Plant responses to temperature stress modulated by microRNAs
Abstract
Due to the increasing impact of worldwide environmental changes, temperature stress has become a major factor resulting in crop yield losses. The discovery of temperature-stress-responsive protein-coding genes has made significant progress in understanding plants' complex stress response systems involving microRNAs (miRNAs). The miRNAs are triggered by heat or cold, thus confirming their significant functional role in cold or heat tolerance. Such dependable recommendations significantly broaden our understanding of the regulatory role of miRNAs in plant stress responses. This article presents novel perspectives on the substantial roles of plant miRNAs in responding to and acclimatizing to heat and cold stress. It comprehensively elaborates on miRNAs responsive to temperature stress, their regulatory mechanisms, and their targeted functions in plants. Additionally, the article investigates how miRNAs contribute to safeguarding plant reproductive tissues, mitigating damage caused by reactive oxygen species, modulating heat shock proteins, transcription factors, and phytohormones in the context of temperature stress. The conclusion outlines potential avenues for future research, highlighting the utilization of miRNAs and their regulatory functions to develop economically vital crops with enhanced tolerance to temperature stress, thereby ensuring future food security.
1 INTRODUCTION
The global distribution and productivity of plants are significantly impacted by temperature. Excessively high or low temperatures can lead to a broad spectrum of adverse effects on plant productivity (Vogel et al. 2019; El Bilali et al. 2020). Reproduction is the most sensitive phase of a plant's life cycle. Even a slight fluctuation in temperature during flowering time can have disastrous consequences, resulting in heavy yield losses (Cohen et al. 2021b; Farooq et al. 2023). Given the projected rise in average surface temperature by 2.0 to 4.6°C by the end of the 21st century, plants are anticipated to encounter significant challenges, especially with the more frequent onset of warmer seasons in the coming years (Buzan and Huber 2020; Shakoor et al. 2023).
The effects of global warming will not only increase the average yearly temperatures globally but also lead to a higher risk of extreme temperature episodes happening more often and with greater intensity (Shakoor et al. 2021a, 2021b). With the increasing frequency and persistence of temperature stress, it becomes essential to pinpoint genes linked to tolerance under such conditions. Furthermore, acquiring a more profound comprehension of the mechanisms and networks governing these genes is pivotal (Zhang et al., 2022). Numerous research studies have investigated the significant roles of microRNA (miRNA) in controlling plant development after transcription (Pandita and Wani 2019; Bhakta et al. 2021). miRNAs have been employed to post-transcriptionally regulate genes through diverse mechanisms, such as guiding the degradation of target mRNAs or inhibiting translation (Movahedi et al. 2018).
miRNAs play a pivotal role in supporting plant development and growth, encompassing processes like organ formation, hormone signalling, and adaptation to various environmental circumstances (Mallory and Vaucheret 2006; Islam et al. 2017, 2018b, 2018a, 2019a, 2022c; Chen et al. 2018). While substantial research has been conducted on miRNAs and their role in mitigating temperature stress, the identification and profiling of these miRNAs across diverse plant species remain limited. Furthermore, there is an incomplete understanding of the specific targets, related genes, and regulatory networks. Additionally, there is limited knowledge regarding the downstream effects on key physiological processes such as photosynthesis, flowering, and senescence, which are influenced by miRNA-mediated responses to temperature stress. Gaps in understanding persist regarding how miRNA-mediated post-transcriptional regulation integrates with temperature stress and signalling pathways within different plant species. This review underscores the importance of miRNAs and aims to provide comprehensive insights into those responding to temperature stresses in plants. We further elucidate critical miRNA mechanisms involved in the plant response to temperature changes, encompassing both cold and heat stress, including their vital roles in phytohormone signalling and other genetic pathways. The review concludes by proposing future research directions to harness the regulatory roles of miRNAs, thereby facilitating the development of temperature stress-tolerant crops and ensuring future food security.
2 BIOGENESIS OF miRNAs IN PLANTS
Plant miRNAs are essential in controlling various stages of development, such as germination, leaf formation, and flowering time. They also have a crucial function in modulating plant response to environmental stressors. The miR genes in plants produce approximately 22 nucleotide-long RNAs, and these precursor RNAs have a stem-loop structure that is partially double-stranded. Dicer-like (DCL) proteins are responsible for processing these precursor RNAs to release mature miRNAs (Fig. 1)(Fard et al. 2020).
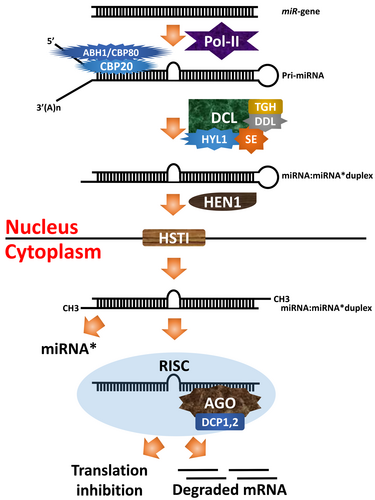
However, recent research has unveiled the structural arrangements of Arabidopsis DCL1, a pri-miRNA, and a pre-miRNA. According to this study, the PAZ domain exhibits the ability to alter its conformation, a critical aspect for recognizing pri-miRNA and pre-miRNA. Furthermore, the study suggests that the helicase module functions as an engine, shuttling the substrate between two cleavage stages (Chen 2005; Wei et al. 2021). Following post-transcriptional regulation, mature miRNAs disperse throughout the cytoplasm, including RNA granules, mitochondria, and endo-membranes. Recent research has also provided evidence that newly synthesized miRNAs exist in the nucleus, potentially playing significant roles in epigenetic regulation (Li and Yu 2021). The propensity of miRNAs to influence epigenetic regulation, such as DNA and histone methylation and control targets at the post-translational phase, makes them a vital tool in regulating gene expression post-translationally (Wang et al. 2019). Numerous reviews have comprehensively explained the development of miRNAs in plants (Chen 2005; Voinnet 2009; Rogers and Chen 2013; Budak and Akpinar 2015; Gao et al. 2021).
3 ROLE OF microRNAs IN TEMPERATURE STRESS
3.1 miRNAs and heat stress
miR156 is an extensively expressed miRNA in plants and is highly conserved. It participates in various functions (Wang et al. 2020), and its regulatory module with squamosa promoter binding protein-like (SPL) helps in a sequence of responses from the vegetative to reproductive phases (Feyissa et al. 2019) (Fig. 2). Observations by Feyissa et al. (2020) indicate that higher concentrations of miR156 are present in young shoot tissues. As the plant germination process unfolds, its expression diminishes, resulting in a decrease in quantity, thus fostering a conducive environment for flowering (Zheng et al. 2019; Islam et al. 2023). On the other hand, SPL levels trigger the flowering MADS-box gene called FRUITFULL (FUL). Notably, the miR156-SPL regulatory module was discovered to be vulnerable to natural temperature variations, particularly during the timing of flowering (Xie et al. 2020) (Fig. 2). Delayed flowering occurs as a result of miR156 overexpression in plants exposed to a lower temperature of 16°C. In contrast, the excessive production of SPL3, which is resistant to miR156, enhances early flowering, irrespective of temperature fluctuations. This is a common occurrence that highlights the significance of temperature changes in the thriving and survival of plants (Liu et al. 2017a; Waheed and Zeng 2020).
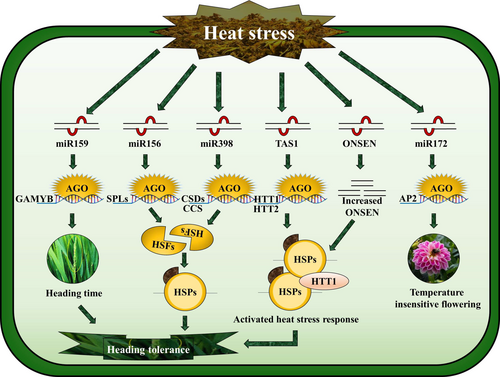
It was discovered that miR156 is heat-inducible, which means that its activation can last for days to weeks. miR156 is particularly important for heat stress memory, and it is increased to a level that promotes and strengthens memory (Stief et al. 2014) (Fig. 2). In Arabidopsis, the involvement of this miRNA in heat stress memory was further linked to the suppression of two key SPL variants (SPL2 and SPL11)(Stief et al. 2014). The study results additionally unveiled that extended periods of heat stress can amplify the expression of heat stress-responsive genes, ultimately augmenting thermo-tolerance. In this context, the expression of miR156 also contributes to this phenomenon (Stief et al. 2014).
miR172 is another miRNA with an opposite function to miR156. Unlike miR156, miR172 exhibits a lower juvenile phase expression but a higher adult phase expression (Silva et al. 2019), which is influenced by two target genes (the target of eating 1 and 2; TOE1 and TOE2, respectively) (Wu et al. 2009). miR172 abundance is less heat stable than miR156; for example, heat-responsive changes at 16°C differ significantly from those at 23°C (Wu et al. 2009). Furthermore, miR172 could act in thermo-sensory flowering as overexpressing of this particular miRNA results in temperature-insensitive flowering (Zhu and Helliwell 2011). Flowering time control protein (FCA) increases miR172 abundance during the flowering and vegetative phases (Fig. 2). The FCA binds to the principal miR172 transcripts in response to a transitory increase in temperature (Airoldi et al. 2015). More specifically, miR172's crucial regulatory role in controlling blooming time under heat stress has been discovered (Ó'Maoiléidigh et al. 2021). Notably, due to their unique gene targets, miR156 and miR172 played a significant opposite role in heat-sensory blooming in opposite directions.
miR398 was found to be expressed more at 23°C than at 16°C (Zhu and Helliwell 2011). This suggests that miR398 contributes to Arabidopsis' response to temperature stress (Chen et al. 2013). Numerous additional investigations have also validated the heat-stress inducibility of the miR398 genes (Guan et al. 2013; Lu et al. 2013; Li et al. 2020c). The mechanism by which heat stress tolerance is imparted involves specific reductions at active sites within the copper chaperone for superoxide dismutase (SOD) transcripts of their target genes, CCS, CSD1, and CSD2 (Wang et al. 2017) (Fig. 2). Gossypium hirsutum mutant plants exhibited lesser susceptibility to heat stress compared to genetic models expressing miR398-resistant variants of CSD1, CSD2, and CCS. This observation suggests that miR398 expression has a direct impact on plant responses to heat stress. In contrast, wild-type (WT) plants displayed higher heat stress tolerance (Wang et al. 2017). Moreover, a pivotal aspect of the plant's reaction to heat stress involves the induction of a critical mitochondrial protein known as heat shock proteins-60 and 10 (HSP60 and HSP10), triggered by the activation of heat stress transcription factors (HSFs) (David et al. 2021). The rate of heat change dramatically alters the transcription level of HSFs and HSPs (Samakovli et al., 2020). The HSFA1 and HSFA7 proteins could potentially target the promoter regions of miR398, activating their expression. This accomplishment lays the groundwork for a thermo-tolerance method (Zhao et al. 2021a).
In contrast to miR156 and miR398, miR159 participates in intercommunication with diverse plant hormones, including gibberellic acid, and governs the heat stress response through a group of transcription factors known as GAMYB-like (Dermastia 2019; Islam et al. 2019b). After being exposed to heat stress, the CSA-miR159 genes (b-variant) of Cucumis sativus plants were discovered to be repressible (Li et al. 2016). However, in Arabidopsis, the reverse effect was observed upon overexpressing the same gene (Szaker et al. 2020). Compared to WT C. sativus plants, overexpression of another gene variant termed tamilR159 was observed to respond to minor temperature differences (Li et al. 2016). In summary, the preceding data established miR159's detrimental role in heat tolerance, regardless of the temperature (Li et al. 2016).
miR396 stimulates the cleavage of HaWRKY6 in Helianthus annuus (Giacomelli et al. 2012). Plants overexpressing HaWRKY6, particularly the variant resistant to miR396, displayed vulnerability to heat stress. This observation underscores the critical involvement of miR396 regulation in the early responses to elevated temperatures (Giacomelli et al. 2012). Growth-regulating transcription factor (GRF) targeted by miR396 also controls leaf/shoot development and proliferation (Wang et al. 2011). This finding demonstrated that miRNA, which regulates development under normal conditions, is also engaged in protecting H. annuus during high-temperature conditions (Wang et al. 2011).
Alternative splicing (AS), is a well-known process observed during gene expression in different species of plants as a potential temperature regulator (dubbed "molecular thermometer") (Sajjanar et al. 2017). AS allows plants to adapt the number of functional transcripts to environmental disturbances and stressors transitory and regulate accumulated miRNA in Arabidopsis under heat stress (Godoy Herz and Kornblihtt 2019; Dikaya et al. 2021). In Arabidopsis, heat stress induces the downregulation of intronic miR400, which is co-transcribed with its host gene, reducing the production of primary transcripts for miR400 (Yan et al. 2012). The heat induction caused by the AS event was very apparent. The AS occurrences happened explicitly in the interionic region's nitrogenous base pair (306 bp). Following the deletion of the 100 base pair fragment, the intron, which was 206 base pairs long and contained the target transcripts, remained connected to the host gene. Overexpression of miR400 transgenic plants promotes reduced germination rates, hypocotyl and root growth retardation compared to WT plants. This, in essence, demonstrated that heat stress harmed miR400 in plants (Yan et al. 2012).
3.2 miRNAs and cold stress
Approximately ten years ago, a genome-wide sequencing study utilizing microarray technology identified numerous miRNAs responsive to temperature stress across various plant species (Sansaloni et al. 2011). For example, within specific Oryza sativa varieties, a conserved miR319 was discovered to play a positive regulatory role in cold tolerance. This was achieved by facilitating the expression of crucial transcription factors, namely OsTCP21 and OsPCF6 (Wang et al. 2014b)(Fig. 3). Apart from O. sativa, the significant role of miR319 in cold stress response has been demonstrated in both flowering and non-flowering plants (Cheng et al. 2020). Following a 24-hour exposure to a temperature of 4°C, there was a significant elevation observed in the levels of miR319 within both the root and shoot tissues of Saccharum officinarum (Thiebaut et al. 2012; Yang et al. 2016). Furthermore, miR319 has been implicated in cold stress responses in Solanum lycopersicum (Zhao et al. 2015), Arabidopsis (Song et al. 2016) and Triticum aestivum (Wang et al. 2014a), underscoring its involvement in cold stress response across different plant species.
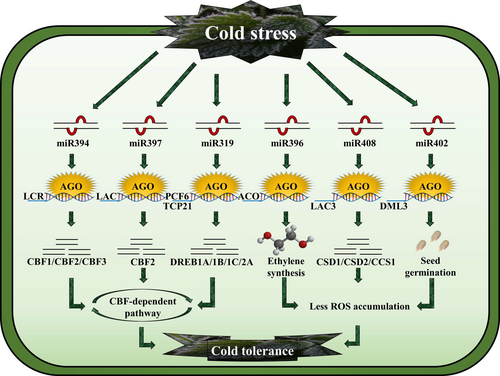
As previously stated, reducing transcription factor activity increased cold stress resistance, possibly due to changes in reactive oxygen species (ROS) levels. In contrast, enhancing OsmiR156K expression in certain species led to reduced cold tolerance (Cui et al. 2015). Moreover, Northern blot analysis demonstrated that increased miR397 levels enhance Arabidopsis' ability to endure cold stress. Overexpressing this miR397 variant impacts the expression of cold-regulated genes and subsequent downstream genes like COR15A, COR47A and RD29A (Dong and Pei 2014).
The leaf curling responsiveness (LCR) gene, a target of miR394 variants (Song et al. 2012), plays a critical role in influencing leaf morphological evolution and stem cell identity (Liu et al. 2018a) via encoding F-BOX protein. miR394 modulates plant responses to salinity, cold, and drought stress (Kumar et al. 2021). Cold stress significantly influences miR394 and LCR transcription. In comparison to WT plants, increased cold tolerance was observed in the miR394 overexpressing (35S: miR394) and lcr-mutant Arabidopsis plants; however, the LCR over-expressing (miR394 cleavage-resistant variant) plants showed an oversensitive phenotype (Fig. 3) (Dong and Pei 2014). Hydrophobic proline and total soluble sugars in the 35S: miR394 and LCR plants differed from WT plants under cold stress conditions (Dong and Pei 2014). The C-repeat binding factors (CBFs)/dehydration-responsive element-binding factors 1 (DREB1s)-dependent cold response pathway is essential for cold tolerance and acclimatization (Li et al. 2020b; Song et al. 2021) (Fig. 3).
Overexpressing miR396 in Citrus spp. enhances resistance to cold stress. This is achieved by suppressing the expression of ACO (1-aminocyclopropane-1-carboxylic acid oxidase), an enzyme essential for ethylene production (Zhang et al. 2016). Poncirus trifoliate miR396b regulates 1-aminocyclopropane-1-carboxylic (ACC), which triggers the expression of genes involved in ethylene polyamine homeostasis, contributing to cold tolerance (Zhang et al. 2016). miR396 positively influences cold tolerance by reducing ACO transcript levels (Fig. 3). Consequently, ethylene synthesis is suppressed, leading to the activation of polyamine synthesis, which aids in scavenging ROS (Zhang et al. 2016).
Another miRNA/ROS modulating agent in plants is miR408 (Ma et al. 2015; Jiang et al. 2018). The elevation of miR408 levels in plants has been associated with improved cold tolerance by reducing electrolyte leakage and enhancing the efficiency of photosystem II (Sun et al. 2018). In contrast to WT O. sativa plants, the knockout of miR408 resulted in heightened cold sensitivity (Sun et al. 2018). As indicated by biochemical and molecular analysis, reduced ROS and increased expression levels of genes linked to anti-oxidative functions, such as Glutathi-one-S-transferase (GST-U25) and copper/zinc SOD (CSD1 and CSD2), suggested that miR408-induced cold tolerance is due to increase in the capacity of cellular antioxidant (Taier et al. 2021).
Finally, there is miR404, which has been linked to seed germination (Schiessl et al. 2020) (Fig. 3). Through DNA methylation, miR404 acts as a promoter of seedling germination in Arabidopsis when subjected to cold stress. Demeter-like protein-3 (DML3) mRNA catalyzes the methylation process (Pratt et al. 2005; Zhou et al. 2008). When compared to WT plants exposed to 12oC, it was discovered that seed germination was faster in both the miR402 overexpressing and dml3 mutant plants. This explains the vital role of miRNA-mediated DNA demethylation regulation and its remarkable adaptation to cold stress (Zhou et al. 2008). To conclude, despite the computational discovery of numerous miRNAs responsive to cold stress, the functions and mechanisms of the majority of these miRNAs remain elusive. However, miRNAs play a multifaceted role in driving various changes in plant species, encompassing molecular, biological, and metabolic modifications that lead to repression, overexpression, oxidation, and reduction, depending on the context.
4 miRNAs MECHANISMS IN TEMPERATURE STRESS
4.1 miRNAs protect reproductive tissues
The reproductive phase in angiosperms represents a particularly vulnerable stage when confronted with elevated temperatures, as even a singular day of high temperatures can prove detrimental to the success of reproductive processes. One of the primary repercussions of heat stress on reproductive tissues, leading to a suboptimal seed set, is the occurrence of either premature or delayed flowering in plants (Buajan et al. 2018; Cohen et al. 2021a). As mentioned earlier, prior studies have established the pivotal roles of miR156 and miR172 in the regulation of flowering time. These two miRNAs have also been observed to undergo induction under heat stress conditions, signifying their crucial involvement in heat tolerance (Cohen et al. 2021a). This underscores the potential significance of microRNAs in the intricate signalling pathways that connect temperature perception to the initiation of flower set. Apart from miR156 and miR172, an array of five other miRNAs responsive to heat (miR159, miR169, miR393, miR397, and miR399) has been implicated in regulating flowering time in diverse plants (Zhang and Chen 2021; Zhou et al. 2021). Additionally, a group of seven heat-responsive miRNAs (miR159, miR164, miR166, miR167, miR171, miR172, and miR319) has been identified, directly influencing flower development or male and female fertility in plants (Table 1). This underscores their pivotal roles in governing the development of reproductive tissues under heat-stress conditions. In total, twelve heat-responsive miRNAs have been demonstrated to coordinate flowering time or influence flower development in plants. Furthermore, the heat-induced retro-transposition of ONSEN takes place during flower development and precedes gametogenesis, specifically avoiding vegetative tissues (Waheed and Zeng 2020; Haider et al. 2021) (Fig. 2). This emphasizes the critical regulatory role at the flowering stage when plants are exposed to heat stress. Remarkably, mutant plants such as csd1, csd2, and ccs, resembling those overexpressing miR398, exhibit heightened tolerance to heat stress, evidenced by a reduction in flower damage (Guan et al. 2013). In summary, it can be deduced that miRNAs play a significant role in ensuring the continuity of flower production despite challenging heat stress conditions, thereby enhancing transgenerational seed production (Liu et al. 2020a).
| miRNA | Target | Plant Species | Target Function | Regulation | Temperature Stress | Reference | |
|---|---|---|---|---|---|---|---|
| Cold | Heat | ||||||
| miR156 | SPLs | Triticum aestivum | Phase transition | Down | √ | (Xin et al. 2010) | |
| Prunus persica | Phase transition | Up | √ | (Barakat et al. 2012) | |||
| Arabidopsis thaliana | Flowering timing | Up | √ | (Stief et al. 2014) | |||
| Oryza sativa | Flowering timing | Down | √ | (Liu et al. 2017b) | |||
| Ipomoea batatas | Flowering timing | Down | √ | (Yu et al. 2020) | |||
| Arabidopsis thaliana | Flowering timing | Down | √ | (Zhou and Tang 2019) | |||
| miR157 | SPLs | Prunus persica | Unknown | Up | √ | (Barakat et al. 2012) | |
| Laminariales, Phaeophyta | Unknown | Down | √ | (Liu et al. 2015) | |||
| miR159 | MYBs, TCPs | Solanum lycopersicum | Flowering time | Up | √ | (Cao et al. 2014) | |
| Triticum aestivum | Flowering time | Down | √ | (Kumar et al. 2015) | |||
| Oryza sativa | Anther development | Down | √ | (Liu et al. 2017b) | |||
| Brassica rapa subsp. pekinensis | Flowering time | Down | √ | (Ahmed et al. 2020) | |||
| Raphanus sativus | Unknown | Up | √ | (Yang et al. 2019) | |||
| miR160 | ARFs | Triticum aestivum | Seed germination | Up | √ | (Xin et al. 2010) | |
| Populus tomentosa | Seed germination | Down | √ | (Chen et al. 2012) | |||
| Hordeum vulgare | Stress response | Down | √ | (Kruszka et al. 2014) | |||
| Oryza sativa | Stress response | Down | √ | (Liu et al. 2017b) | |||
| Arabidopsis thaliana | Seed germination | Up | √ | (Lin et al. 2018) | |||
| miR162 | DCLs | Cucumis sativus | MiRNA biogenesis | Down | √ | (Li et al. 2016) | |
| Oryza sativa | MiRNA biogenesis | Down | √ | (Liu et al. 2017b) | |||
| miR164 | NAC | Gossypium hirsutum | Root and leaf development | Up | √ | (Wang et al. 2016) | |
| Oryza sativa | Root and leaf development | Up | √ | (Liu et al. 2017b) | |||
| Manihot esculenta | Root and leaf development | Up | √ | (Li et al. 2020a) | |||
| miR166 | HD-ZIP | Solanum lycopersicum | Axillary meristem initiation | Up | √ | (Cao et al. 2014) | |
| Hordeum vulgare | Axillary meristem initiation | Down | √ | (Kruszka et al. 2014) | |||
| Panicum virgatum | Floral organ polarity | Up | √ | (Hivrale et al. 2015) | |||
| Musa × paradisiaca | Floral organ polarity | Up | √ | (Vidya et al. 2018) | |||
| miR167 | ARFs | Solanum lycopersicum | Male and female fertility | Down | √ | (Cao et al. 2014) | |
| Gossypium hirsutum | Male and female fertility | Up | √ | (Wang et al. 2016) | |||
| Solanum tuberosum | Metabolic pathways | Up | √ | (Yan et al. 2021) | |||
| Hemerocallis fulva | Metabolic and cellular processes | Up | √ | (Huang et al. 2023) | |||
| Vitis vinifera | Thermostability | Up | √ | (Zhang et al. 2023) | |||
| miR168 | AGOs | Panicum virgatum | MiRNA biogenesis | Up | √ | (Hivrale et al. 2015) | |
| Zea mays | MiRNA biogenesis | Down | √ | (He et al. 2019) | |||
| miR169 | NF-YA | Brachypodium spp. | Anther development; flower timing | Up | √ | (Zhang et al. 2009) | |
| Triticum aestivum | Anther development; flower timing | Up | √ | (Xin et al. 2010) | |||
| Triticum aestivum | Drought tolerance | Up | √ | (Wang et al. 2014a) | |||
| Laminariales, Phaeophyta | Drought tolerance | Down | √ | (Liu et al. 2015) | |||
| Arabidopsis thaliana | Root growth recovery | Down | √ | (Aslam et al. 2020) | |||
| miR171 | GRFs | Triticum aestivum | Floral development | Down | √ | (Kumar et al. 2015) | |
| Solanum lycopersicum | Floral development | Down | √ | (Cao et al. 2014) | |||
| Panicum virgatum | Abiotic stress response | Down | √ | (Hivrale et al. 2015) | |||
| miR172 | AP2 | Vitis vinifera | Lowering timing; floral organ identity | Up | √ | (Sun et al. 2015) | |
| Gossypium hirsutum | Lowering timing; floral organ identity | Down | √ | (Wang et al. 2016) | |||
| Solanum lycopersicum | Lowering timing; floral organ identity | UP | √ | (Keller et al. 2020) | |||
| Musa itinerans | Lowering timing; floral organ identity | Up | √ | (Liu et al. 2018b) | |||
| Vitis vinifera | Abiotic stress tolerance | Up | √ | (Chen et al. 2022) | |||
| miR319 | TCPs | Cucumis sativus | Leaf development; biosynthesis of JA | Up | √ | (Li et al. 2016) | |
| Gossypium hirsutum | Leaf development; biosynthesis of JA | Down | √ | (Wang et al. 2016) | |||
| Solanum habrochaites | Leaf development; biosynthesis of JA | Up | √ | (Shi et al. 2019) | |||
| miR390 | ARF | Populus spp. | Auxin signaling | Up | √ | (Lu et al. 2008) | |
| Solanum lycopersicum | Auxin signaling | Down | √ | (Cao et al. 2014) | |||
| miR393 | TIRs, AFBs | Arabidopsis thaliana | Disease resistance; flowering timing | Up | √ | (Sunkar and Zhu 2004) | |
| Prunus persica | Disease resistance; flowering timing | Down | √ | (Barakat et al. 2012) | |||
| Solanum lycopersicum | Disease resistance; flowering timing | Up | √ | (Zhou et al. 2020) | |||
| Oncorhynchus mykiss | Disease resistance; flowering timing | Up | √ | (Ma et al. 2019) | |||
| Saccharum officinarum | Ethylene signaling | Up | √ | (Yang et al. 2018) | |||
| Oryza sativa | Grain development | Down | √ | (Payne et al. 2023) | |||
| miR394 | F-box | Populus spp. | Abiotic stress response | Up | √ | (Lu et al. 2008) | |
| Oryza sativa | Abiotic stress response | Down | √ | (Barrera-Figueroa et al. 2012) | |||
| miR395 | ST | Populus tomentosa | Response to sulphate deprivation | Up | √ | (Chen et al. 2012) | |
| Apium graveolens | Response to sulphate deprivation | Down | √ | (Li et al. 2014a) | |||
| miR396 | GRF | Panicum virgatum | Leaf and cotyledon development | Up | √ | (Hivrale et al. 2015) | |
| Gossypium hirsutum | Leaf and cotyledon development | Down | √ | (Wang et al. 2016) | |||
| Populus trichocarpa | Enhances photosynthetic efficiency | Up | √ | (Zhao et al. 2021b) | |||
| miR397 | Laccases | Laminariales, Phaeophyta | Lignin biosynthesis; flowering timing | Up | √ | (Liu et al. 2015) | |
| Oryza sativa | Lignin biosynthesis; flowering timing | Down | √ | (Liu et al. 2017b) | |||
| Glycine max | Regulation of male fertility | Down | √ | (Ding et al. 2021) | |||
| miR398 | CSDs | Solanum lycopersicum | Copper homoeostasis | Up | √ | (Cao et al. 2014) | |
| Triticum aestivum | Copper homoeostasis | Down | √ | (Wang et al. 2014a) | |||
| Triticum aestivum | Oxidative stress | Up | √ | (Kumar et al. 2015) | |||
| miR399 | PS | Gossypium hirsutum | Response to phosphate starvation | Up | √ | (Wang et al. 2016) | |
| Oryza sativa | Response to phosphate starvation | Down | √ | (Liu et al. 2017b) | |||
| miR408 | Laccase; TaCLP | Apium graveolens | Various abiotic stress responses | Up | √ | (Li et al. 2014a) | |
| Laminariales, Phaeophyta | Various abiotic stress responses | Down | √ | (Liu et al. 2015) | |||
| Helianthus annuus | Various abiotic stress responses | Up | √ | (Kouhi et al. 2020) | |||
| Oryza sativa | Various abiotic stress responses | Down | √ | (Sun et al. 2018) | |||
| miR528 | Peroxidas, MYB | Oryza sativa | Elimination of ROS | Up | √ | (Liu et al. 2017b) | |
| Oryza sativa | Increase cell viability | Up | √ | (Tang and Thompson 2019) | |||
| miR535 | SPLs | Musa × paradisiaca | FLowering | Down | √ | (Zhu et al. 2019) | |
| miR824 | HSF | Arabidopsis thaliana | Flowering transition | Down | √ | (Szaker et al. 2019) | |
| miR827 | NAD(P); SPX | Panicum virgatum | signal transduction | Up | √ | (Hivrale et al. 2015) | |
| miR5175 | Acc-likeoxidase | Laminariales, Phaeophyta | Unknown | Up | √ | (Liu et al. 2015) | |
| miR9748 | bZIP, NFP | Cucumis sativus | Signal transduction | Up | √ | (Li et al. 2022) | |
- Here:- GRFs (Growth regulation factors); ST (Sulphate transporter); PS (Phosphate transporter); HSF (Heat shock factor); CSD ( Cold shock domain); ARF (Auxin response factor); AGO (Argonaute); SPL (SQUAMOSA-promoter binding protein-like); DCL (Dicer-like); MYB (myeloblastosis); HD-Zip (Homeodomain-leucine zipper), TCP (TEOSINTE BRANCHED 1, CYCLOIDEA, PCF1); NAC <(NAM (no apical meristem, Petunia), ATAF1–2 (Arabidopsis thaliana activating factor), and CUC2 (cup-shaped cotyledon, Arabidopsis)>; AP2 (APETALA2); ST (Sulphate transporter); PS (Phosphate transporter); HSF (Heat shock transcription factor); NFYA (Nuclear Factor Y-sub unit A); TIR (TRANSPORT INHIBITOR RESPONSE); AFB (AUXIN-SIGNALING F-BOX).
4.2 miRNAs modulate HSF/HSP/CBF genes
All living things have HSPs induced by HSF activation (Fragkostefanakis et al. 2015). Many miRNAs target and activate HSF and HSP genes (Yan et al. 2012), therefore, heat tolerance may be provided through HSF and HSP synergy (Liu et al. 2020b). For example, Arabidopsis seedlings that undergo miR156 overexpression exhibit increased levels of HSP17.6A, HSFA2, and HSP22.0 (Stief et al. 2014; Ding et al. 2020). The expression of HSPs and HSFs also experiences elevation in heat-tolerant mutant plants csd1, csd2, and ccs; however, the converse is observed in heat-sensitive plants expressing miR398 (Guan et al. 2013). Amid heat stress, HTT1, which is regulated by TAS-associated small RNA, directly interacts with HSP70 and HSP40 (Li et al. 2014b). miR166e-3p target gene SGT1 is postulated to engage directly with HSP90 and HSP70, governing O. sativa flowering's response to heat stress (Liu et al. 2017b) (Fig. 2). These findings underscore the significance of HSFs and HSPs in the context of miRNA-mediated heat stress. HSFs have been demonstrated to directly bind to miRNA or target gene promoter regions to stimulate the expression (Liu et al. 2017b). miRNAs regulate heat tolerance in plants by recruiting HSFs and HSPs. It also exhibited an HSFA7b feedback loop when miR398 was expressed (Guan et al. 2013). Plants' CBF/DREB1-dependent response mechanism mediates cold tolerance and acclimatization. In Arabidopsis, miR394 and miR397 were previously linked to CBF activation and cold-responsive genes (Tiwari et al., 2020). Tolerance to cold is promoted by miR319/DREB1 module in O. sativa (Wang et al. 2014b). We think that changing the CBF-dependent mechanism in plants may improve miRNA-mediated cold tolerance.
4.3 miRNAs reduce ROS damage
Under normal biological circumstances, plants maintain an equilibrium between ROS generation and elimination. However, under harsh conditions such as drought, cold, salinity, and heat stress, there is an excessive production of ROS that can lead to oxidative damage in plant cells, affecting membrane lipids, proteins, and nucleic acids (Islam et al. 2022a; Zhang et al. 2022). A very efficient antioxidant system that comprises enzyme scavengers to remove ROS in temperature-stressed plants has evolved (Seleiman et al. 2020; Islam et al. 2022d). SOD is an enzyme that converts superoxide radicals into less harmful hydrogen peroxide (H2O2). The cleavage of SOD genes (CSD1 and CSD2) by miR398 is well-established in response to oxidative stress. Arabidopsis plants possessing the miR398-resistant CSD2 gene exhibit enhanced resistance to ultraviolet radiation, heavy metals, and other oxidative stresses (Xu et al. 2014; Noman et al. 2019; Islam et al. 2022b). miR398-mediated thermotolerance is linked to alterations in ROS levels during heat stress (Liu et al. 2017a). The influence of ozone, an abiotic inducer of ROS, results in the differential regulation of 12 miRNA families in Arabidopsis, including miR156, miR159, miR160, and miR166 (Liu et al. 2017a). Many plant species use conserved miRNAs to respond to cold or heat stress (Table 1). The standard regulatory module is miRNA-directed gene silencing for both oxidative and thermal stress responses. Conversely, the ability to withstand high temperatures through miRNA regulation may rely on the plant's defence mechanisms against oxidative stress. Studies suggest that exposure to heat stress induces changes in genes associated with ROS elimination and maintaining ROS balance in O. sativa (Mittal et al. 2012). These findings suggest that miRNAs aid plants in withstanding temperature stress and mitigating ROS-related damage.
4.4 miRNAs regulate phytohormone signalling pathways
Scientific investigations have demonstrated that plant hormones are pivotal in governing plants' reactions to cold stress. Various plant species, including wheat, utilize plant hormones like Abscisic acid (ABA), auxin, Gibberellic acid (GA), and ethylene to convey heat stress signals (Ritonga and Chen 2020). Research on wheat has revealed that diverse genes related to phytohormone metabolism or signalling display differential expression patterns during heat stress across cultivars with varying degrees of heat tolerance (Qin et al. 2008). These findings underline the critical involvement of hormone pathways signalling in plant heat tolerance and acclimatization. Temperature-sensitive miRNAs also participate in multiple hormone pathways, implying interplay between transcripts under such conditions (Megha et al. 2018). For instance, in Arabidopsis seedlings experiencing stress, ABA causes miR159 to accumulate, resulting in the degradation of MYB33 and MYB101 transcripts through a homeostatic mechanism (Reyes and Chua 2007). Additionally, ABA influences many other miRNAs, including the downregulation of miR167, miR169, and miR398 and the upregulation of miR160, miR319, and miR393 (Li et al. 2017). It has been established that miR160, miR167, and miR393 directly target components of auxin signalling (ARFs, TIR1, and AFBs) (Bai et al. 2017; De-la-Peña et al. 2017; Hou et al. 2020). Furthermore, miR396's enhancement of cold tolerance in P. trifoliata has been linked to its targeting of the ethylene synthase gene ACO (Zhang et al. 2016). Similarly, miR5175 has been confirmed to target another ethylene-producing enzyme, ACC-like oxidase, with its mRNA expression decreasing in barley plants exposed to 24 hours of heat stress (Ijaz et al. 2020). Mutants of ethylene signalling components, such as ethylene resistant 1 (etr1) and ethylene insensitive 2 (ei2), have shown susceptibility to heat stress (Singh et al. 2021). miR5175 reduces an enzyme that is similar to ACC-like oxidase, making barley plants more vulnerable to heat stress (Feng et al. 2017). This underscores the potential association between miRNA-mediated responses to temperature stress and hormone signalling, highlighting the influence of climate-sensitive miRNAs and genes involved in hormone signalling on plants' capacity to endure temperature stress.
5 CONCLUSIONS AND PERSPECTIVES
In the past decade, many genomic investigations have pinpointed diverse miRNAs influenced by various environmental stressors, encompassing temperature fluctuations within plant systems. This observation underscores the pivotal involvement of miRNAs in reacting to extreme temperature shifts. However, the intricacy of miRNA reactions to temperature stress hinges on variables such as plant species, genotype, tissue type, and developmental phase. While plant retorts to temperature stress exhibit considerable diversity, only a fraction of temperature-responsive miRNAs have undergone validation. The imperative to verify the functional roles of temperature stress-responsive miRNAs is pressing. Likewise, a dearth of knowledge persists concerning the miRNA-mediated temperature stress regulatory networks and the interconnected pathways. Gaining a comprehensive comprehension of miRNA functions is a prerequisite to harnessing their potential benefits for plants. With the rapid strides in genome sequencing technology, an array of temperature stress-linked miRNAs is anticipated to gain validation, thereby illuminating their regulatory networks within plant systems in the future. The established significance of miRNAs in orchestrating plant growth, development, and reactions to diverse external environmental cues is incontrovertible. While miRNAs offer a promising target for augmenting plant development, the strategies for miRNA selection and implementation demand refinement. Given that miRNAs often target numerous genes, a single miRNA can exert multiple influences. Some target genes might exhibit pleiotropic effects, participating in numerous functions. Conversely, specific miRNA traits might yield adverse outcomes. Thus, validating the broader impacts of miRNAs beyond their intended targets is essential before harnessing them to enhance plant growth. A spectrum of methods rooted in miRNAs has been devised for genetically improving crops to elevate their agricultural and agronomic attributes. Various methodologies, including artificial miRNAs, target mimics, and miRNA-resistant targets, have been employed. Another promising tool is the CRISPR-Cas9 technology, which enables RNA-guided genome editing and targeted gene knockdown without the need for transgenic modifications. Although the utilization of CRISPR-Cas9 for editing miRNAs in plants is not extensively studied, we anticipate that continuous advancements in this technology will enhance miRNA-mediated gene regulation, ultimately bolstering crop resilience to temperature stress.
AUTHOR CONTRIBUTIONS
WI collected the data and prepared the initial draft. MA, MQ and AW performed editing and helped in the preparation of the table. WI prepared the figures. MOA revised and MMA completed the English language editing to improve the article. FZ supervised. WI and FZ approved the final version of the revised article.
ACKNOWLEDGEMENT
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through General Research Project under grant number G.R.P (244 /44).
FUNDING
Financial support for this project was generously provided by the collaborative initiative of the National Natural Science Foundation of China and the Xinjiang Uygur Autonomous Region of China (Grant No. U1903102), the National Natural Science Foundation of China (Grant No. 41977050), as well as the Ministry of Science and Technology (Grant No. QN2022045001L).
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




