Comprehensive analysis of Trichoderma ressei mediated CO2 stress attenuating responses and de novo transcriptome sequencing of rice flag leaf
Abstract
The objective of this study was to investigate the alterations in physiological, biochemical, and transcriptional reactions in the flag leaf of rice following the application of Trichoderma as a biofertilizer (BF) at both ambient (aCO2; 395 ppm) and enhanced (eCO2; 550 ppm) carbon dioxide levels. A comparatively smaller percentage change was observed in the photosynthetic parameters, including photosynthetic rate, respiration, and stomatal conductance, subsequent to the introduction of BF in the elevated carbon dioxide (eCO2) environment. Furthermore, biochemical analysis revealed a noteworthy elevation in photosynthetic pigments, total sugar, proline, and ascorbate peroxidase concentration levels in plants treated with BF under ambient and increased carbon dioxide conditions. The buildup of indole-3-acetic acid (IAA) was shown to be increased in rice treated with BF under both conditions. Moreover, ICP-MS analysis demonstrated an augmentation in the levels of vital micronutrients in the experimental group while observing no alterations in the carbon-to-nitrogen ratio within the flag leaf. The examination of transcriptional responses in the flag leaf demonstrated an increase in the expression of genes associated with the photosynthetic system, amino acid metabolism, several transporters, including aquaporin, and the phosphate pathway, as well as carbon and nitrogen metabolism. Hence, our work elucidates the effective role of Trichoderma in mitigating the adverse consequences associated with elevated levels of atmospheric carbon dioxide (eCO2).
1 INTRODUCTION
Atmospheric CO2 concentrations (CO2) have significantly increased since pre-industrial times, approximately from 280 ppm to 414.5 ppm by 2020 (https://gml.noaa.gov/ccgg/trends/). The current anticipated models for future greenhouse scenarios forecast that the increase in CO2 concentration will not stabilize but rather increase gradually over the coming decades (IPCC, 2020).To try and get optimum crop yields with the frequent changes in climate, various studies have been conducted on plants as well as ecosystems (De Boeck et al., 2011; Smith, 2011; Bauweraerts et al., 2013). There is a need to study the effect of continuously increasing environmental CO2 on rice productivity and yield. For this, a natural open system is required, which allows researchers to study its effects, known as the Free Air Concentration Enrichment System (FACE) (Lesaulnier et al., 2008, Mishra et al., 2019).
Rice is cultivated on 160 million hectares of land in approximately 112 countries (Bruinsma, 2003; FAOSTAT, 2016). According to a report by FAOSTAT 2018, world rice production was around 760 million tons in 2017–2018 but was reduced to 750 million in 2018–2019. The fate of the grain is decided by the last leaf that emerges on a mature flowering stem, the so called flag leaf. The flag leaf exhibits higher photosynthetic capacity due to its position at the top of the canopy, which facilitates higher interception of light than the lower canopy leaves (Adachi et al., 2017). The remaining photosynthates utilized during grain filling are provided by the leaf present below the flag leaf and by the remobilization of reserve carbohydrates in leaf sheaths and older senescing leaves (Li et al., 2017; Lin et al., 2018). This enhanced rate of photosynthesis employs a physiological load on the plants through improper assimilation and allocation of photosynthetic assimilates to the various organs of the plant, resulting from an increase in plant biomass (Makino & Mae, 1999, Kimball, 2016). The effects of elevated carbon dioxide CO2 (eCO2) have been investigated at physiological, biochemical and genome-wide transcription levels in Arabidopsis thaliana, which mitigates the effects of heat waves as well as drought conditions (Zinta et al., 2014).
Several agronomic strategies are adopted to enhance and mitigate the negative impacts of biotic as well as abiotic stresses. In recent years, there has been an increase in the use of bio-fertilizers (BFs), which have emerged as a solution to the problem of nutrient supply systems as well as yield enhancement of crops during stress conditions. Bacillus amyloquifaciens positively modulates the defense system in coordination with genome-wide altered transcription in rice to combat salt stress (Chauhan et al., 2019). Among the various microorganisms used as BFs, several species of the fungi Trichoderma have established in plant growth promotion through various direct and indirect mechanisms, such as mycoparasitism, nutrient uptake facilitation by mobilizing elements such as carbon, phosphorous, nitrogen, and sulfur to the plants. The amendment of Trichoderma as BFs in the soil has been reported to enhance the yield and nutritional value of tomatoes with only minimal use of chemical fertilizer (Molla et al., 2012). Contreas-Cornejo et al. (2014) revealed the suppression of the pathogen Botrytis cinera in A. thaliana by 12%, which causes necrosis, chlorosis and death in 80% of infected seedlings. Trichoderma-treated white maize plants were found to be more robust and greener in the field of the Democratic Republic of the Congo, possibly due to an increase in nitrogen use efficiency (Harman, 2011). Trichoderma is well known for its diverse nature (Mahfooz et al., 2016). The role of Trichoderma at the transcript level was studied in wheat and found to accelerate nitrogen uptake in coordination with other metabolic pathways (Rubio et al., 2019). The root treatment of plants with Trichoderma caused significant changes in the transcripts during the multiple stresses (Brotman et al., 2013). One of the studies reported an alteration in signal transduction pathways that were controlled by phytohormones, such as salicylic acid (SA), jasmonic acid (JA), and auxins, after the treatment of Trichoderma during salt stress. It was found that IAA, antioxidants, and osmoprotectants were enhanced in plants (Contreras–Cornejoet al., 2014).
The effects of carbon enrichment have been studied in many crops at physiological and biochemical levels at several growth stages (Ainsworth et al., 2005, Thompson et al., 2017). However, the role of microbes, especially fungi, in the flag leaf of rice has never been investigated in elevated CO2 conditions at the transcriptomic level. This study was conducted on two contrasting rice varieties, Heena (drought resistant) and Kiran (drought sensitive), with supplementation of Trichoderma (BF) at the vegetative stage during ambient CO2(aCO2) and elevated CO2(eCO2) at the Free Air Carbon-di-oxide Enrichment (FACE) site. Our previous study revealed adaptive behaviour in BF-treated plants during eCO2 by reducing percent change in yield-related traits, increasing photosynthetic capacity and significant changes in roots and microbial diversity in the rhizospheric region (Mishra et al., 2019). Since the flag leaf is a major yield determinant in rice, our current study elaborates on the multifaceted role of BFs in adaptive response at the physiological, biochemical and transcriptional levels of the rice flag leaf (panicle stage) during eCO2 conditions.
2 MATERIALS AND METHODS
2.1 FACE site
The experiments were carried out at the FACE facility, which was established at the National Botanical Research Institute in Lucknow, India (26°51′28.55′′N, 80°56′52.22′′E) (Pandey et al., 2017, Surabhi et al., 2020). The FACE system is made up of six hexagonal rings, each with a diameter of 10 m, three of which were randomly assigned to elevated CO2 treatments (eCO2) and the remaining three to ambient CO2 treatments (aCO2). The compressed CO2 gas is released from pipes for the maintenance of CO2 concentration at the perimeter of therings during daylight hours. The rings arecovered with nylon nets for protection from birds, and the wind direction was continuously directed towards the upwind side of the ring. The computer program, SCADA was used to measure and control the amount of CO2 in the elevated and ambient CO2 rings.
2.2 Crop cultivation, treatments and sampling-
The seedlings of two contrasting rice cultivars, Heena(drought resistant) and Kiran (drought-sensitive) (Oryza sativa sp. indica) (Agarwal et al., 2016) were transplanted manually into the rings of aCO2 and eCO2 on August 8th 2017 and July 17th 2018. The transplantation process involved the utilization of rice seedlings that were approximately 22–25 days old. The seedling roots underwent a pretreatment process in which they were exposed to Trichoderma ressei at a concentration of 6.0 × 106 colony-forming units per milliliter in sterile distilled water for 60 min (Roberts et al., 2010; Mishra et al., 2019). During the weeding procedure, the soils were regularly aerated at specific intervals. The control and BF-treated seedlings were transplanted in both aCO2 and eCO2 rings in pots and plots at a density of 3–5 seedlings per hill in four cone-shaped plots (36 m2). The plots and pots were regularly watered with brief aeration by ploughing for BF to maintain the aerobic conditions. The average temperature and relative humidity (RH) of the two seasons were found to be 32.0°C and 44.6%, respectively. The average CO2 in the surroundings was 400.7 ppm, whereas in the elevated rings, it was around 550.0 ppm. Different treatments of both varieties used for the study have been defined as Heena Ambient Control (CHA), Heena Ambient Treated (THA), Heena Elevated Control (CHE), Heena Elevated Treated (THE) and Kiran Ambient Control (CKA), Kiran Ambient Treated (TKA), Kiran Elevated Control (CKE), Kiran Elevated Treated (TKE). All the studies were conducted at the flag leaf at the panicle stage for both rice cultivars.
2.3 Gas exchange and biochemical parameters of rice plant grown under eCO2
Fully expanded flag leaves at an early flowering stage were used for the measurement. Three plants from different pots of each set were randomly selected for measurements. LICOR model 6400 equipped with CO2 control modules and LED light sources at a photosynthetic photon flux density (PPFD) of 1200 μmol m−2 s −1was used to study the light-saturated rate of CO2 assimilation (Asat), stomatal conductance (gs), maximal efficiency of PSII (Fv/Fm) and intercellular CO2 (Ci). Leaf temperature was maintained at 25°C, and water pressure deficit was kept constant at 1.0 to 1.5 kPa. Sample cell H2O and flow rates were 20 mmol H2O mol−1 and 500 μmols−1, respectively. Water use efficiency (WUE) was calculated by the ratio of AN (net photosynthesis) to transpiration rate (E). The data was recorded of fully grown flag leaves from all treatments at 6 to 9 am (Guha et al., 2018). Total chlorophyll estimation was done according to Arnon (1949). Sugar estimation in flag leaf was performed using 0.2 g of fresh leaves crushed with 80% methanol as per the protocol of Cross et al. (2006).
Proiline estimation: 100 mg of fresh samples was homogenized for proline estimation in 1 mL of 70% ethyl alcohol and centrifuged. The 50 μL of ethanolic extract from the supernatant was taken and further allowed for reaction. The reaction mixture comprised of ninhydrin (1%w/v) prepared in acetic acid (60%v/v)and ethanol (20%v/v) and incubated at 95°C for 20 min. After completion of the reaction, the absorbance was recorded at 520 nm (Carillo & Gibbon, 2011).
Total Phenolic Content: TPC was quantified according to Zheng and Shetty (2000) by using Folin–Ciocalteu reagent. The obtained absorbance at 725 nm was quantified by the calibration curve of Gallic acid and converted to mM Gallic acid equivalent (GAE) g−1 FW.
Lipid peroxidation: The level of lipid peroxidation in the leaf tissue was measured as content of malondialdehyde (MDA) equivalents using the 2-thiobarbituric acid (TBA) method (Heath and Packer et al., 1968).
2.4 Enzymatic and non-enzymatic assays
These assays were performed on rice flag leaf of both cultivars with and without treatment under ambient and elevated CO2. Plant tissue weighing 1 g was taken and mixed with 1% polyvinyl pyrrolidone (w/v), 0.3 mM phenylmethyl sulfonyl fluoride (PMSF), and 1 mM EDTA in a 10 mM sodium phosphate buffer (pH 6.0) at 4°C. Homogenized samples were centrifuged at 17000 g for 10 min to eliminate the impurities and plant debris from the enzymatic extract. The total protein content of the enzymatic extract was evaluated according to the Bradford method (Bradford, 1976) and calculated by using the calibration curve of Bovine Serum Albumin (BSA). All the enzymatic assays were performed in triplicate and documented as the average of three parallel measurements. The activities of all the enzymes were assessed spectrophotometrically.
Superoxide dismutase activity - (SOD, EC 1.15.1.1) was determined by measuring the inhibition of photochemical reduction of nitro blue tetrazolium (NBT) (Beyer & Fridovich, 1987). The photo reduction of NBT was measured at 560 nm, and one unit of SOD was defined as being equivalent to the volume of extract that caused inhibition of the photo-reduction of NBT by 50%.
Catalase activity - CAT activity was determined on the basis of H2O2 degradation method according to Rao (1996). Furthermore, the oxidation rate of ascorbic acid was measured by the decrease in absorbance at 290 nm, which was the principle based on which ascorbate peroxidase (APX, EC 1.111.11) activity was measured. The method described by Nakano and Asada (1981) was followed, and activity was calculated using the extinction coefficient of 2.8 mM−1 cm−1.
Phytohormone analysis - For phytohormone analysis, fully expanded flag leaves of both rice varieties were collected aseptically for the estimation of endogenous phytohormones viz. gibberellic acid (GA), indole- 3-acetic acid (IAA), and abscisic acid (ABA) by using the prescribed method of Pan et al., (2010). The phytohormone estimation was performed on a HPLC system (Waters 2474) using a reverse phase C-18 column (250 × 9.4 mm; 5 μm; Phenomenex Luna). The mobile phase contained 30 mM orthophosphoric acid in HPLC-grade water (component A) and acetonitrile (component B) in a 70:30 ratio. HPLC was run at 0.5 mLmin−1 for 40 min at 280 nm wavelength. The different concentrations of phytohormones were examined using external standards (Sigma Aldrich).
2.5 Microelement analysis
Micro-elemental deposition in all the treatments was analyzed by Inductively Coupled Plasma Mass Spectrometry (Agilent 7500CX, Agilent Technologies). Flag leaves of both varieties were oven dried and finely powdered (0.1 g) and digested with 3.0 mL of HNO3 (Sigma) and 1.0 mL of H2O2 (Sigma). Reference standards of chromium (Cr), manganese (Mn), iron (Fe), nickel (Ni), copper (Cu), zinc (Zn), selenium (Se) and molybdenum (Mo) (E-Merck) were used for calibration.
The C and N content analysis of flag leaf was performed on IRMS by following the Singh et al. (2016) and Upreti et al. (2021) method. For the analysis of carbon isotope composition, flag leaf was oven-dried at 70°C for 72 h and fine powdered. This was analyzed with a mass spectrometer, Delta V Plus IRMS isotope ratio mass spectrometer (Thermo Electron Corporation), which was interphased with a combustion device, Flash EA 1112, via TC/EA gas control unit (Thermo Finnigan).
2.6 Total RNA isolation and cDNA library preparation and sequencing
Frozen flag leaves were ground in liquid nitrogen and total RNA was extracted using the Trizol method. The quality and quantity of total RNA were analyzed by agarose gel and spectrophotometric analysis (ND1000 NanodropTechnologies). An equal amount of total RNA from three different preparations was pooled and used for further processing. The integrity of RNA samples, with a concentration of approximately 0.2–1.5 μgμl−1, were checked on an Agilent Bioanalyzer 2100 (Agilent Technologies) using the RNA 6000 Nano kit. RNA samples with a RIN-value ≥8.0 were used for sequencing analysis. RNA true seq libraries were prepared using True Seq RNA library preparation Kit v2 (Illumina) and subjected to sequencing on the Illumina HiSeq2000 platform (Illumina) (Langmead & Salzberg, 2012). All the sequencing files were generated in the fastaq file format and deposited in the National Centre for Biotechnology Information (NCBI) database with the accession number (SUB10388612).
2.6.1 For assembly, mapping and assessment of gene expression
Low-quality Illumina reads and adapters were trimmed with the trimmomatic software (Bolger et al., 2014). The filtered good-quality reads of all samples of Heena and Kiran were mapped on to the Oryza sativa Nipponbare reference genome version (ftp://ftp.ensemblgenomes.org/pub/plants/release-51/fasta/oryza_sativa/dna/) using STAR aligner (Dobin et al., 2013). The uniquely mapped reads of each library were extracted through samtools (Li et al., 2009) and used for further analysis. Uniquely mapped reads were further subjected to the assembly using the Cufflinks program with the rice genome annotation file (ftp://ftp.ensemblgenomes.org/pub/plants/release-49/gff3/oryza_sativa) (Shannon et al., 2003; Trapnell et al., 2009). The pair-wise differential expression analysis (Control vs Elevated Carbon, Control vs Ambient Trichoderma, Control vs Elevated Trichoderma) was conducted using the edgeR program in the R package for both the Heena and Kiran variety (Robinson et al., 2010). Differentially expressed genes (DEGs) were identified for downstream analysis with the p-value <0.01,q-value (FDR value) < 0.05, and log2 fold change ≥ +/− 1 parameters. The genes whose log2fold change >1 were considered upregulated genes while log2fold change<1 was represented as downregulated genes in our analysis.
Gene Ontology (GO) terms for significant DEGs for all samples were retrieved from the AgriGO database (Du et al., 2010) for biological, molecular and cellular processes. The involvements of all sets of genes in different pathways were analyzed through the KEGG (Kyoto Encyclopedia of Genes and Genomes) analysis. The detailed fine mapping of pathway analysis was performed with Page Man analysis (http://mapman.mpimpgolm. mpg.de/pageman/) with an average value using Benjamini Hochberg testing correction using Mapman (Benjamini & Hochberg, 1995).
2.7 Quantitative real-time (qRT)-PCR analysis
Real-time PCR was done on Stratgene Mx3005P instrument (Agilent Technologies) using diluted cDNA by using qPCR mix cyber green of Thermofisher Scientific. Each sample was prepared in a reaction mixture containing 5 μl power SYBR® Green PCR Master mix, 10 μM of forward and reverse primer, and 1 μL 5-fold diluted cDNA, making the volume up to 10 μL with DNase/RNase free water. Primers for qRT-PCR analysis were designed using IDT software (Table S1). An endogenous control of the Actin gene was used for normalization of Ct values of the selected genes. The thermal cycling conditions were kept as mentioned in Shivhare and Lata (2016). The reactions were performed in three biological replicates and three technical replicates for every biological sample. The heat maps for expression profiles of genes were produced using the MEV4 software package already described earlier (Saeed et al., 2003).
2.8 Statistical analysis
The statistical analysis of the data was performed using SPSS software version 18.0. Statistical analysis of the data was performed for each parameter. Mean values and standard deviations were calculated for all the datasets. Turkey's multiple comparison tests were done in more than one group by using one-way ANOVA. The results were considered significant at p < 0.05. A three-way analysis of variance (ANOVA) was performed, including variety, CO2 and Trichoderma as fixed factors to find the effect on flag leaf physiological and biochemical responses.
3 RESULTS
3.1 Photosynthetic parameters of rice grown under elevated CO2 condition after treatment of Trichoderma
The impact of CO2 stress on physiology and the possibility of its amelioration by Trichoderma on the two-rice varieties, Heena and Kiran, were studied. Photosynthetic rate, respiration, stomatal conductance, transpiration and water use efficiency were measured as percentage changes between control and treated varieties grown under aCO2 and eCO2 conditions. Our data showed a reduced percent change in photosynthetic rate (9.7%), respiration (12%) and water use efficiency (15.6%) after the addition of BF in eCO2 conditions in Heena as compared to controlled aCO2 conditions, where the photosynthetic rate (15.3%), respiration (19%) and water use efficiency (30.5%) were recorded higher. Contrary to Heena, the Kiran variety responded with a higher percent change in photosynthetic rate (11.3%), respiration (14.1%), stomatal conductance (−35.6%), water use efficiency (23.9%) and transpiration (−20.8%) as compared to the Heena variety. The three-way ANOVA revealed a significant influence of interactions (cultivar × CO2× BF treatment) on the photosynthetic rate and water use efficiency in both the variety (Table 1).
| Varieties | Treatments | Photosynthesis | Respiration | Stomatal Conductance | Water Use Efficiency | Transpiration |
|---|---|---|---|---|---|---|
| Heena | Ambient control | 15.6 ± 1.08 | −1.2 ± 0.2 | 0.09 ± 0.04 | 12.1 ± 1.8 | 3.5 ± 1.5 |
| Elevated Control | 18 ± 1.34 | −1.5 ± 0.2 | 0.06 ± 0.03 | 15.8 ± 2.1 | 2.2 ± 1.3 | |
| % change | 15.3 | 19 | −35.2 | 30.5 | −36.7 | |
| Ambient Treated | 19.6 ± 2.2 | −1.4 ± 0.2 | 0.12 ± 0.02 | 16.5 ± 1.7 | 3 ± 0.7 | |
| Elevated Treated | 21.5 ± 0.8 | −1.6 ± 0.1 | 0.06 ± 0.02 | 19.1 ± 1.6 | 2.3 ± 0.9 | |
| % change | 9.7 | 12.3 | −1.1 | 15.6 | −22.1 | |
| Kiran | Ambient control | 15.8 ± 0.3 | −1.4 ± 0.1 | 0.15 ± 0.00 | 11.4 ± 0.2 | 4.4 ± 0.08 |
| Elevated Control | 17.6 ± 0.9 | −1.6 ± 0.6 | 0.1 ± 0.02 | 14.1 ± 1.2 | 3.5 ± 0.5 | |
| % change | 11.3 | 14.1 | −35.6 | 23.9 | −20.8 | |
| Ambient Treated | 13.7 ± 0.7 | −1.2 ± 0.08 | 0.13 ± 0.03 | 9.9 ± 1.1 | 3.8 ± 1.0 | |
| Elevated Treated | 19.6 ± 1.2 | −1.4 ± 0.2 | 0.09 ± 0.03 | 16 ± 2.4 | 3.6 ± 1.5 | |
| % change | 11.3 | 10.3 | −29.6 | 61.5 | −4.1 | |
| Cultivar | *** | *** | *** | *** | *** | |
| CO2 | *** | ns | *** | *** | *** | |
| Treatment | *** | ns | ns | *** | ns | |
| Cultivar x CO2 | ** | ns | ns | ns | ns | |
| Cultivar x treatment | *** | ns | ns | ns | ns | |
| CO2X treatment | * | * | ns | *** | ns | |
| Cultivar x CO2 x treatment | * | ns | ns | * | ns | |
- % Change was determined as (elevated − ambient)/ambient × 100%.
- NS non-significant.
- * P < 0.05;
- ** P < 0.001;
- *** P < 0.0001.
3.2 Role of Trichoderma in alteration of biochemical enzymes of rice grown under elevated CO2 condition
To observe the impact of Trichoderma, biochemical enzymes were estimated further in all treatments. The level of photosynthetic pigments was higher in Trichoderma treated plants in both ambient and elevated CO2 conditions and in both varieties compared to their respective controls. In Heena, the level of photosynthetic pigments (0.21and 0.23 mgg−1 in aCO2and 0.22 and 0.27 mgg−1 in eCO2 for control and BF treated, respectively) was higher than in Kiran (0.09 and 0.09 mgg−1 in aCO2 and 0.16and 0.19 mgg−1 in eCO2 for control and BF treated, respectively). In Heena, the total soluble sugar content was higher after treatment in both aCO2 (186.6 mgg−1) and eCO2 (256.6 mgg−1), whereas in Kiran, the opposite trend was observed in both conditions aCO2 (266.4 mgg−1) and eCO2 (196.1 mgg−1). The three-way ANOVA interactive model exhibited a significant difference in the photosynthetic pigments and total soluble sugars (Cultivar ×CO2× BF treatment) in both the cultivars after the treatment of Trichoderma in aCO2 and eCO2conditions. Proline content was also higher in treated plants grown in eCO2 (67.6 M) conditions than in aCO2 (58.9 M) conditions in Heena but lower in Kiran eCO2 (41.5 M) conditions than in aCO2 (48.8 M) conditions. There was no significant difference in the total phenolic content in various treatments or in two varieties. There was no significant difference in the total phenolic content in various treatments or in two varieties. There was no increment in the level of superoxide dismutase and catalase activity after the treatment of Trichoderma in aCO2 and eCO2conditions in either of the varieties. We could not find any statistically significant difference in any of the models designed, whereas ascorbate peroxidase showed a significant difference (Table 2).
| Treatments | Biochemical Activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chl(A) (mg/g) | Chl (B) (mg/g) | Total Chlorophyll (mg/g) | Xanthophyll and carotenoids (mg/g) | Sugar (μg/gm) | Proline (μM) | Total Phenolics (mg/g F.W.) | LPX (nmole/gF.W) | Total Protein (mg/g F.W) | SOD (U/G FW) | APX (Unit/mg Protein) | CAT (Unit/mg Protein) | |
| CHA | 0.15 ± 0.008 | 0.06 ± 0.003 | 0.21 ± 0.000 | 0.03 ± 0.001 | 339.0 ± 2.1 | 48.87 ± 3.4 | 8.0 ± 0.001 | 0.75 ± 1.7 | 7.45 ± 1.5 | 129.3 ± 6.6 | 0.73 ± 0.14 | 0.009 ± 0.001 |
| THA | 0.16 ± 0.007 | 0.07 ± 0.001 | 0.23 ± 0.006 | 0.04 ± 0.001 | 186.6 ± 1.3 | 58.96 ± 1.4 | 11.5 ± 0.624 | 0.93 ± 2.4 | 7.58 ± 0.42 | 121.9 ± 10.7 | 0.69 ± 0.14 | 0.012 ± 0.002 |
| CHE | 0.17 ± 0.005 | 0.05 ± 0.000 | 0.22 ± 0.010 | 0.03 ± 0.000 | 377.6 ± 2.6 | 49.20 ± 2.4 | 11.6 ± 0.001 | 1.72 ± 4.1 | 7.40 ± 0.77 | 116.2 ± 14.8 | 0.65 ± 0.13 | 0.007 ± 0.001 |
| THE | 0.2 ± 0.001 | 0.03 ± 0.001 | 0.27 ± 0.002 | 0.01 ± 0.001 | 256.6 ± 1.5 | 67.68 ± 1.5 | 12.4 ± 0.000 | 1.48 ± 3.3 | 8.69 ± 0.39 | 116.8 ± 4.7 | 0.51 ± 0.14 | 0.011 ± 0.002 |
| CKA | 0.10 ± 0.007 | 0.06 ± 0.000 | 0.09 ± 0.002 | 0.03 ± 0.002 | 304 ± 1.7 | 46.78 ± 2.5 | 7.2 ± 0.000 | 0.51 ± 1.2 | 7.29 ± 0.38 | 113.8 ± 4.7 | 0.73 ± 0.13 | 0.006 ± 0.001 |
| TKA | 0.14 ± 0.001 | 0.05 ± 0.000 | 0.09 ± 0.000 | 0.02 ± 0.000 | 266.4 ± 1.1 | 48.87 ± 2.0 | 7.3 ± 0.000 | 0.55 ± 1.3 | 7.71 ± 0.96 | 129.6 ± 6.5 | 0.62 ± 0.15 | 0.006 ± 0.006 |
| CKE | 0.13 ± 0.003 | 0.02 ± 0.001 | 0.16 ± 0.004 | 0.02 ± 0.000 | 237.2 ± 1.5 | 39.49 ± 1.6 | 8.2 ± 0.001 | 0.86 ± 1.9 | 8.12 ± 0.30 | 154.5 ± 14.7 | 0.71 ± 0.16 | 0.004 ± 0.001 |
| TKE | 0.12 ± 0.001 | 0.02 ± 0.000 | 0.19 ± 0.001 | 0.009 ± 0.000 | 196.1 ± 1.9 | 41.54 ± 4.3 | 6.0 ± 0.000 | 1.30 ± 2.9 | 8.38 ± 0.62 | 122.6 ± 4.9 | 0.68 ± 0.17 | 0.005 ± 0.001 |
| Cultivar | ns | ** | ns | ns | ** | ns | ns | ns | ns | ns | ns | ns |
| CO2 | ** | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Treatment | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Cultivar x CO2 | ns | ns | *** | ns | ns | ns | *** | ns | ns | ns | ns | ns |
| Cultivar x treatment | ns | ns | *** | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| CO2 x treatment | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | *** |
| Cultivar x CO2 x treatment | *** | *** | ns | *** | *** | *** | ns | *** | *** | ns | *** | ns |
- NS = Non-significant, P < 0.05 = *, P < 0.001 = *, P < 0.0001 = ***.
3.3 Impact of Trichoderma inoculation in phytohormones during eCO2 condition
The concentration of GA3 and IAA varied significantly after the treatment of Trichoderma in Heena and Kiran. In Trichoderma treated plants, GA3 accumulation was significantly higher in aCO2 and eCO2 of Heena in comparison to respective controls, whereas no significant change was observed in Kiran. During carbon dioxide enrichment conditions, IAA accumulation was found to be higher in the BF-treated both rice varieties, as shown in (Figure 1A–B).

3.4 Mineral element, carbon and nitrogen estimation in rice after treatment of Trichoderma during aCO2 and eCO2 condition
Micro-elements play an important role in plant growth and development. Micro elemental analysis of rice flag leaf revealed the higher accumulation of Mn, Fe, Co, Cu, Zn, Mo, and Pb in Trichoderma-treated plants in aCO2 as well as eCO2 conditions in both the varieties. In our investigation, we utilized inductively coupled plasma mass spectrometry (ICP-MS) to quantify a variety of crucial micronutrients across all treatment groups. Figure 2A shows a notable increase in the concentration of essential micronutrients in the treated condition compared to the control (CHA and THA) under aCO2 conditions. Similarly, Figure 2B illustrates a significant increase in the concentration between CHE and THE in the Heena rice variety under eCO2 conditions. The findings obtained in the Kiran variety are also consistent (Figures 2C, D and 3-D).
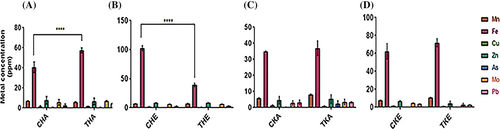
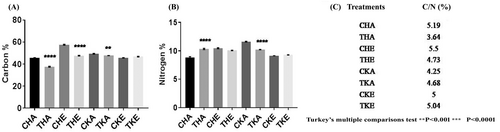
The Fe content in the flag leaf was significantly increased in the BF-treated rice variety (Figure 2A-D). The carbon content was significantly increased in treated plants as compared to their respective controls in both aCO2 and eCO2 conditions, whereas nitrogen content was significantly increased in treated ambient conditions of both the rice varieties (Figure 3A-C).
3.5 Extensive study of transcript abundance and differential gene expression analysis
3.5.1 Transcriptional changes in ambient and elevated condition of control and Trichoderma treated rice
A total of 160 million raw reads were generated, with an average of 16 million reads in Heena and 21 million reads for the Kiran variety (Table 3). After the preprocessing, an average of ~150 million high qualities reads (q-value ≥30) were mapped on the rice genome for Heena and Kiran varieties (Table 3). Approximately 86.3 and 92.2% of uniquely mapped high-quality reads were mapped for the Heena and Kiran varieties, respectively and for the control and Trichoderma treatment in ambient and elevated condition (Table 3).
| Heena | Input Reads | Uniquely mapped reads | Uniquely mapped reads % | Multiple mapped reads % | Kiran | Input Reads | Uniquely mapped reads | Uniquely mapped reads % | Multiple mapped reads % |
|---|---|---|---|---|---|---|---|---|---|
| Ambient Control | 19241465 | 17320523 | 90.02% | 95.50% | Ambient Control | 23866028 | 21691833 | 90.89% | 96.00% |
| Elevated Control | 14972666 | 13560057 | 90.57% | 95.27% | Elevated Control | 26180787 | 23886961 | 91.24% | 96.01% |
| Ambient Trichoderma | 13062442 | 9680946 | 74.11% | 79.11% | Ambient Trichoderma | 18189005 | 16967221 | 93.28% | 96.74% |
| Elevated Trichoderma | 20121713 | 18248639 | 90.69% | 93.62% | Elevated Trichoderma | 18517908 | 17265223 | 93.24% | 96.47% |
Combinatorial datasets were used to investigate the differentially expressed genes in all conditions. These datasets compare ambient control to ambient treatment for Heena and Kiran (CHA_THA, CKA_TKA), ambient control to elevated control for Heena and Kiran (CHA_CHE, CKA_CKE), and ambient control to elevated Trichoderma treatment for Heena and Kiran variety of rice (CHA_THE, CKA_TKE). With log2fc > ± 1 and FDR ≤0.05, Differentially Expressed Genes (DEGs) were identified for all combinatorial datasets in both varieties (Figure 4). A total of 2030, 2461 and 2103 genes were upregulated in the Heena, and 864, 1189 and 1878 genes were upregulated in the Kiran variety for the CHE condition, THA and THE conditions, respectively.
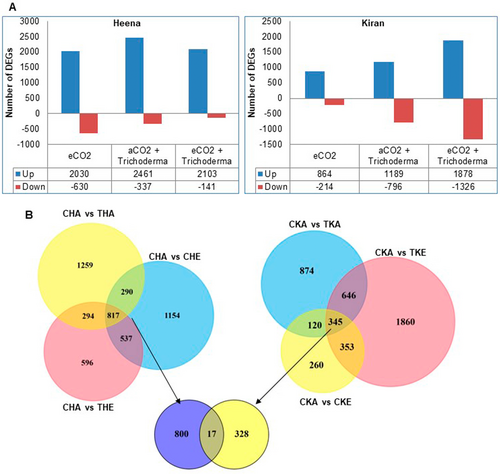
Overall, the maximum number of DEGs was observed in the Heena variety for all three conditions as compared to the Kiran variety, which showed a higher number of DEGs only in THE condition. For a comparative view, up-and downregulated DEGs of the Heena and Kiran varieties were represented through a Venn diagram (Figure S1) in which 127, 178 and 199 genes were commonly upregulated, whereas 6, 19 and 15 genes were commonly downregulated in elevated control, ambient treated and elevated treated conditions respectively (Figure S1). It represented the significant changes in both the rice variety after the intervention of Trichoderma in ambient and elevated conditions. Among the DEGs between all three combinatorial datasets, 817 and 345 genes were commonly regulated in the Heena and Kiran variety, suggested that they might be affected with a similar mechanisms in all conditions. Unlikely, only 17 genes were commonly regulated in Heena and Kiran varieties suggested that their working mode in all three conditions was different for two selected varieties (Figure 4A–B).
3.5.2 Gene ontology, metabolic and regulatory pathway analysis of identified DEGs
The gene ontology (GO) analysis revealed the role of DEGs in various metabolic processes in both rice varieties. The major pathways studied through GO analysis by statistically significantly upregulated genes during the CHE condition in the Heena variety were photosynthesis, electron transport chain, developmental process, reproductive process, and in response to different stress stimuli (Figure 5). In HET, we found similar observations like CHE along with the addition of pathways involved in different secondary metabolites like terpenoid, isoprenoid and phenylpropanoid (Figure 5) while during HAT condition they also showed their involvement in different RNA metabolic processes, nitrogen related metabolic pathways and different catabolic pathways (Figure 5). In the GO analysis of Kiran variety in CKE and KET conditions, we found a higher number of enrichments in multicellular organism development and different types of stimulus like – organic, hormonal and chemical responses (Figure 5B). While in KAT condition, upregulated genes were enriched as were the cellulose, polysaccharide and glucan related metabolism processes that were unique to the Kiran variety (Figure 5B).
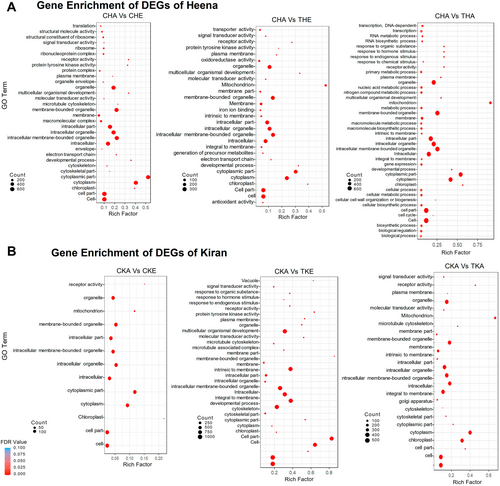
Kyoto Encyclopedia of Genes and Genome analysis of DEGs augmented the GO enrichment information where the CHE, THA and THE showed higher involvement in metabolism and regulation-related pathways followed by hormone signaling, transport and metabolism-related pathways in both the Heena and Kiran varieties (Figure S2). Among all three conditions for Heena, HAT showed higher regulation in all biological pathways with some exceptions like flavonoid and phenyl propanoid biosynthesis. Surprisingly, only in THA conditions for Heena, some DEGs show their involvement in IAA biosynthesis, Gibberellins signaling and flower development. In the Kiran variety, no such involvement was seen for any condition, especially for CKE, in which no genes were involved in any hormonal signalling (Figure S2). The pathways of common DEGs of both the Heena and Kiran varieties were further analyzed and visualized through Mapman (Figure S3). The major pathways studied were related to photosynthesis, carbohydrate metabolism, amino acid metabolism, hormone signalling, developmental process and secondary metabolic pathways. The Trichoderma-treated plant samples showed higher expression in both varieties. Genes (Os08g0200300, Os03g0333400, and Os03g0592500) involved in the photosynthetic process showed higher expression in treated samples in both Heena and Kiran. Similarly, other genes involved in several metabolic pathways showed significantly higher expression after the intervention of Trichoderma (Figure S4).
3.5.3 Validation of RNA seq data using qRT-PCR
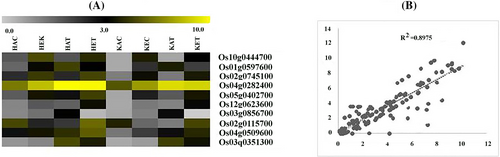
The significant differentially expressed genes in all the datasets during the treatment were selected and further validated using qRT-PCR (Figure 6). The selected genes were involved in photosynthesis, carbohydrate metabolism, phytohormone, amino acid transporters, aquaporins and nitrogen metabolism-related pathways. The data obtained through qRT-PCR showed expression is in accordance with the RNA seq experimental data sets, which further confirms the results obtained from the Illumina sequencing. A good correlation with a coefficient value of 0.8975 (Figure 6A–B) was found between expression profiling of all the selected genes and RNA seq data, which suggested the accuracy of identification of DEGs in all datasets.
4 DISCUSSION
In the coming years, extreme climatic events are expected to become more frequent due to global climate change (Meehl & Tebaldi, 2004; IPCC, 2012), hampering plant growth and production (Knapp et al., 2008; Smith, 2011). There are numerous ecosystem manipulation experiments recommended to correlate future climate situations as realistically as possible (Beier et al., 2012). The effect of eCO2 on various crops, including rice, has previously been described (Abdelrahman et al., 2016). Recent agricultural practices emphasize the use of PGPRs to alleviate a variety of environmental stresses while also improving yield enhancement in a variety of crop plants such as rice, maize, soybean, and barley (Tapias et al., 2012; Sharma et al., 2013; Sen & Chandrasekhar, 2014; Suarez et al., 2015). Concurrently, Trichoderma spp. is a ubiquitous fungal organism that has been preferably used in bio-control as well as in the enhancement of plant growth-promoting activity during environmental stress (Chepsergon et al., 2014). It is well proven that Trichoderma inoculation and colonization in rice roots helps increase the biomass and promote the different growth support mechanisms by promoting nutrient bio-availability through solubilisation, chelating the minerals (particularly phosphate) and boosting nutrient uptake efficacy (Doni et al., 2014).
However, the role of Trichoderma has yet to be explored in reducing abiotic stress at the molecular level in C3 crops (Mastouri et al., 2010; Ahmad et al., 2015; Tripathi et al., 2015). Our present study emphasizes the efficacy of Trichoderma reesei MTCC5659 as a bio-fertilizer, which provides an adaptive mechanism to rice at the panicle stage by modulating various genes in metabolic pathways. The flag leaf has been taken for the study, indicating its important role as it is the major site for the accumulation of metabolites and oxidative stress markers/enzymes in many crops, including rice (Yoshida, 1972). We have found positive responses of the Heena rice variety in comparison with Kiran at physiological, biochemical and transcript levels during eCO2 conditions (Mishra et al., 2019) as well as in nutrient-deficient conditions after the introduction of Trichoderma as a bio fertilizer (Singh et al., 2019). During gas exchange conditions, we found a minor percent change in physiological parameters of the Heena variety as compared to the Kiran variety as well as reduced stomatal conductance and transpiration rate in both varieties during eCO2 after the BF treatment. This could be a possible mechanism to restrict the entry of excess carbon dioxide through stomata in the leaves to prevent excess carbon gain during the elevated CO2 in the rice. The results also revealed a positive interaction between Cultivar × CO2 × Treatment in photosynthetic rate, demonstrating the efficacy of Trichoderma in reducing the negative effects of stress caused by disturbed physiological parameters. The root colonization property of Trichoderma enhanced the water use efficiency in rice which can positively help in minimizing the effect of other environmental factors like drought stress. The observed increase in proline content might also be related to the mitigation of the drought stress due to eCO2 condition.
The accumulation of ROS species takes place during abiotic stress, which destroys the cellular and molecular components of plants. The antioxidant system is comprised of the enzymatic and non-enzymatic systems, which protect the plant from this damage. The photosynthetic pigments chlorophyll a and chlorophyll b accumulate in the chloroplast and mediate the photosynthetic light reactions. Due to oxidative stress, the chlorophyll content of plants gets degraded, which causes chlorosis in plants. Our results showed a significant increase in photosynthetic pigments chla and chlb, including xanthophyll and carotenoids, after the treatment of Trichoderma in both varieties under aCO2 and eCO2 conditions (Table 2). Xanthophylls and carotenoids protect plants from oxidative damage. This is in accordance with previous findings where Trichoderma boosts the accumulation of photosynthetic pigments by different PGP traits, mitigating the negative impacts of nutrient deficiencies under hydroponic conditions along with field conditions (Nieto-Jacoba et al., 2017; De Santiago et al., 2009).
The increased level of carbohydrates is prominent during eCO2 conditions (Aranjuelo et al., 2008) and is important for normal plant growth under ambient CO2 conditions (Smith et al., 2013). The increased level of sugar has been found and is well interrelated with previous studies in both varieties after the treatment of Trichoderma in eCO2 conditions. The increased level of total phenolic content in the flag leaf of rice can be correlated with the higher APX content, as both are involved in polyphenol biosynthesis. The result shows a relationship with the previous study, in which higher phenolic and ascorbic acid content were found in the early sown wheat cultivars and favored polyphenol biosynthesis (Pellegrini et al., 2018). Previous research found that under eCO2, Oryza sativa showed a significant reduction in total phenolics and total flavonoid content at all initial growth stages but increased at maturity, leading to photosynthetic acclimation (Goufo et al., 2014).
Our results showed that no significant change was found in the activity of SOD and CAT in both rice varieties, which is well correlated with the previous work found in some plant species, such as mung bean, under eCO2 showed a marked reduction in ROS levels (O·2−, H2O2) and some antioxidant enzymes such as CAT and SOD (Mishra & Agrawal, 2014). Some C3 legumes (Medicago lupulina, and Lotus corniculatus) did not show any significant change in the ascorbtae glyoxalate cycle, while lipid peroxidation superoxide dismutase, and catalase activity were found to be decreased (AbdElgawad et al., 2015). Trichoderma has already been reported for enhanced IAA production for stress mitigation in plants. Here, our results also suggest enhanced IAA production (Figure 1), which helps in lateral root development for proper nutrient uptake in aCO2 as well as eCO2 condition (Contreas-Cornejo et al., 2009). GA3 helps in the regulation of plant growth processes and development, including seed and flower development.
In the era of climate-smart agriculture, nitrogen supply has been considered a supreme factor (Lipper et al., 2014). Nitrogen uptake and assimilation are well coordinated with carbon metabolism, including photosynthesis, photorespiration and respiration (Stitt et al., 2002). This is important for the efficient synthesis of organic N- compounds using C- skeletons, energy being provided by respiration and inorganic N from the soil. Trichoderma spp. are powerful rhizospheric microorganisms that can colonize the outer epidermal layer of roots and aid in the solubilization of nutrients such as N, Fe, Cu, Zn, and Mn in soils (Singh et al., 2014). In the sameway, T. harizanium T22 has been studied to increase nitrogen use efficiency in maize (Harman, 2000). We found a significant percent change in carbon and nitrogen concentration during eCO2, as well as at aCO2, after Trichoderma treatments which facilitated the nitrogen use efficiency. Our results showed a reduced C/N ratio after the treatment of Trichoderma in the Heena variety during aCO2 as well as eCO2conditions, whereas contrasting results were found in the Kiran variety. Our findings show that Fe accumulation is greater in Trichoderma-treated Heena variety grown in aCO2 than in eCO2 conditions. Fe plays an important role in photosynthesis, respiration and nucleic acid synthesis.
Generally, plant nutrient concentration (PNC) has been seen to decline under eCO2 condition which is the impact of the dilution effect due to the increment of biomass in plants (Conroy, 1992; Gifford et al., 2000; Pang et al., 2006). The concentration of N was found to be decreased by 15.8% in a field study of rice (Kim et al., 2011), and it was created due to the dilution effect of increased C integration during CO2 enrichment (Coleman et al., 1993; Norby et al., 1999). The N concentration decreases during the atmospheric CO2 enrichment of the grain and leaf of rice (Cheng et al., 2010). The allocation of nitrogen has also been lessened into leaf blades of rice during eCO2 conditions, which afterwards affects the Rubisco and other protein synthesis (Seneweera et al., 2011). The change in the C:N ratio could be seen in the whole plant due to an increase in C concentration by 0.8–1.2% and a decline in N concentration by 7.4–10.7% for rice and wheat, along with the reduction in P concentration by 10.0% in rice (Wang et al., 2019).
Transcriptomic studies have been performed to differentiate the expression of genes in two biologically different conditions by researchers (Brautigam & Gowik, 2010). Furthermore, synchronic monitoring of photosynthetic rate, soil mineral availability, and factors associated with abiotic and biotic stress necessitates precise mechanisms that can adjust starch turnover in response to these stimuli (Thalmann and Santelia, 2017), for which transcriptional and post-translational levels are critical (Stitt & Zeeman, 2012; Santelia et al., 2015). Our RNA sequencing results showed that Trichoderma has a positive effect on transcript levels in several pathways. GO analysis suggested that during the elevated carbon conditions in Heena; all energy goes to the different developmental processes. The genes related to photosynthesis like Os08g0200300, Os03g0592500 Os09g0439500, Os03g0333400 (light reactions, dark reactions, photosynthetic pigments and electron transport chain) Os01g0720500, Os09g0439500, Os07g0562700 (photosynthetic pigments), were found to be upregulated which is attributed to minimizing the stress and yield enhancement in rice.
Trichoderma has also been reported to induce changes directly in stress-related genes and proteins in the host plants (Brotman et al., 2013). According to Brotman et al., (2013), Trichoderma upregulated the SOD (Mn) and SOD (Cu) genes in cucumber plants during salt stress. eCO2 sometimes increases the levels of antioxidants such as ASC and phenols as found in Beta vulgaris (Kumari et al., 2013), and it has been proven that ASC synthesis increases due to excess carbohydrate synthesis (Smirnoff and Wheeler, 2000; Zinta et al., 2014). The increased level of antioxidants in eCO2 helps in other biological processes of senescence, but it varies from species to species. After treating Trichoderma under eCO2 conditions; we found that genes involved in secondary metabolic processes were turned up. Flavonoids, tocopherols, tocotrienols, oryzanol and phytic acids have been previously reported for antioxidant properties (Goufo et al., 2014). Trichoderma harizanium has been reported to ameliorate stress in several rice genotypes through the up-regulation of aquaporin, dehydrin and malonialdehyde pathway-related genes along with different other physiological parameters (Pandey et al., 2016). We have also found the expression of various transporters of amino acids, and water uptake, like Os02g0745100, Os02g0666200 (Aquaporin), Os09g0538400 (Secondary metabolic process), and Os04g0401000 Proline-rich protein for blast resistance) phosphate uptake Os10g0444700 (Pi homeostasis) Os12g0637100 (Regulation of growth under phosphate deficient condition) after the treatment of Trichoderma in CO2 enrichment. The genes involved in flower development also showed significant change, viz., Os06g0157500 and Os04g0282400 (flowering time and development). This could be one of the possible mechanisms for the proper allocation of nutrients in the flag leaf as it regulates the fate of plants in multiple ways, such as effects grain yield, synthesis and translocation of photoassimilates in different organs as well as remobilization of minerals for the seeds.
GO analysis has revealed the significant up-regulation of genes mainly involved in RNA metabolic processes, nitrogen-related metabolic processes and different catabolic processes after the treatment of Trichoderma in aCO2 as well as eCO2 conditions. Overall, this transcriptomic study revealed the mechanisms that evolved in the panicle stage of rice and their response in the presence of PGP fungus (Trichoderma) to future extreme climate changes in CO2. The data has also identified a competent set of genes discussed above after the treatment of Trichoderma involved in the regulation of several metabolic pathways, which is of great importance to the agricultural sector as well as ecosystem management.
5 CONCLUSION
A biological mitigation strategy is necessary in the current climate change situation to increase crop yields by combating all adverse effects. In the flag leaf of rice during CO2 enrichment, Trichoderma-mediated physiological, biochemical, and transcriptional regulation is predicted by this work to take a new mechanistic approach. Trichoderma ability to regulate photosynthesis, secondary metabolic processes, immune responses, and resource allocation is supported by a number of scientific studies. For crops to continue to grow and develop, several mechanisms like, photosynthesis, nitrogen uptake, and transport must be maintained. Trichoderma's function as a BF has demonstrated its multifarious significance in rice flag leaf, which marks the change from crop growth to grain production and raises the yield potential of the crop under stressed environments.
Authorship contribution statement
Nishtha Mishra: Investigation, Methodology, Data Curation, Formal analysis, Visalization, Writing original draft, Priti Prasad: Transcriptomic data analysis, Sahil Mahfooz: Investigation, Data analysis, Writing, Radha Shivhare: Methodology and data analysis, Pratibha verma: Investigation, Data analysis. Satyendra Pratap Singh: Investigation, Seema Mishra: Investigation, Review, Shashank Kumar Mishra: Investigation, Ramakant Bajpayee: Investigation, Aradhana Mishra: Conceptualization, Supervision, Writing, Review and editing, Funds acquisition.
ACKNOWLEDGMENTS
This study was supported by Science and Engineering Research Board, New Delhi (GAP-3349). NM is grateful to Department of Science and Technology, New Delhi, India for awarding the Senior Research Fellowship. SM is grateful to the SEED division of DST, New Delhi for the project grant (SEED/TIASN/2018/74). Authors are also grateful to Dr. Vivek Pandey for providing the FACE facility. Thanks are due to Prof. S.K. Barik, Director, National Botanical Research Institute, Lucknow, for providing all the necessary support. This manuscript has been approved by institutional ethical committee with (MS number CSIR-NBRI_MS/2022/07/08).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.




