to growing areas through the phloem and also act as regulators of N absorption by the roots (Iqbal et al. 2020; The et al. 2021; Trovato et al. 2021).
In addition to soluble AAs, the foliar concentration of soluble proteins in our experiment showed an increase in all Cytolan® and Cytolan® Stress treatments, except for Cytolan® Stress at a high dose (500 mL/hL) (Table 6). The increase in soluble proteins, especially with the application of the dose of 300 mL/hL for Cytolan® and Cytolan® Stress, would explain the increase in plant growth caused by these treatments (Table 1) since soluble proteins represent the levels of cellular enzymes involved in essential processes such as N assimilation, photosynthesis, carbon metabolism, etc. (Iqbal et al. 2020; The et al. 2021). The concentration of soluble proteins also indicates that the application of Cytolan® Stress at a high dose is not suitable for inducing plant growth because a similar soluble protein concentration was observed in this treatment compared with control plants (Table 6).
3.5 Mineral element concentrations
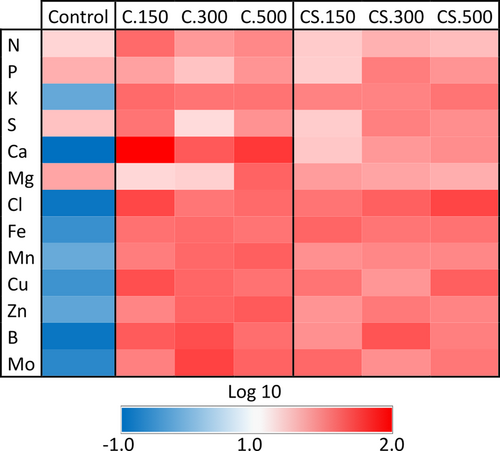
Another positive effect of biostimulant application is the improvement of the nutritional state of plants through the increased accumulation of essential mineral elements (Jardin 2012; Papa et al. 2022). The concentration of mineral nutrients in the leaves is presented in Figure 2 and Table S1. In general, the values obtained can be considered normal, and nutrients were not in a range of excess or deficit that could limit the normal growth of plants (Epstein and Bloom, 2005). There were no differences in the concentration of any nutrient between the Cytolan® and Cytolan® Stress treatments, but K, Ca, and micronutrients concentrations were higher compared to control plants with the application of both biostimulants (Figure 2 and Table S1). The increment in K and Ca could be significant given their crucial physiological functions and the fact that they contribute to tolerance to stresses such as salinity stress (Capula-Rodríguez et al. 2016; Navarro-León et al. 2020). Furthermore, the increase in micronutrient concentration can contribute to the enhanced functionality of antioxidant enzymes, photosynthesis, and N assimilation, as these nutrients play pivotal roles in these processes (Epstein and Bloom, 2005; Hänsch and Mendel, 2009).
3.6 Antioxidant compounds
Several studies have also demonstrated that the application of biostimulants can increase the accumulation of compounds with antioxidant activity in plants (Francesca et al. 2020; Franzoni et al. 2022; Ma et al. 2022). The results showed that the application of Cytolan® Stress, which represents an extra contribution of a phenolic compound such as SA and of AsA, determined a higher foliar concentration of total phenols and AsA. In addition, the maximum values were presented in the Cytolan® Stress treatment at a dose of 500 mL/hL. Likewise, the application of Cytolan® also increased the foliar concentration of these compounds compared with control plants, but to a lesser extent than the application of Cytolan® Stress (Table 8).
| Treatment | Total phenols | Flavonoids | Anthocyanins | AsA |
|---|---|---|---|---|
| (mg g−1 FW) | (mg g−1 FW) | (mg g−1 FW) | (mg g−1 FW) | |
| Control | 1.27 ± 0.05e | 0.98 ± 0.05f | 0.44 ± 0.01e | 0.21 ± 0.002e |
| Cytolan® 150 mL/hL | 1.29 ± 0.06e | 1.12 ± 0.08e | 0.54 ± 0.01c | 0.23 ± 0.04e |
| Cytolan® 300 mL/hL | 1.42 ± 0.13d | 1.26 ± 0.07d | 0.54 ± 0.01c | 0.29 ± 0.04d |
| Cytolan® 500 mL/hL | 1.61 ± 0.05c | 1.34 ± 0.14d | 0.49 ± 0.02d | 0.31 ± 0.03d |
| Cytolan® Stress 150 mL/hL | 2.45 ± 0.25c | 1.69 ± 0.08c | 0.68 ± 0.05b | 0.41 ± 0.02c |
| Cytolan® Stress 300 mL/hL | 3.21 ± 0.22b | 2.07 ± 0.15b | 0.73 ± 0.02b | 0.48 ± 0.003b |
| Cytolan® Stress 500 mL/hL | 4.32 ± 0.31a | 3.31 ± 0.14a | 0.96 ± 0.08a | 0.60 ± 0.02a |
| p-value | *** | *** | *** | *** |
- Ascorbate (AsA). Values are means ± standard deviation (n = 9 data). The level of significance was represented as.
- *** (p < 0.001). Values with different letters indicate significant differences according to Fisher's least significant differences test.
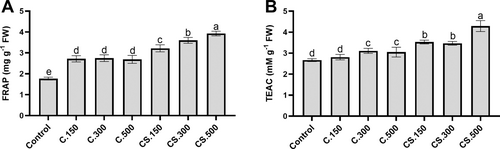
The results clearly suggest that the application of Cytolan® Stress was more effective in inducing the antioxidant capacity of lettuce plants because the application of all doses showed a very significant increase in both the values of the FRAP and TEAC tests. The maximum values were found in plants supplied with Cytolan® Stress applied at 500 mL/hL. Likewise, the application of Cytolan® at all doses increased the values of FRAP and TEAC to a lesser extent than Cytolan® Stress (Figure 3). Therefore, another additional positive effect of the assayed biostimulants could be the attainment of plants that are more adept at facing oxidative stress (Rodrigues de Queiroz et al. 2023). Furthermore, Cytolan® application could deliver plants with enhanced nutritional quality for human consumption; AsA and phenolics being of significant interest in preventing specific diseases (Baslam et al. 2013).
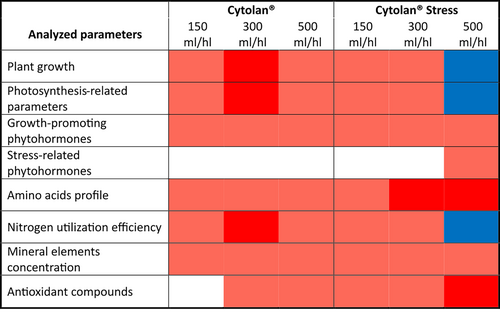
In conclusion, this study identified the application of Cytolan® Stress at a dose of 300 mL/hL as the most advantageous treatment for lettuce cultivation. Thus, this biostimulant substantially improved lettuce growth, probably by inducing photosynthetic activity and enhancing phytohormone, AAs, and mineral nutrient accumulation. Furthermore, the biostimulant improved NutE; thereby, this product could allow the reduction of N fertilizers without impairing the yield of the crops, reducing costs and environmental impact. Additionally, it substantially augmented the antioxidant capacity and concentration of various antioxidant compounds, leading to increased lettuce quality and potential stress tolerance. However, it is worth noting that applying Cytolan® Stress at doses exceeding 300 mL/hL, although still elevating antioxidant capacity, eliminated the beneficial effect on plant biomass production, underscoring the importance of adhering to recommended dosages (Figure 4).
AUTHOR CONTRIBUTIONS
JMR and BB conceived and designed the research. DVC and SAC conducted experiments. ENL, SAC, and DVC analyzed the data. JMR and ENL wrote the manuscript. JMR and BB did a critical revision of the article. All authors read and approved the manuscript.
FUNDING INFORMATION
This work was supported by the PAI program (Plan Andaluz de Investigación, Grupo de Investigación AGR282).
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.




