Modulation of phosphorylation status of MAP3 kinases under abiotic stress responses
Abstract
Plants must face various environmental stresses that they cannot avoid, and they have developed numerous mechanisms to survive these challenges. Phosphate-mediated signal transduction is a common method for regulating gene expression and protein activity in response to stresses. Phosphate groups can be transferred to other proteins by proteins called kinases. MAP3Ks are the largest kinase family in plants, and they comprise various group and individual pathways collectively known as MAPK cascades. When plants encounter environmental stresses, several MAP3Ks act as positive or negative regulators by phosphorylating downstream proteins within the MAPK cascade, as well as transcription factors, cytoskeletal proteins, enzymes, and kinases, through their kinase activity. In addition, other upstream kinases and phosphatases regulate MAP3Ks by adding or removing phosphate groups. Various MAP3Ks have been isolated in many plant species, and evidence suggests that they function as post-translational modulators under various abiotic stresses, including ABA responses as well as salinity, osmotic, drought, oxidative, cold, and heat stress. This review focuses on how plant MAP3Ks affect various abiotic stresses as regulators and substrates through post-translational phosphorylation.
1 INTRODUCTION
As sedentary organisms, plants have evolved strategies to alleviate the impacts of different environmental stresses, such as drought, that affect their growth, survival, distribution, and crop productivity (Yoshida et al., 2015; Soma et al., 2020; Takahashi, Y et al., 2020). These mechanisms encompass a broad range of molecular, physiological, and biochemical responses, which have facilitated the evolution of drought tolerance (Shinozaki & Yamaguchi-Shinozaki, 2006; Yoshida et al., 2015; Takahashi et al., 2020). The phytohormone abscisic acid (ABA) plays a crucial role in regulating plant responses to abiotic stresses, including drought, salinity, heat, and cold stresses (Li et al., 2017; Li et al., 2021; Chen et al., 2022; Muhammad Aslam et al., 2022). Under stress conditions, ABA levels are enhanced, leading plants to enter a state of stress adaptation and triggering stress response mechanisms (Tuteja, 2007; de Zelicourt et al., 2016; Sah et al., 2016). In particular, ABA elicits various drought-tolerant responses, including the regulation of seed dormancy/germination, plant growth/development, and stomatal closure, to prevent water loss under drought stress conditions (Cutler et al., 2010; Raghavendra et al., 2010; Cheng et al., 2017; Sato et al., 2018; Lin et al., 2021).
The core signalling pathway of ABA is made up of three major components—specifically ABA receptors [pyrabactin resistance1 (PYR1)/PYR1-like (PYL)/regulatory component of ABA receptor (RCAR)], co-receptor protein phosphatase 2Cs (PP2Cs), and SNF1-related protein kinase 2 s (SnRK2s)—and is tightly regulated through phosphorylation and dephosphorylation (Fujii et al., 2009; Cutler et al., 2010). The ABA receptors PYR1/PYL/RCARs interact with ABA to initiate the ABA response (Gonzalez-Guzman et al., 2012; Santiago et al., 2012). When ABA is present, ABA receptors undergo a change in which two loop regions, known as the gating loops (the loops ß3-ß4 and ß5-ß6), bind to ABA and enclose it (Santiago et al., 2012). These altered forms of receptors can interact with the surface of PP2Cs and directly bind to the active site of PP2Cs to inhibit phosphatase activity, which acts as a negative regulator of the ABA response (Santiago et al., 2012; Lee et al., 2013; Ali et al., 2020). The inhibition of PP2Cs releases and activates SnRK2s in the plant cell (Hirayama & Umezawa, 2010; Manohar et al., 2017). Activated SnRK2s subsequently phosphorylate downstream transcription factors, such as ABA-insensitive 5 (ABI5)/ABA-responsive element binding factor (ABF), as well as other proteins, including stomatal response proteins and MAPK cascade proteins (Dai et al., 2013; Fujita et al., 2013; Lee et al., 2013; Umezawa et al., 2013; Kamiyama et al., 2021a). Phosphorylation by SnRK2s can then regulate the function of downstream proteins by either activating or deactivating proteins, and this process ultimately leads to an increase in plant stress resistance (Wang et al., 2013; Kamiyama et al., 2021a).
Protein phosphorylation is known to play a crucial role in early plant responses to environmental changes (Arsova et al., 2018; Zhang et al., 2020). Phosphorylation affects the aggregation, localization, conformational flexibility, interaction, and stability of proteins (Nishi et al., 2011; Nishi et al., 2014; Yalinca et al., 2019). Protein phosphorylation is one of the most prominent PTMs currently reported in more than 460 different forms (Arsova et al., 2018; Vu et al., 2018; Ramazi & Zahiri, 2021). This process is an extremely dynamic and reversible process mediated by kinases and phosphatases, and it contributes to signal transduction from the cell surface to the nucleus to regulate protein functions under stress conditions (Cohen, 2002). In the Arabidopsis genome, over 1,000 genes encode protein kinases (Wang et al., 2003), and approximately 150 genes encode protein phosphatases (Kerk et al., 2008). Generally, in eukaryotes, phosphorylation of proteins takes place on serine (Ser) and threonine (Thr) residues, while phosphorylation on tyrosine (Tyr) residues is comparatively infrequent (van Bentem & Hirt, 2009). Several types of kinases, including mitogen-activated protein kinases (MAPKs), sucrose non-fermenting-related kinases (SnRKs), calcium-dependent protein kinases (CPKs), and receptor-like kinases (RLKs), participate in this process (Zhu, 2016). In particular, the MAPK cascade is a highly conserved signalling pathway that responds to extracellular stimuli, including stress responses, growth, differentiation, survival, and apoptosis (Plotnikov et al., 2011; Guo et al., 2020). This pathway is exclusive to eukaryotic organisms and consists of three successive steps involving Ser/Thr kinases, specifically MAP3K, MAP2K, and MAPK (Jagodzik et al., 2018; Guo et al., 2020). The mechanism behind the MAPK cascade pathway involves the transfer of phosphate from an upstream kinase to the next kinase in the cascade, ultimately leading to the phosphorylation of target substrate proteins such as other kinases, enzymes, cytoskeletal proteins, or transcription factors (Jagodzik et al., 2018). The initiation of the MAPK cascade takes place upstream of the MAPKKK via transmembrane receptors (Lee et al., 2012), receptor-like kinases (Wang & Gou, 2020), other protein kinases (Kim et al., 2012), and heterotrimeric GTPases (Guo et al., 2009; Cheng et al., 2015; Su et al., 2015), among others (Komis et al., 2018; Sun & Zhang, 2022). MAP2Ks are then activated through the phosphorylation of the serine or threonine residue of S/T-X5-S/T within the MAP2K activation loop by activated MAP3Ks (Yin et al., 2021). Subsequently, MAP2K phosphorylates threonine or tyrosine residues of the T-X-Y motif within the MAPK activation loop (Yanagawa et al., 2016). Finally, MAPKs activate the final substrate protein, which can be a transcription factor, enzyme, or other kinase, by phosphorylating Ser/Thr of the target protein (Jagodzik et al., 2018; Liu et al., 2018).
Plant hormones such as auxin (AUX), ABA, jasmonate (JA), salicylic acid (SA), ethylene, brassinosteroids (BRs), and gibberellins (GAs) can influence MAPK cascades (Jagodzik et al., 2018; Lin, L et al., 2021). Recent studies suggest that plant MAP3Ks are associated with ABA core components in ABA signalling and drought stress responses (Katsuta et al., 2020; Lozano-Juste et al., 2020; Takahashi et al., 2020; Jeong et al., 2021; Kamiyama et al., 2021a; Jeong et al., 2023). This association is primarily based on the phosphorylation of ABA core components, specifically SnRK2s and PP2Cs, which are involved in ABA signal transduction and play a crucial role in the plant drought stress response. In this review, we focus on the phosphorylation relationship between MAP3K and the ABA core components SnRK2s and PP2Cs as they relate to ABA signalling and plant responses to abiotic stresses, including drought stress.
2 IDENTIFICATION OF MAP3K PROTEINS IN PLANTS
The first MAP3K gene to be isolated in plants was NICOTIANA TABACUM PROTEIN KINASE 1 (NtNPK1). NPK1 has been studied for its role in cell growth and division as a homolog of STE11 and BCK1, which are MAP3Ks of Saccharomyces cerevisiae and Saccharomyces pombe that function as temperature-dependent regulators of cell lysis (Banno et al., 1993; Xie et al., 2023). Later, Arabidopsis NPK1-related protein kinase (ANP) 1/2/3, which are part of the MAP3K family, were identified as homologs of NPK1 (Nishihama et al., 1997). Members of the MAP3K protein family have been isolated in various plants, including Arabidopsis, strawberry, maize, cotton, rice, barrel clover, tomato, soybean, grape, and pepper (Ichimura et al., 1998; Neupane et al., 2013; Singh et al., 2014; Wang et al., 2014; Wu et al., 2014; Liu et al., 2015; Li et al., 2016; Zhou et al., 2017; Bokros et al., 2019; Lim et al., 2020). The MAP3K family comprises more proteins than MAP2Ks and MAPKs (Ichimura et al., 2002). In Arabidopsis, there are 80 genes encoding MAP3Ks, but only 10 genes encoding MAP2Ks and 20 genes encoding MAPKs (Jonak et al., 2002; Su & Krysan, 2016). The strawberry genome contains 73 genes encoding MAP3Ks, seven genes encoding MAP2Ks, and 12 genes encoding MAPKs (Zhou et al., 2017). In maize, there are 74 MAP3K genes (22 MEKK, 46 Raf, and 6 ZIK, by subfamily), while there are just 8 MAP2K and 17 MAPK genes (Kong et al., 2013). Similarly, cotton (Gossypium hirsutum) has 215 genes encoding MAP3Ks (52 MEKK, 136 Raf, and 27 ZIK, by subfamily), 23 genes encoding MAP2Ks, and 52 genes encoding MPAKs (Bokros et al., 2019; Yin et al., 2021). Canola has 66 genes encoding MAP3Ks (18 MEKK, 39 Raf, and nine ZIK, by subfamily), eight genes encoding MAP2Ks, and 18 genes encoding MAPKs (Liang et al., 2013; Sun et al., 2014). Pepper plants also have 60 MAP3K genes (17 MEKK, 37 Raf, and six ZIK by subfamily), compared to only five MAP2K and 14 MAPK genes (Iftikhar et al., 2017; Lim et al., 2020). While the precise reasons for the greater number of MAP3Ks compared to MAP2Ks or MAPKs in the cascade remain elusive, it is postulated that each MAP3K may respond to a variety of stimuli. In contrast, downstream MAP2Ks and MAPKs exhibit more limited responses to stimuli. Consequently, the fine-tuning and activation of the downstream MAPK cascade by MAP3K are necessary to achieve high efficiency with minimum energy (Chen et al., 2002; Stronach et al., 2014).
3 CLASSIFICATION OF MAP3K PROTEINS IN PLANTS
In Arabidopsis, MAP3K proteins are classified into three major groups: MEKKs, Raf-like kinases, and ZIKs (Colcombet & Hirt, 2008; Jagodzik et al., 2018). MEKKs and Raf-like kinases are subdivided once more, based on sequence similarities (Ichimura et al., 2002; Jagodzik et al., 2018; Paul et al., 2021) (Figure 1). Plant MEKKs (Subgroup A) are members of MAP3K subfamily that have most homology with animal MEKKs and yeast MAP3Ks members (such as STE11 and BCK1) (Ichimura et al., 2002). Plant MEKKs include conserved sequence, G(T/S)PX(W/Y/F)MAPEV, in their kinase domain (Jonak et al., 2002; Paul et al., 2021). Plant Raf-like kinases (Subgroup B and C)are related to the Raf kinases, whose sequences differ from those of MEKK/STE11/BCK1 (Ichimura et al., 2002). Plant Raf-like kinases include conserved sequence, GTXX(W/Y)MAPE, in their kinase domain (Jonak et al., 2002; Paul et al., 2021). Among the Raf kinases, subgroup B kinases have a particularly extended N-terminus domain, whereas subgroup C does not (Ichimura et al., 2002). Plant ZIK-like kinases are also members of a similar group to animal MEKKs, STE11, and BCK1 (Jonak et al., 2002). However, plant ZIK-like kinases include a different sequence, TPEFMAPE(L/V)Y, in their kinase domain compared to MEKKs (Jonak et al., 2002; Paul et al., 2021). Additionally, plant ZIK-like kinases lack a catalytic lysine residue in subdomain II within their kinase domain, which sets them apart from MEKKs and RAF-like kinases (Wang et al., 2008).
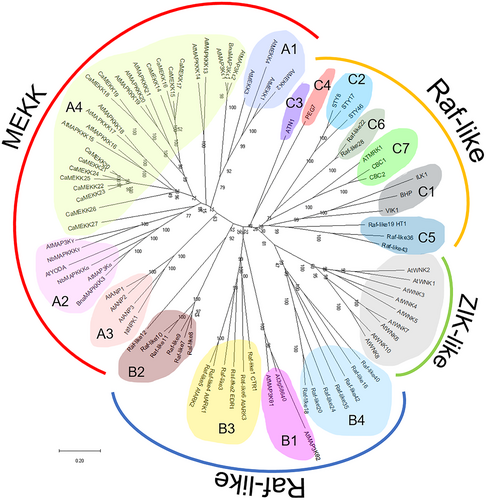
3.1 MEKKs
First, MEKKs are called subgroup A, which can be further divided into four subgroups (A1–A4) (Ichimura et al., 2002; Danquah et al., 2014).
Subgroup A1: In Arabidopsis, four MAP3Ks (AtMEKK1–4, also called AtMAPKKK8–11) belong to subgroup A1 (Jagodzik et al., 2018). Expression of these proteins is linked to programmed cell death (PCD), stress, and defence response (Asai et al., 2002; Hashimoto et al., 2012). In particular, AtMEKK4 has several functional domains, including a glycine-rich region, paired-amphipathic-helix repeat, WRKY domain, TIR domain, and NB-ARC domain, as well as a Leu-rich repeat (LRR) in the N′ terminus that is not found in AtMEKK1–3 (Ichimura et al., 2002).
Subgroup A2: Subgroup A2 includes three MAP3Ks (AtMAP3Kα, AtYODA, and AtMAP3Kγ, also known as AtMAPKKK3–5) in Arabidopsis, as well as BnaMAPKKK3, NbMAPKKKα, and NbMAPKKKγ in other plant species (Jagodzik et al., 2018). AtMAP3Kα is required for localized cell death (the hypersensitive response; HR) and resistance against Pseudomonas syringae (del Pozo et al., 2004). AtYODA has been demonstrated to operate in non-canonical immune pathways as a component of the ER/ERLs/TMM/BAK1-YODA signalling cascade. Additionally, the YODA-MKK4/MKK5-MPK3/MPK6 cascade regulates stomatal development and indices (Bergmann et al., 2004; Téllez et al., 2020). NbMAPKKKα and NbMAPKKKγ are essential components in plant defence mechanisms, particularly in the induction of HR-like and programmed cell death, which involves the generation of hydrogen peroxide (Hashimoto et al., 2012).
Subgroup A3: NtNPK1 (Nicotiana protein kinase 1 in tobacco) is expressed in the root, stems, and mature leaves of tobacco. Moreover, the expression of NtNPK1 is induced in mature tobacco leaves following treatment with both auxin and cytokinin (Banno et al., 1993; Nishihama et al., 1997). In Arabidopsis, AtANP1, AtANP2, and AtANP3 (also known as AtMAPKKK1, 2, and 12, respectively) are homologs of NtNPK1 (Nishihama et al., 1997). NtNPK1 and AtANP genes are involved in the control of cellular growth and division as positive regulators of cytokinesis (Krysan et al., 2002). Furthermore, the AtANP family is associated with oxidative stress and auxin responses (Kovtun et al., 2000; Krysan et al., 2002).
Subgroup A4: AtMAP3Kε1 and AtMAP3Kε2 (also known as AtMAPKKK7 and 6, respectively, in Arabidopsis) and BnaMAP3Kε1 (in Brassica napus) (Ichimura et al., 2002; Danquah et al., 2014) are members of subgroup A4 that share functional similarities with cdc7p and cdc15p from Schizosaccharomyces pombe and Saccharomyces cerevisiae, respectively (Jouannic et al., 2001). The cdc proteins involved in cell division control are not MAPKKKs, but BnaMAP3Kε1 partially complements a cdc7p loss of function in S. pombe (Jouannic et al., 2001). Furthermore, AtMAP3Kε1 is a member of the septation initiation network (SIN)-related pathway, which shares similarities with the SIN pathway of S. pombe and is highly expressed in dividing Arabidopsis cells during G2-M phases; BnaMAP3Kε1 also shares similar characteristics (Jouannic et al., 2001; Champion et al., 2004). However, the mechanisms of their kinase activity remain unknown in plants (Jouannic et al., 2001; Ichimura et al., 2002; Champion et al., 2004).
AtMAPKKK13–21 are also involved in this subgroup and have been studied in the context of abiotic stress. AtMAPKKK14–19 expression is known to be regulated during various abiotic stress treatments, including cold, osmotic, salt, drought, genotoxic, oxidative, heat, UV-B, and wounding stress (Menges et al., 2008). In pepper, CaMEKK14–27 exhibit sequence homology with AtMAPKKK15–21, and the expression levels of CaMEKK15–17 and CaMEKK20–25 are altered in response to ABA and drought stress (Lim et al., 2020).
3.2 Raf-like kinases
Raf-like kinases are divided into subgroups B and C (Ichimura et al., 2002; Jagodzik et al., 2018). Subgroup B kinases are further divided into subgroups B1–B4 in Arabidopsis (Ichimura et al., 2002).
Subgroup B1 - In Arabidopsis, AtMAP3Kθ1, AtMAP3Kθ2, and At3g58640 are included in subgroup B1, but their biological functions are currently unknown (Ichimura et al., 2002).
Subgroup B2 - Subgroup B2 kinases include Raf-like kinases 7–12, which are involved in the activation of 100-kDa osmotic stress-activated protein kinases (OK100) (Ichimura et al., 2002; Fàbregas et al., 2020; Lin et al., 2020; Lozano-Juste et al., 2020). The N′ terminal non-kinase region of these six kinases contains both the Per, Arnt, and Sim (PAS) domain, as well as the PAS-associated C-terminal (PAC) motif (Ichimura et al., 2002; Fàbregas et al., 2020; Lin et al., 2020). The PAC motif is present at the C-terminus of the PAS domain and consists of 40–45 amino acid residues, which can contribute to the PAS structural domain (Ponting & Aravind, 1997). Notably, PAS domains are primarily found in proteins involved in signal transduction, either directly or indirectly, and may serve as signal sensors and facilitate protein–protein interaction in all kingdoms of life (Taylor & Zhulin, 1999; Möglich et al., 2009; Henry & Crosson, 2011). In plants, PAS domain-containing proteins are known to be associated with regulating responses to environmental changes (Vogt & Schippers, 2015). Among the B2-Raf kinases, the loss-of-function of RAF10 and RAF11 abolished ABA response in Arabidopsis seed germination (Lee et al., 2015; Nguyen et al., 2019).
Subgroup B3 - Subgroup B3 kinases include Raf-like kinase 1–6 (Raf1/Raf2/Raf4–6, also called CTR1/EDR1/AtARK1–3) (Ichimura et al., 2002; Fàbregas et al., 2020; Katsuta et al., 2020; Lin et al., 2020; Lozano-Juste et al., 2020). Among these kinases, CTR1/EDR1 are associated with ethylene and disease resistance signalling (Kieber et al., 1993; Frye et al., 2001). Notably, Raf3–6 are involved in ABA and osmotic stress responses: The raf3/4/5 triple knock-out Arabidopsis showed ABA-hyposensitive phenotype at the seedling stage and reduced activity of SnRK2.6/OST1 in response to ABA (Takahashi et al., 2020). Raf3-5 directly phosphorylate SnRK2.6/OST1. Under ABA treatment, HAB1 dephosphorylates and inactivates SnRK2.6/OST1-mediated Raf5 phosphorylation, which can be subsequently re-activated. Similarly, the triple mutant of raf4/raf5/raf6 displayed moderate ABA insensitivity during seedling establishment, stomatal closure, and gene expression, however, it exhibited significantly impaired responses to osmotic stress and desiccation (Katsuta et al., 2020). RAF6 can phosphorylate SnRK2.6/OST1, but its phosphorylation activity is weaker than that of RAF4 and RAF5 (Fàbregas et al., 2020).
Subgroup B4 - Raf-like kinases 16, 18, 20, 24, 35, 40, and 42 are classified as part of the activation of 130-kDa osmotic stress-activated protein kinases (OK130), which are activated by osmotic stress responses (Lin et al., 2020). Subgroup B MAP3Ks, except for subgroup B1, are associated with osmotic stress and SnRk2s, and they activate SnRK2 signalling pathways, whether they be ABA-dependent or -independent (Fàbregas et al., 2020; Katsuta et al., 2020; Lin et al., 2020; Lozano-Juste et al., 2020; Soma et al., 2020; Takahashi et al., 2020).
Subgroup C1 - Subgroup C kinases are divided into seven groups (C1–C7) and related to Dictyostelium discoideum developmentally regulated protein-tyrosine kinase 1 (DRYK1) (Ichimura et al., 2002; Kamiyama et al., 2021a). Subgroup C1 in Arabidopsis includes six kinases, which all have ankyrin repeat sequences in their N′ terminus domain, a motif that is known to play a role in protein–protein interaction (Gorina & Pavletich, 1996; Ichimura et al., 2002). Among subgroup C1, three kinases have been studied. VH1-interacting kinase (VIK) influences leaf venation by interacting with VH1/BRL2, a receptor-like kinase of the BRI1 family with a role in vascular development (Ceserani et al., 2009). Integrin-linked kinase 1 (ILK1) interacts with the high-affinity K+ transporter HAK5, and it influences PTI-immunity and growth inhibition in a kinase-independent manner; in addition, ILK1 promotes HAK5 activity in response to ionic osmotic stress, including limited K+, and enhances resistance (Brauer et al., 2016). Blue light-dependent H+-ATPase phosphorylation (BHP) is involved in blue light-dependent stomatal opening by phosphorylating H+-ATPase (Hayashi et al., 2017).
Subgroup C2 - Protein kinase Ser/Thr/Try (STY) 8, 17, and 46 belong to subgroup C2 in Arabidopsis (Kamiyama et al., 2021a). They have an aspartokinase, chorismite mutase, and TyrA (ACT) domain, which are involved in responding to amino acid concentrations and regulating a variety of metabolic enzymes (Aravind & Koonin, 1999; Ichimura et al., 2002). STY8, 17, and 46 are involved in chloroplast differentiation, which is crucial for chlorophyll accumulation, biosynthesis of nucleus-encoded chloroplast proteins, and establishment of photosynthetic capacity (Lamberti et al., 2011). In addition, STY46 transcript levels are altered under various abiotic stresses, including darkness, osmotic and salt stresses, and ABA treatment.
Subgroup C3 - Only one subgroup C3 kinase, ATN1, has been characterized. ATN1 displays gene expression in each organ and N-myristoylation motif, but its phenotypic effect remains unknown (Tregear et al., 1996; Ichimura et al., 2002).
Subgroup C4 - Within subgroup C4, paternally expressed imprinted gene 7 (PEG7) is involved in seed development in Arabidopsis, but its kinase activity is unclear (Wolff et al., 2015).
Subgroup C5 - Three subgroup C5 kinases, Raf19 (HT1), Raf43, and Raf36, are represented in Arabidopsis (Kamiyama et al., 2021a). Raf19 (also called high temperature 1, HT1) is a negative controller and interacts with SnRK2.6/OST1 for red light- and CO2-induced stomatal opening (Hashimoto et al., 2006; Matrosova et al., 2015). Raf43 is required for tolerance to multiple abiotic stresses, including osmotic, drought, salinity, and oxidative stresses, and ABA response, but it is not necessary for biotic resistance to Botrytis cinerea and P. syringae pv. tomato DC3000 (Virk et al., 2015). Raf36 is an ABA-negative regulator with partial functional redundancy with a subgroup C6 kinase, Raf22 (Kamiyama et al., 2021a).
Subgroup C6 - Raf22 is an ABA negative regulator, like Raf36. In addition, Raf22, together with Raf28, is an essential component of embryogenesis, and auxin polar transport may be involved in this regulation (Wang et al., 2018).
Subgroup C7 - Among the five subgroup C7 kinases, only three kinases, including convergence of blue light and CO2 1–2 (CBC1–2) and Arabidopsis thaliana MLK/Raf-related protein kinase 1 (ATMRK1), have been characterized (Hiyama et al., 2017; Hayashi et al., 2020; Kamiyama et al., 2021a). CBC1–2 negatively regulates stomatal closure by plasma membrane H+-ATPase phosphorylation in dark, blue-light, and low [CO2] conditions (Hiyama et al., 2017; Hayashi et al., 2020). ATMRK1 has only been studied for its sequence similarity to the catalytic domain of mammal mixed-lineage kinase and Raf kinase homologs in Arabidopsis (Ichimura et al., 1997). In general, almost all subgroup C kinases are related to light responses and multiple abiotic stress responses, including ABA responses (Hashimoto et al., 2006; Matrosova et al., 2015; Virk et al., 2015; Brauer et al., 2016; Hayashi et al., 2017; Hiyama et al., 2017; Dong et al., 2020; Hayashi et al., 2020; Sun et al., 2022). Moreover, several subgroup C kinases play a role in vascular, chloroplast, and seed development (Ceserani et al., 2009; Lamberti et al., 2011; Wolff et al., 2015; Wang et al., 2018).
3.3 ZIK-like kinases
ZIK-like kinases were first discovered by (Xu et al., 2000) and named WNKs (with no lysine in subdomain II of kinase). Since then, they have been classified as a new MAPKKK family in mammals (Wang et al., 2008). ZIK-like kinases include 10 members in Arabidopsis and are suggested to regulate plant flowering time, growth, and development (Wang et al., 2008; Kumar et al., 2011; Zhang et al., 2013). Among ZIK-like kinases, AtWNK1 phosphorylates the putative circadian clock component APRR3 (Kumar et al., 2011). AtWNK8 has been shown to auto-phosphorylate and then phosphorylate Arabidopsis subunit C (AtVHA-C) of vacuolar H+-ATPase (Hong-Hermesdorf et al., 2006). In addition, AtWNK8 is related to severe salinity and osmotic stresses as a negative regulator (Zhang et al., 2013). Conversely, AtWNK9 is a positive regulator in ABA-mediated dehydration responses (Xie et al., 2014). In D-glucose sensing, AtWNK1, 8, and 10 play specific roles in the plant's metabolism, stress response, and developmental signalling by distinguishing the low concentration-long exposure duration (AtWNK1) from the high concentration-momentary exposure duration (AtWNK8 and 10) of D-glucose (Fu et al., 2014). Overall, ZIK-like kinases play a role in regulating developmental signalling in response to abiotic stress.
4 FUNCTIONAL INVOLVEMENT OF MAP3KS IN ABA SIGNALING RESPONSES
ABA is an essential stress hormone in the plant kingdom (Sun et al., 2019). ABA core signalling depends upon phosphorylation and dephosphorylation (Hauser et al., 2017). ABA-mediated SnRK2s, classified into subclass III and including SnRK2.2, SnRK2.3, and OST1 in Arabidopsis, are essential for the direct phosphorylation of downstream target proteins (Fujii & Zhu, 2009). Sometimes, SnRK2s are phosphorylated by other kinases as well as themselves (Nguyen et al., 2019; Lozano-Juste et al., 2020). Together, subgroup B2 and B3 kinases are classified as activation of 100-kDa osmotic stress-activated protein kinases (OK100). OK100 members phosphorylate subclass III SnRK2s in ABA-mediated and ABA-unmediated osmotic stress responses (Nguyen et al., 2019; Katsuta et al., 2020; Lozano-Juste et al., 2020; Takahashi et al., 2020). Within the OK100 group, Raf6 activates the kinase activity of OST1 through phosphorylation, even when OST1 has no auto-phosphorylation and is dephosphorylated by HAB1 during ABA-mediated osmotic stress responses (Takahashi et al., 2020).
Conversely, subclass III SnRk2s phosphorylate Raf36, 22, and 28 in response to ABA during the post-germinative growth stage. Raf22 is dephosphorylated by ABI1, a negative regulator of the ABA signalling pathway, and this dephosphorylation leads to the activation of the kinase activity of Raf22, which was previously inhibited by SnRK2-mediated phosphorylation. Afterwards, activated Raf22 enhances the phosphatase activity of ABI1 by phosphorylation, thereby inhibiting SnRK2s during the post-germinative growth stage (Kamiyama et al., 2021a; Sun et al., 2022). In pepper plants, the proteins CaADIK1 and CaMEKK23 are inhibited through dephosphorylation by pepper PP2C proteins, and CaMEKK23 is also phosphorylated by pepper SnRK2.6 in ABA-mediated drought response (Jeong et al., 2021; Jeong et al., 2023).
Plant MAP3Ks are also shown to be associated with the ABA signalling pathway, although it is unclear whether the MAPKs are linked to ABA core components directly or indirectly. The expression patterns of AtMAPKKK16–18 and AtWNK9 are known to be altered in response to ABA treatment. AtMAPKKK16-OX Arabidopsis showed an ABA-insensitive phenotype during germination and cotyledon greening but demonstrated ABA-hypersensitive root growth; relatedly, AtMAPKKK16 phosphorylates ABR1, a negative regulator of the ABA response (Choi et al., 2017). In contrast, AtMAPKK18-OX and AtWNK9-OX Arabidopsis showed ABA-sensitive phenotypes (Xie et al., 2014; Li et al., 2017). AtMAPKKK18 positively regulates drought stress resistance by phosphorylating AtMAPKK3, and its expression is induced by osmotic stress in the ABA-dependent signalling pathway (Li et al., 2017). There is also evidence that AtMAPKKK18 is directly associated with PP2Cs and SnRK2s: the ABA response of AtMAPKKK18 is negatively regulated through ABI1, which dephosphorylates AtMAPKKK18, and AtMAPKKK18 interacts with SnRK2.6 (Mitula et al., 2015; Tajdel et al., 2016). In addition, the drought stress tolerance of AtMAPKKK18 is negatively modulated by the RING finger proteins RGLG1 and RGLG2 through poly-ubiquitination, leading to the degradation of AtMAPKKK18 proteins via the 26 s-proteasomal pathway (Yu et al., 2021). In pepper, the expression patterns of CaMEKK15–17 and CaMEKK20–25, which belong to subgroup A5 of pepper MAP3Ks, are altered by ABA treatment (Lim et al., 2020). Silencing of CaAIMK1/CaMEKK24 and CaDIMK1/CaMEKK21 leads to an ABA-sensitive phenotype. In contrast, CaAIMK1-OX and CaDIMK1-OX plants showed more ABA-sensitive phenotypes than wild-type plants, and CaAIMK1K32N-OX and CaDIMK1K32N-OX plants showed a similar ABA-response phenotype as the wild type, indicating that their kinase activity is required for ABA responses (Jeong et al., 2020; Kim et al., 2021).
5 FUNCTIONAL INVOLVEMENTS OF MAP3KS IN ABIOTIC STRESSES
Most subgroups of MAP3Ks are associated with stress responses (Table 1). Among these stresses, salinity, osmotic, dehydration, drought, oxidative, and temperature stresses are closely related to signalling transduction through the phosphorylation or kinase activity of MAP3Ks.
| Species | MAP3K | Upstream Regulator | Downstream regulator | Stress | ABA-dependent/independent | references |
|---|---|---|---|---|---|---|
| Arabidopsis thaliana | AtMEKK1 | - | AtMKK2 | Salinity/Osmotic/Drought/Cold | Independent | (Mizoguchi et al., 1996; Kim et al., 2011; Zhao et al., 2017) |
| B2 Raf-like kinases (7–12) | - | Subclass III SnRK2s | Osmotic | Dependent | (Lin et al., 2020; Lin et al., 2021) | |
| B3 Raf-like kinases (1–6) | - | Osmotic | Dependent/independent | (Takahashi et al., 2020; Katsuta et al., 2020; Lin et al., 2021) | ||
B4 Raf-like kinases (16, 18, 20, 24 35, 40 and 42) |
- | Subclass I SnRK2s | Osmotic | Independent | (Soma et al., 2020) | |
| Raf-like 43 | - | - | Salinity/Osmotic/Drought/Oxidative | Dependent | (Virk et al., 2015) | |
| Raf-like 36 | OST1 | - | Drought | Dependent | (Kamiyama et al., 2021) | |
| Raf-like 22 | OST1,ABI1 | ABI1 | Drought | Dependent | (Kamiyama et al., 2021; Sun et al., 2022) | |
| Raf-like 28 | - | - | Drought | Dependent | (Kamiyama et al., 2021) | |
| AtMAPKKK16 | - | ABR1 | Salinity | Dependent | (Seo-wha et al., 2017) | |
| AtMAPKKK17 | - | AtMKK3 | Drought | Dependent | (Danquah et al., 2015) | |
| AtMAPKKK18 | ABI1,RGLG1/2 | AtMKK3 | Drought | Dependent | (Mitula et al., 2015; Yu et al., 2021)) | |
| STY46 | - | - | Salinity/Osmotic | Dependent | (Dong et al., 2020) | |
| AtWNK8 | - | - | Salinity/Osmotic | - | (Zhang et al., 2013) | |
| AtWNK9 | - | - | Drought | Dependent | (Xie et al., 2014) | |
| Capsicum annuum | CaMEKK15-17 | - | - | Drought | Dependent | (Lim et al., 2020) |
| CaMEKK20 | - | - | Drought | Dependent | (Lim et al., 2020) | |
| CaDIMK1 | - | - | Drought | Dependent | (Kim et al., 2021) | |
| CaADIK1 | CaADIP1 | - | Drought | Dependent | (Jeong et al., 2021) | |
| CaMEKK23 | CaADIP1/CaAIPP1/ CaAITP1, CaSnRK2.6 | - | Drought | Dependent | (Jeong et al., 2023) | |
| CaAIMK1 | - | - | Drought | Dependent | (Jeong et al., 2020) | |
| CaMEKK25 | - | - | Drought | Dependent | (Lim et al., 2020) | |
| Gossypium hirsutum | GhRaf19 | - | - | Salinity/Drought/Oxidative/Cold | Dependent | (Jia et al., 2016) |
| GhMAP3K15 | - | GhMKK4 | Drought | Independent | (Li et al., 2017) | |
| GhMAP3K40 | - | Putative GhMKK4 | Oxidative | Dependent | (Chen et al., 2015; Xie et al., 2023) | |
| GhMAP3K62 | GhMKK16 | Drought | Independent | (Chen et al., 2022) | ||
| Nicotiana tabacum | NtNPK1 | - | - | Salinity/ Oxidative/Cold | - | (Kovtun et al., 2000) |
- * - : not determine
5.1 Salinity stress
Salinity stress is an environmental stress that can significantly impact plant growth and development. In plants, accumulation of sodium can lead to reduced photosynthesis, reduced turgor pressure for expansion growth (known as osmotic stress), adverse cellular water relations in the apoplast, changes in enzyme activities, damage from reactive oxygen species, and alterations in hormonal concentrations (Flowers et al., 2014; Zhao et al., 2021). Several MAP3Ks have been demonstrated to act as negative regulators in salt stress responses. As a member of the ZIK family, AtWNK8 gene overexpression in Arabidopsis plant caused less growth and lower fresh weight than wild-type plants in the presence of NaCl, as opposed to AtWNK8 knock-out Arabidopsis, which exhibited more growth and higher fresh weight, catalase, and peroxidase activity than wild-type plants (Zhang et al., 2013). AtWNK8 knock-out Arabidopsis plants also showed an ABA-sensitive phenotype compared to WT plants (Li et al., 2021). However, it remains unclear whether enhanced tolerance of AtWNK8 knock-out plants to salt is associated with ABA signalling. In cotton, the expression level of GhRaf19 transcripts and the protein level of GhRaf19 were reduced by NaCl and ABA treatment (Jia et al., 2016). Furthermore, GhRaf19-OX tobacco plants exhibited lower growth and survival rates than wild-type plants in the presence of NaCl. These results suggest that GhRaf19 could be involved in the inhibition by salinity stress via the ABA signalling pathway. On the other hand, there is evidence that MAP3Ks are positive regulators in salinity stress responses. For example, the expression levels of STY46, AtMAP3K16, and Raf43 are increased by salinity stress, and their knock-out Arabidopsis mutants have shown a salt-tolerant phenotype (Virk et al., 2015; Choi et al., 2017; Dong et al., 2020). Overexpression of STY46 (STY46-OE25) accumulates more sugars in leaves and accelerates root growth under salinity stress (Dong et al., 2020). The STY46 transcripts were up-regulated earlier by ABA treatment than by the salt stress, which suggests that STY46 may be involved in an ABA-dependent salt stress (Dong et al., 2020). The AtMAP3K16 and Raf43 knock-out Arabidopsis mutants exhibited an ABA-sensitive phenotype in the germination rate, and this pattern was similarly shown in the germination rate under salt stress (Virk et al., 2015; Choi et al., 2017). These observations suggested that AtMAP3K16 and Raf43 are involved in ABA-dependent salt stress responses. In the MAPK cascade, the AtMEKK1-AtMKK2-AtMAP4/6 cascade is established in response to ABA-independent salt stress: AtMEKK1 expression is upregulated under salt stress, but not ABA and the AtMEKK1 protein has been shown to interact with AtMKK2. In turn, AtMKK2 phosphorylates AtMAPK4/6 in response to salt stress (Covic et al., 1999; Teige et al., 2004).
5.2 Osmotic stress
Osmotic stress inhibits cell division, expansion, and glucose transport during leaf growth (Roth et al., 1985; Kalve et al., 2020). Osmotic stress can also be imposed by drought, salinity, and cold stress (Xiong & Zhu, 2002). Osmotic regulation is maintained by osmolytes, such as potassium ions [K+], sugars, amino acids, and secondary metabolites (SMs) (Osakabe et al., 2013; Isah, 2019). The main sensors of osmotic stress are subclass I and III SnRK2s (Lozano-Juste et al., 2020). All SnRK2s except for SnRK2.9 are activated by osmotic stress, and subclass III SnRK2s are specifically ABA-activated kinases, while subclass I SnRK2s are not (Lozano-Juste et al., 2020; Kamiyama et al., 2021b). In ABA-unmediated osmotic stress responses, subclass I SnRK2s are related to subgroup B4 Raf-like kinases (OK130) (Fàbregas et al., 2020; Lin et al., 2020; Lozano-Juste et al., 2020; Soma et al., 2020). In these pathways, subclass I SnRK2s are activated by OK130 Raf-like MAP3Ks, which occur independently of downstream MAP2Ks in the MAPK cascade. In response to osmotic and drought stress, Raf 18/20/24 phosphorylate ABA-unresponsive subclass I SnRK2s (Soma et al., 2020).
5.3 Drought stress
Drought stress affects plant growth, leaf and stem dry weights, root growth, and plant height. In response to drought stress, plants show changes in stomatal conductance, transpiration rate, photosynthetic rate, and water potential, possibly caused by osmotic stress (Mahmood et al., 2020). The AtMAPKKK17/18-AtMKK3-AtMPK1/2/7/14 cascade is a representative example of the ABA-mediated drought response (Danquah et al., 2015; Matsuoka et al., 2015). Many homologues of AtMAPKKK17/18 have been reported to function in drought stress responses, whether they be ABA-mediated or -unmediated. In cotton, the GhMAP3K62-GhMKK16-GhMPK32 cascade is activated under drought stress, and the transcription factor GhEDT1, which binds to the promoter of GhNCED3 and activates GhNCED3 expression, thereby improves drought tolerance by regulating ABA accumulation and stomatal closure (Chen et al., 2022). The GhMAP3K15 cascade progresses through the GhMAP3K15-GhMKK4-GhMPK6 pathway, related to the ABA-unmediated drought stress response. The final target of this pathway is the transcription factor GhWRKY59, which binds to W-boxes of GhDREB2. GhDREB2 encodes a transcription factor of drought response genes and is a positive feedback regulator of GhMAP3K15, which increases the expression level of GhMAP3K15 as a transcription factor (Li et al., 2017). Although the MAPK cascade pathway has not been established in pepper, several MEKKs are shown to play a critical role in drought stress responses. For example, CaAIMK1/MEKK24 and CaDIMK1/MEKK21 act as positive regulators in ABA-mediated drought responses through their kinase activities (Jeong et al., 2020; Kim et al., 2021). Similarly, overexpression of the kinase-dead mutants CaADIK1/CaMEKK22K32N and CaMEKK23K32N did not alter drought tolerance, although CaADIK1-OX and CaMEKK23-OX plants showed a more drought-tolerant phenotype (Jeong et al., 2021; Jeong et al., 2023). The kinase activity of CaADIK1 is negatively modulated by the phosphatase activity of CaADIP1, one of the PP2C proteins in pepper. In response to drought stress, CaADIK1/CaADIP1-OX plants exhibited lower ABA/drought stress-responsive gene expression than CaADIK1-OX plants but showed a similar expression pattern to CaADIP1-OX plants, suggesting that CaADIP1 suppresses the drought-tolerant phenotype driven by CaADIK1 (Jeong et al., 2021). For CaMEKK23, kinase activity is inhibited by CaADIP1 and other pepper PP2Cs such as CaAIPP1 and CaAITP1. CaMEKK23- and CaAITP1/CaMEKK23-silenced peppers showed ABA-mediated drought-sensitive phenotypes, unlike CaAITP1-silenced pepper, which exhibited a drought-tolerant phenotype (Jeong et al., 2023). CaMEKK23 is also phosphorylated by CaSnRK2.6 in vitro, suggesting that CaMEKK23 is associated with the ABA core signalling components CaPP2Cs and CaSnRK2.6 (Jeong et al., 2023).
5.4 Oxidative stress
Oxidative stress can damage cell components and cause dysfunction by inducing reactive oxygen species (ROS) generation under abiotic and biotic stresses (Demidchik, 2015; Casartelli et al., 2019; Erol et al., 2020). The change in gene expression induced by oxidative stress aims to prevent the overproduction of ROS and improve resistance to abiotic and biotic stress (Demidchik, 2015; You & Chan, 2015). Among MAP3Ks, Arabidopsis Raf43 and GhMAP3K40 are shown to be involved in oxidative stress responses (Chen et al., 2015; Virk et al., 2015). In Arabidopsis, the expression level of Raf43 was increased by oxidative stress (Virk et al., 2015). Raf43-knockout Arabidopsis showed less tolerance to oxidative stress and consistently had lower chlorophyll content than wild-type plants after treatment with methyl viologen (MV) (Virk et al., 2015). In addition to oxidative stress, Raf43 is possibly associated with both ABA response and ABA-mediated signalling pathway (Virk et al., 2015). Loss-of-function of Raf43 led to enhanced ABA sensitivity, suggesting that Raf43 might be involved in oxidative stress response in an ABA-dependent manner. In N. benthamiana plants, overexpression of GhMAP3K40 results in an oxidative stress-sensitive phenotype (Chen et al., 2015). This sensitivity is attributed to the plants' weakened ability to handle ROS under the conditions of MV treatment, drought stress, and biotic stress. The activation of the GhMAP3K40-GhMAPKK-GhMAPK pathway, possibly the GhMAP3K40-GhMAPKK4-GhMAPK4 cascade, is implicated in this response. The existence of this cascade is supported by the interaction between GhMAP3K40 and GhMAPKK4, as well as that between GhMAPKK4 and GhMAPK4. Additionally, there was an effect of ABA treatment on the expression of these three kinase genes, and similar expression was observed in response to oxidative stress, however, GhMAP3K40 expression remained unaffected (Li et al., 2014; Chen et al., 2015). These findings suggest that the GhMAP3K40-GhMAPKK4-GhMAPK4 cascade may participate in oxidative stress response through an ABA-dependent pathway.
5.5 Cold stress
Chilling (0–15°C) and freezing (0°C) can cause cold stress in plants. Chilling affects membrane stiffening in plant cells and disturbs protein stability, protein complexes, and enzymes involved in ROS scavenging (Ding et al., 2019). In contrast, freezing stress is more damaging to plants and may lead to cell death (Ding et al., 2019). During freezing stress, ice crystals form within plant cells and spread into the apoplast, leading to water efflux, cell dehydration, and irreversible cell damage (Ninagawa et al., 2016; Ding et al., 2019). In Arabidopsis, the MKK4/5-MPK3/6 pathway inhibits CBF-dependent cold signalling, but not SnRK2.6-dependent cold signalling associated with ABA signalling. In this cascade, MPK3/6 phosphorylate ICE1, leading to the 26 s-proteasomal degradation of ICE1 through poly-ubiquitination under cold stress conditions (Li et al., 2017). In contrast, the MEKK1-MKK2-MPK4 pathway, which is activated by CaM-regulated receptor-like kinase (CRLK)1/2, positively regulates ABA-unmediated cold stress signalling (Teige et al., 2004; Furuya et al., 2013). MPK4 inhibits the kinase activity of MPK3/6, but the detailed mechanism behind this process remains unknown (Zhao et al., 2017; Guo et al., 2018).
6 CONCLUSION
MAP3Ks are important for plant growth, survival, and various abiotic stress responses, including responses to drought, salinity, osmotic stress, and changes in leaf temperature. In this review, we aimed to present examples illustrating the diverse regulatory effects of MAP3Ks on abiotic stress responses through different signalling pathways (Figure 2). Under normal conditions, MEKKs are inhibited by PP2Cs, leading to suppressed stress resistance responses (Mitula et al., 2015; Jeong et al., 2021; Jeong et al., 2023). In addition, subgroup C Raf-like kinases are released by PP2Cs and, in turn, activated subgroup C Raf-like kinases phosphorylate PP2Cs to enhance PP2C phosphatase activity (Sun et al., 2022). Subgroup C Raf-like kinases also phosphorylate downstream substrates (transcription factors, cytoskeletal proteins, kinases, etc.) through the MAPK cascade, which induces normal growth (Wang et al., 2018). Under stress conditions, stress-activated subgroup B Raf-like kinases activate SnRK2 kinases through phosphorylation (Takahashi et al., 2020), and, in turn, MEKKs are phosphorylated by SnRK2s (Jeong et al., 2023). Finally, each MAPK cascade is triggered and phosphorylates downstream substrates, which regulate abiotic stress responses (Umezawa et al., 2013).
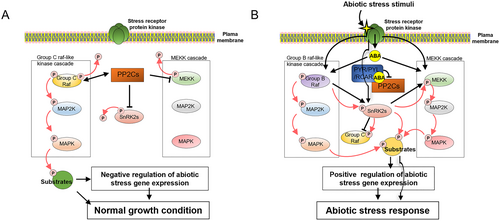
However, many components of our current understanding of these processes are still unclear. First, certain details of the MAP2K-MAPK pathway, specifically those related to subgroup C Raf-like kinases, remain unknown (Wang et al., 2018). In addition, though the association between group B Raf-like kinases and various SnRK2s under osmotic and drought stress has been established, the relationship between MEKKs and SnRK2s has only been studied in the case of SnRK2.6 (Tajdel et al., 2016; Lozano-Juste et al., 2020; Jeong et al., 2023). Finally, the involvement of subgroup B Raf-like kinases in responses to drought and osmotic stress has been observed specifically in Arabidopsis, but their roles in responding to other stresses and in other species have not yet been investigated (Lozano-Juste et al., 2020; Soma et al., 2020). Further research is necessary to elucidate the roles of MAPK cascades, the SnRK2 signalling pathway, and the interplay between MAPK cascades and SnRK2 signalling in responding to abiotic stress. A more comprehensive understanding of the intricate mechanisms underlying plant responses to various abiotic stresses can be achieved by resolving these knowledge gaps.
AUTHOR CONTRIBUTIONS
SJ, CWL, and SCL: conceptualization; SJ, MK and CWL: data analysis; SJ, CWL, and SCL: writing.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Agriculture & Technology Development (Project No. RS-2024-00322140) and the Chung-Ang University Graduate Research Scholarship in 2023.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




