The Brassica napus boron deficient inflorescence transcriptome resembles a wounding and infection response
Abstract
Oilseed rape and other crops of Brassica napus have a high demand for boron (B). Boron deficiencies result in the inhibition of root growth, and eventually premature flower abortion. Understanding the genetic mechanisms underlying flower abortion in B-limiting conditions could provide the basis to enhance B-efficiency and prevent B-deficiency-related yield losses. In this study, we assessed transcriptomic responses to B-deficiency in diverse inflorescence tissues at multiple time points of soil-grown plants that were phenotypically unaffected by B-deficiency until early flowering. Whilst transcript levels of known B transporters were higher in B-deficient samples, these remained remarkably stable as the duration of B-deficiency increased. Meanwhile, GO-term enrichment analysis indicated a growing response resembling that of a pathogen or pest attack, escalating to a huge transcriptome response in shoot heads at mid-flowering. Grouping differentially expressed genes within this tissue into MapMan functional bins indicated enrichment of genes related to wounding, jasmonic acid and WRKY transcription factors. Individual candidate genes for controlling the “flowering-without-seed-setting” phenotype from within MapMan biotic stress bins include those of the metacaspase family, which have been implicated in orchestrating programmed cell death. Overall temporal expression patterns observed here imply a dynamic response to B-deficiency, first increasing expression of B transporters before recruiting various biotic stress-related pathways to coordinate targeted cell death, likely in response to as yet unidentified B-deficiency induced damage-associated molecular patterns (DAMPs). This response indicates new pathways to target and dissect to control B-deficiency-induced flower abortion and to develop more B-efficient crops.
1 INTRODUCTION
In boron (B) limited Brassica napus plants, mature flowers often abort prior to seed-setting, which can lead to considerable yield losses (Wang et al., 2007; Broadley et al., 2012). Although B is already one of the most frequently deficient and actively managed micronutrients in modern agriculture (Wimmer et al., 2020), B limitations are likely to become increasingly common. Projected climate change is predicted to result in more frequent contrasting weather extremes, even within the vegetative period of a single crop. For instance, in Central Europe, a region typically characterized by sufficient rainfall, winters with increased precipitation rates followed by persistent drought periods in spring and summer are increasingly observed (Hänsel et al., 2019). Such a weather sequence strongly challenges the water and nutrient acquisition and subsequent yield of both winter- and spring-type crops. Under these conditions, soil-mobile macronutrients such as nitrate and sulfate, and particularly the micronutrient B, are prone to leaching during winter. Even if resupplied through targeted fertilization during a dry spring, any B present in the soil may still be unable to sufficiently reach plants due to the lack of soil water mass flow towards the roots (Broadley et al., 2012). This is especially detrimental for crop performance as corresponding growth stages often align with the highest nutrient demands of winter and spring crop types. Any B that does reach the roots may also not effectively reach the shoot during dry periods, as B uptake and translocation within plants strongly depend on water availability in the soil (Wimmer and Eichert, 2013). At a molecular level, B is known to form borate di-ester crosslinks with apiose residues of the polysaccharide rhamnogalacturonan II (RG-II; O'Neill et al., 1996, 2001, 2004; Goldbach and Wimmer, 2007; Lewis, 2020; Wimmer et al., 2020). The formation of crosslinked RG-II dimers in the pectin layer of primary cell walls is crucial for the integrity and stability of cell walls, which is, therefore, determined by B availability. Whilst this is the only widely accepted function of B in plants, confirming its essentiality in plant mineral nutrition, Lewis previously hypothesized that B is also required for regulating cell wall enzymes involved in lignification (Lewis, 1980a). It is also likely that further functions of B within plants exist that we do not yet fully understand (Wimmer et al., 2020).
Oilseed rape and other crucifers within the Brassica genus have the highest demand for B among crop species (Gupta, 1980; Sharma et al., 2014). Brassica napus is an allopolyploid formed by ancestral hybridization between ancestors of B. rapa (A Genome) and B. oleracea (C Genome; Chalhoub et al., 2014). Oilseed rape is intensively cultivated in several regions of the world and has become one of the major oil/protein crops used for human and animal consumption, industrial products, and as a biofuel source (Snowdon et al., 2007). In Europe alone, annual oilseed rape production consistently exceeds 20 million tons (FAO, 2023). Oilseed rape is highly sensitive to B-deprivation, and deficiency symptoms resulting in yield losses occur frequently. Boron deficiency causes immediate inhibition of primary and lateral root growth with irreversible anatomical changes. Most detrimental for yield, oilseed-rape exhibits a “flowering-without-seed-setting” phenotype when B-deficiency occurs during reproductive growth (Wang et al., 2007). Mature flowers may appear phenotypically normal but remain sterile, or flower bud development may be arrested for yet unknown molecular reasons. Boron-deficiency-related yield and quality losses in oilseed rape mainly occur in countries of Northern Europe, Canada, and China (Shorrocks, 1997). Brassica napus accessions have B requirements higher than 0.5 mg B kg−1 soil, which exceeds bioavailable concentrations in many agricultural sites (Shorrocks, 1997). In China, soils in more than 33.3 million hectares of land where crops are planted have B concentrations below this threshold (Xu et al., 2001), representing over 25% of cropland in China (FAO, 2023). Systematic breeding approaches aimed at developing B-efficient or coupled water-efficient and B-efficient crop varieties would represent a sustainable strategy to ensure yield stability in the context of a changing climate.
Identification of mechanisms and underlying genes which increase B efficiency has proven difficult despite dedicated efforts since the 1990s (Xue et al., 1998; Stangoulis et al., 2001; Xu et al., 2001, 2002; Yang et al., 2013; Zhang et al., 2014). More recently, a gene encoding a root-localized B uptake channel belonging to the Nodulin26-like intrinsic protein (NIP) aquaporin subfamily was identified in a QTL fine mapping analysis and found to significantly contribute to B efficiency in the Asian semi-winter type Qingyou 10 (Hua et al., 2016). Multiple members of the NIP subfamily play a role in plant B uptake and translocation (Takano et al., 2006; Tanaka et al., 2008; Pommerrenig et al., 2015). Additional B transporter proteins within the BOR transport family are also important for B transport and distribution within plants (Miwa and Fujiwara, 2010; Onuh et al., 2021). Transcript abundance and protein accumulation of individual NIPs and BORs were different between high and low B conditions in many plant species, including Arabidopsis thaliana (Takano et al., 2005; Tanaka et al., 2008; Tanaka et al., 2011; Kobayashi et al., 2018) and Oryza sativa (Hanaoka et al., 2014). Various NIPs and BORs were also found to be differentially expressed in B. napus under B-deficient conditions in a highly tissue-specific manner (Yuan et al., 2017; Diehn et al., 2019). However, NIP and BOR expression assays carried out to date have largely focused on vegetative tissue, with only limited information available on the effects of B-deficiency on NIP and BOR expression levels in inflorescence tissues. This is consistent with wider B-efficiency research, which to date has mainly focused on the characterization of mechanisms regulating root-localized B uptake and translocation and determining B-availability-dependent root developmental processes (Peng et al., 2012; Hua et al., 2016; Kobayashi et al., 2018; He et al., 2021a,b). Despite their obvious economic value, studies focusing on the elucidation of genetic, molecular or physiological mechanisms underlying the “flowering-without-seed-setting” phenotype or aiming to increase the fertility of the highly B-deficiency-sensitive inflorescences of B. napus under B-limiting growth conditions are missing or in their infancy (Zhang et al., 2017). However, such knowledge would provide the basis to potentially enhance B efficiency in oilseed rape and prevent B-deficiency-related yield losses under recurrent or sustained B-deficient growth conditions.
Wider transcriptomic responses to B-deficiency have been previously analyzed in various species. In citrus, a very broad change profile was observed in response to low B conditions, including differential expression of genes involved in protein and nucleic acid metabolism, carbohydrate and energy metabolism, and stress and defence responses (Lu et al., 2015). In A. thaliana, a transcriptome response of genes prominently linked to jasmonic acid has been observed in whole plants (Peng et al., 2012), and differential gene expression resembling a pathogen-induced response has been observed in roots (Kobayashi et al., 2018). The latter forms an interesting observation, especially given that in 1980, Lewis suggested that in B-deficient plants, effects seen on pollen tube formation during germination form a very close analogue to those seen during an infection process (Lewis, 1980b). For spruce, it was indeed confirmed that B-deficiency has a negative effect on pollen tube growth and germination (Wang et al., 2003). Considering that the inflorescence contains very fast developing and highly B-demanding tissues, it appears a reasonable hypothesis that B-deficiency causes developmental delays in inflorescence tissues due to insufficient capacity for B-dependent cell wall formation. Alternatively, B-deficiency may induce stress responses in some tissues, which instead leads to a reduction in inflorescence development even when sufficient B would be present to continue development to some degree (Kobayashi et al., 2018; Peng et al., 2012).
Here, we report on the exploitation of a soil-based growth medium carefully titrated to induce B-deficiency, specifically at the onset of flowering and employ this to analyze the spatiotemporal transcriptome of multiple inflorescence tissues under B-deficient and B-sufficient conditions.
We hypothesized that the generation of a carefully controlled transcriptomics approach targeting B-deficient inflorescence tissues would allow us to gain insights into the molecular mechanisms controlling the early termination of inflorescence tissues and, therefore, the “flowering-without-seed-setting” phenotype and other costly symptoms of B-deficiency in B. napus. Precise analysis of transcriptomic changes in B-deficient inflorescence tissues on a plant that was phenotypically not impacted by B-deficiency until the onset of flowering represents a unique and highly effective setup for analyzing the genetic effects of B-deficiency in a real-world relevant scenario.
2 MATERIALS AND METHODS
2.1 Plant material, cultivation and harvest
Brassica napus cv. Darmor-PBY018 (Schmutzer et al., 2015) plants were cultivated in the glasshouse in a soil substrate (white-peat volcanic clay mixture) cultivation system optimized to generate defined and repeatable B-deficient and B-sufficient growth conditions. Detailed information about soil substrate preparation, growth conditions, plant watering and fertilization regimes, and tissue harvest procedures is provided in the Supporting Information – Methods S1. Plant developmental stages were tracked against the “Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie” (BBCH) growth scale (Lancashire et al., 1991).
2.2 Library preparation, RNA sequencing and in Silico transcriptome analysis
Total RNA was extracted from plant inflorescence and vegetative tissues using NucleoSpin RNA Plant Kits and DNase I treatment according to the manufacturer's instructions (Macherey-Nagel). Library preparation (Illumina TruSeq RNA Sample Prep Kit v2) and sequencing by synthesis using the Illumina HiSeq2500 device (Illumina) involved standard protocols from the manufacturer (Illumina, Inc.). Additional experimental information, workflow specifications and procedures for the library preparation and sequencing are described in detail in the Supporting Information - Methods. Raw data from this study are deposited in the European Nucleotide Archive (ENA) under the study accession number PRJEB48627. Software packages and procedures employed in RNA mapping and in silico transcriptome analysis (including analyzing differential gene expression, kmean clusters, and MapMan functional bin and GO-term enrichment) are described in detail in the Supporting Information - Methods S1.
2.3 HR-ICP-MS analysis
Sample digestion and element quantifications were measured as described in Eggert and von Wirén (2013). More detailed information is provided in the Supporting Information - Methods S1.
3 RESULTS
3.1 Inducing B-deficiency specifically during flower development in Brassica napus in a soil-substrate-based growth system
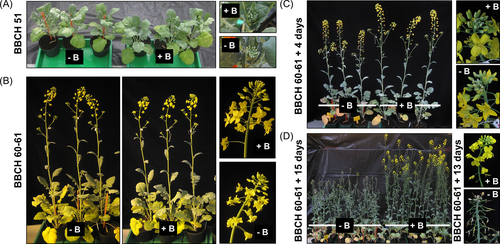
We previously developed a soil-substrate-based growth system using a peat-based ‘Fruhstorfer Nullerde’ with undetectable B concentrations, which enables experimentation with defined and repeatable B-deficiency conditions (Pommerrenig et al., 2018; Diehn et al., 2019). In this study, we utilized this growth set-up to mimic a spring drought-provoked B-deficiency-induced “flowering-without-seed-setting” phenotype in the European winter-type B. napus cv. Darmor-PBY018. The substrate B concentrations were carefully calibrated (see Supporting Information - Methods S1) to allow plants to grow phenotypically identical under B-sufficient and B-deficient conditions up to and including growth stage BBCH 51 (onset of flowering; Figure 1A). The first flowers (first 10–20) of plants grown under B-deficient conditions developed visually normally without any apparent phenotypic B-deficiency defects (Figure 1B). Shortly thereafter (especially from BBCH 60–61 + 4 days; Figure 1C), increasing numbers of abnormal flowers developed on B deficient compared to B sufficient plants, and typical floral B-deficiency symptoms such as exposed stigmata, malformed stamen as well as dried, shrivelled, and aborted floral buds were observed on B-deficient plants, first on the main and then on side racemes. Finally, B-deficient plants developed the “flowering-without-seed-setting” phenotype (Figure 1D), whilst no B-deficiency symptoms such as cracked stems or malformed leaves were yet visually apparent on vegetative plant organs, despite an overall reduction in plant height. This set-up, therefore, allowed analysis of molecular and physiological B-deficiency responses in a developing oilseed-rape inflorescence suffering from B-deficiency on a plant on which other tissues were not phenotypically affected by B-deficiency. Plants which were grown for comparison on B-sufficient conditions developed typically and without any B-deficiency phenotypes (Figure 1A-D).
3.2 Plant transcript patterns and response reactions are triggered by B-deficiency and are not biased by other nutrient disorders
To determine the nutritional status and exclude co-limitations of plants grown under B-deficient and B-sufficient growth conditions, we performed tissue-specific mineral analyses five days after BBCH 60–61. Tissues investigated were bracts, closed flower buds, mature flowers, pedicels of flower buds, pedicels of mature flowers, stems and true leaves (Figure 2). Under B-sufficient conditions, tissue B concentrations of individual samples ranged 6.78-fold from 17.04 mg kg−1 in pedicels of mature flowers, to 115.59 mg kg−1 in bracts (Appendix S1 Datasheet S1). In all tissues except pedicels of mature flowers, B concentrations were statistically lower under B-deficient growth conditions (Two-way ANOVA with post-hoc Tukey tests; total DF = 107; interaction between tissue and B treatment DF = 6, F = 27.55, p < 0.001), ranging 3.82-fold from 1.42 mg kg−1 in stems to 5.44 mg kg−1 in pedicels of flower buds. These concentrations are typical for severely B-deficient oilseed-rape plants showing B-deficiency symptoms (Dell & Huang, 1997; Asad et al.,1997), and are well below shoot B thresholds considered sufficient for healthy growth of between 25 and 32 mg kg−1 in shoots (Rashid et al., 1994; Dinh et al., 2022). On average, across all tissues, B concentrations were 92.38% lower under B-deficient conditions than under B-sufficient conditions. Interestingly, despite below-critical B concentrations across all tissues analysed, no symptoms of B-deficiency were visually apparent in vegetative tissues, in contrast to the severe effects observed in later stages in inflorescence tissues (Figure 1C,D). This indicates a potentially sudden high requirement for B in inflorescence tissues, which could not be met by the very low B concentrations available to B-deficient plants.
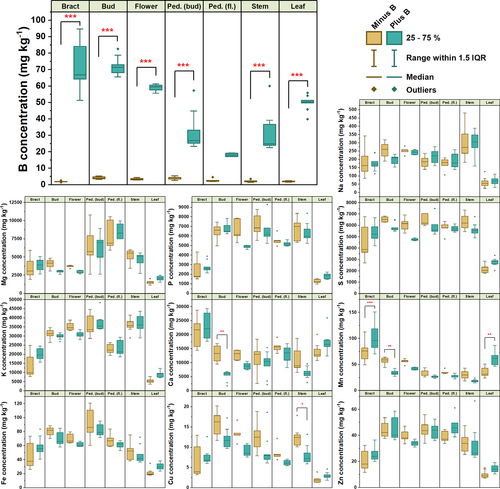
Concentrations of other elements were mostly unaffected by B treatment. Exceptions to this were elevated Ca (q value = 6.38, p = 0.00144) and Mn (q value = 5.68, p = 0.00826) concentrations in buds, and lower Mn concentrations in bracts (q value = 7.64, p < 0.001) and true leaves (q value = 6.37, p = 0.00147) under B-deficient conditions (Figure 2). Stem Cu concentrations were also higher under B-deficient conditions (q value = 5.06, p = 0.03362). However, plant tissue concentrations of all elements, except B, in both B conditions were generally comfortably within ranges suitable for healthy development (Rengel et al., 2023). Overall, these data demonstrate that the B-deficient soil substrate results in a highly B-specific effect on the plant ionome, thus signifying that the observed growth phenotypes are B-deficiency induced.
3.3 Single NIP and BOR transport family members react to B-deficiency, but overall gene family transcript abundances are mostly stable
Proteins of the NIP and BOR families are highly important for B uptake and translocation in plants. Through RNA-sequencing of inflorescence tissues of plants grown in B-deficient and B-sufficient conditions, we characterized the expression of 33 NIP and 20 BOR full-length genes within the B. napus Darmor-bzh v10 genome after removal of heavily truncated or fragmented homologs presumed to be non-functional transporters. These were identified based on homology with the nine NIPs (Johanson et al., 2001) and seven BORs (Takano et al., 2008) previously described in A. thaliana, and scrutiny of the correspondence between B. napus Darmor-bzh v5 and v10 genome annotations (Rousseau-Gueutin et al., 2020). We have harmonized the naming system of both the NIP and BOR gene families for this study, such that individual members are easily identifiable by their position in the genome. (Appendix S1 Datasheet S2).
Considering all NIP homologs together, within specific tissues and developmental stages, there was only limited overall expression changes between B-deficient and B-sufficient growth conditions. However, increased overall NIP expression was observed within B-deficient green buds and mature flowers at growth stages BBCH 60–61 and 60–61 plus 4 days compared to B-sufficient samples (Figure 3A). On an individual gene basis, the NIP5;1 homologs showed the most obvious B response across tissues and developmental stage: expression of four out of six NIP5;1 homologs, BnaA02.NIP5;1a, BnaA03.NIP5;1b, BnaC02.NIP5;1a and BnaC03.NIP5;1b, was higher in all tissues under B-deficient than B-sufficient conditions, ranging from a 1.47- to 22.06-fold increase on a TPM-basis (Figure 3B; Figure S6; Appendix S1 Datasheet S3). Differential expression of NIP1;2 homologs were also observed in shoot heads at growth stage BBCH 60–61 plus 4 days: expression was between 1.85- and 9.55-fold higher under B-deficient than B-sufficient conditions across all four homologs. Expression of NIP3;1 and NIP7;1 homologs, which are permeable to B (Diehn et al., 2019), was restricted only to shoot heads at all time-points sampled.
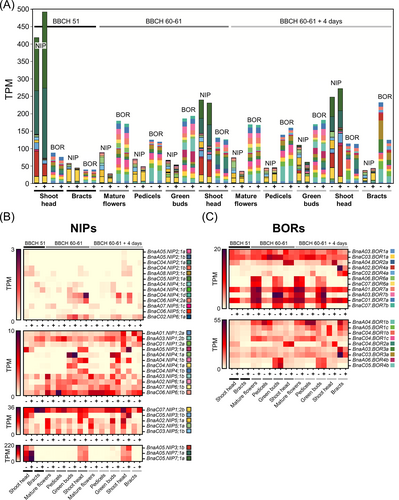
Overall transcript levels of BORs were also remarkably stable, with the exception of bracts at growth stage BBCH 60–61 plus 4 days in which expression of all BORs combined was around 86% higher under B-deficient than B-sufficient conditions (Figure 3A). In addition, the BOR1 homolog BnaA04.BOR1b was expressed 2.15-fold higher in bracts at growth stage BBCH 60–61 plus 4 days in B-deficient conditions than B-sufficient conditions. In shoot heads at the same growth stage, BnaA05.BOR1c and BnaC04.BOR1b were also 9.08- and 6.05-fold upregulated, respectively, under B-deficient conditions. Both BOR2 homologs showed no obvious response to B across tissues until the latest analyzed growth stage, where expression in green buds and shoot heads was between 2.83 and 5.19-fold higher in B-sufficient than B-deficient growth conditions. Both BOR3 homologs had around 2.65-fold increased expression in bracts at growth stage BBCH 60–61 plus 4 days under B-deficient conditions. Expression of two BOR4 homologs, BnaA02.BOR4a and BnaC02.BOR4a, was highly localized to bracts at growth stage BBCH 60–61 plus 4 days, the former of which was expressed 4.03-fold higher under B-deficient conditions. (Figure 3C; Appendix S1 Datasheet S3). In summary, whilst no NIP or BOR family-wide response to low B conditions was observed, individual genes of these families clearly form part of the early response to B-deficiency across various inflorescence tissues, including already at BBCH 51 at which point no visual symptoms of B-deficiency were apparent. The expression of some of these transporters, for example, the NIP5;1 orthologues, remain elevated in response to B-deficiency at the later analysed time points (Figure 3B; Figure S6). However, the lack of a clear trend in expression with B-deficiency severity suggests other mechanisms contribute to later B-deficiency responses.
3.4 Global spatiotemporal transcriptome responses to B-deficiency in B. napus inflorescences
We analyzed the RNA-seq derived transcriptome datasets by various grouping and enrichment methods in order to define the broader B. napus reaction to B-deficiency, to cross-confirm the results obtained, and to prevent ontology-based bias. We compared the B-deficient and B-sufficient samples within each tissue type, and further defined response relationships by determining intersects, defined as groups of differentially expressed genes (DEGs) shared between B-deficient vs. B-sufficient comparisons in different tissues and time points (Figure 4A). We generated k-means clusters, whereby DEGs that showed similar spatiotemporal expression patterns across the analyzed samples were grouped together in order to analyze gene sets which respond distinctly to different stimuli (Figure 4B-E and Figure S2). All gene lists generated as described above were characterized in bulk by gene ontology (GO) enrichment analysis (Figure 4F; Appendix S1 Datasheet S4-S6). In addition, we isolated DEGs between B-deficient and B-sufficient shoot heads at all sampling time points and analyzed them for their presence in MapMan stress-related gene groupings based on the Mercator ontology (Appendix S1 Datasheet S7).
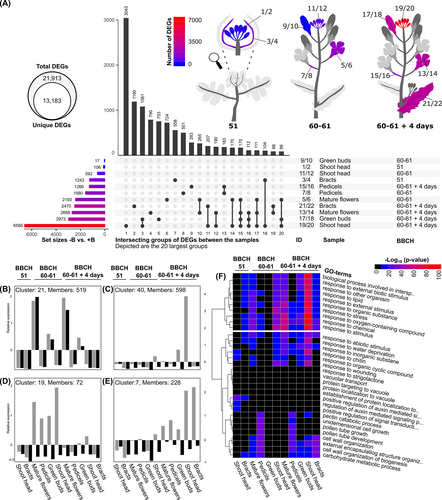
Across all tissue samples at all time points sampled, we detected 21,913 events of differential gene expression between B-sufficient and B-deficient conditions (multiple hypothesis corrected p-value/q-value <0.05; Appendix S1 Datasheet S8). The events were attributed to 13,183 unique genes, corresponding to 12.2% of the genes predicted within the B. napus Darmor-bzh V10 genome (Figure 4A). Overall, 2,033 DEGs were shared between at least three of these contrasts, indicating that the responses to B-deficiency were tissue and time specific. At the growth stage BBCH 51, 106 DEGs were identified in the shoot head between B-deficient and B-sufficient growth conditions, whereas over 1,000 were detected at the same growth stage within the bracts surrounding the shoot head (Appendix S1 Datasheet S8). This suggests that at around the onset of flowering, the plant had started to respond to B-deficiency, but the inflorescence transcriptome had not yet been dramatically affected. A similar pattern was evident at BBCH 60–61, where we observed 592 and 17 DEGs in the shoot head and green buds, respectively (Figure 4A). However, at the same growth stages, we detected 1,580 and 2,193 DEGs in pedicels of open flowers and open flowers, respectively, between B-deficient and B-sufficient growth conditions. At BBCH 60–61 plus 4 days, the opposite pattern was apparent, with the most DEGs found in the shoot head and green buds, followed by mature flowers and bracts. Over six thousand DEGs were detected in the shoot head at growth stage BBCH 60–61 plus 4 days between B-deficient and B-sufficient conditions, double that of the second most affected sample and over 3,000 of which were unique to this specific sample (Figure 4A). The overall patterns indicate that inflorescence tissues show particularly strong changes in transcript abundances for B-deficiency, which escalate as B-deficiency becomes more acute in developing tissues.
We compared the changes induced by B-deficiency with changes induced by development. To this end, we made eight additional comparisons between tissue developmental stages, treating B-sufficient and B-deficient growth conditions as two independent experiments (Figure S1, Appendix S1 Datasheet S9). Development of the plant had a much bigger impact on the number of DEGs than exogenous B concentration did, with a total of 44,174 differential expression events across all eight additional comparisons in B-sufficient and 36,660 differential expression events in B-deficient conditions during development. These were attributed to 31,889 unique genes, as compared to the 13,183 unique DEGs identified between B-deficient and B-sufficient conditions (Figure 4A; Appendix S1 Datasheet S8-S9). With the exception of the contrast between shoot head at BBCH 60–61 and green buds at BBCH 60–61 plus 4 days, all additional comparisons revealed a higher number of DEGs for the B-sufficient samples than the B-deficient samples (Figure S1). The larger numbers of DEGs between developmental stages within the B-sufficient samples suggest a slowed or perturbed development of the B-deficient samples.
3.5 The B. napus B-deficient inflorescence transcriptome is enriched for defence response related genes
Analysis of the most enriched GO-terms across B-deficient to B-sufficient comparisons revealed a mixed picture. In general, if a term was among the top five most enriched in any given contrast, it had a high probability of being also significantly enriched in other contrasts (Figure 4F). Terms related to known functions of B in plants, including “pectin catabolic process”, “pollen tube growth”, and “cell wall organization” (GO:0045490, GO:0009860, GO:0071555) were enriched, mostly in pedicels, but terms related to pectin and cell wall also in other inflorescence tissues. However, additional sets of genes related to biotic stress responses were also highly enriched across tissues, particularly at growth stage BBCH 60–61 + 4 days and in the shoot head (Figure 4F). Many GO-terms related to defence/wounding were enriched across multiple sampling time-points. For example, terms including “response to chitin” and “response to other organism”, and “regulation of auxin mediated signaling” terms were already enriched in bract or shoot head samples at growth stage BBCH 51. The former is a well-known trigger for the pathogen-associated molecular pattern (PAMP) defense response pathway (Bacete et al., 2018). Further enriched GO-terms that can be directly related to defence against pathogens are “response to wounding”, “multi-organism process”, and “response to external biotic stimulus” (Appendix S1 Datasheet S4). Meanwhile, within developmental contrasts, the B-sufficient contrasts were dominated by normal developmental GO-terms. Meanwhile the B-deficient developmental contrasts also included stress and pathogen response related terms, especially for contrasts with the largest temporal separation. This indicated a growing defense-type response with time despite the fact that no biotic disease symptoms were observed in any plants in the experiment (Appendix S1 Datasheet S11).
In order to systematically evaluate the most informative transcriptional changes across tissues and time points, the Upset plot (Figure 4A) was used to identify the gene sets representing the largest unique and shared transcriptome changes. Analysis of GO-term enrichment revealed that the shoot head samples from BBCH 60–61 plus 4 days (intersect 1) possessed the greatest number of unique DEGs and that these were also enriched for biotic stress-related GO-terms, including “response to chitin”, “defense response”, and “immune system process” (GO:0010200, GO:0006952, GO:0002376; Appendix S1 Datasheet S5). Related terms were also observed in intersects 3 and 4, which also corresponded to inflorescence tissues at growth stage BBCH 60–61 + 4 days. In contrast, the unique DEGs between B-deficient and B-sufficient bract (leaf) samples at the same time point (intersect 2) were enriched with GO-terms related to carbohydrate metabolism and the cell wall, for example, “carbohydrate metabolic process”, “cell wall” (GO:0005975, GO:0005618). Similar terms were found in intersect 5, which is specific for green buds at BBCH 60–61 + 4 days, alongside terms more similar to those identified in intersects 1, 3 and 4, including response to chitin and wounding. Significant GO-term enrichment was also observed for intersects 6–13. These intersects comprised samples mostly from earlier time points or gene sets shared between samples and comprised enriched GO-terms more related to known functions of B in plants, including terms related to cell wall maintenance, pollen tube extension and pectin metabolism (Appendix S1 Datasheet S5). These results suggest that the observed defence/wounding type response to B-deficiency was mostly localized to inflorescence tissues at BBCH 60–61 + 4 days.
In the cluster analysis, clusters can be separated between mostly developmental (time- or tissue-specific) and B-deficiency-specific by looking at the patterns of relative expression. If the genes in a cluster follow a similar pattern between B-deficient and B-sufficient conditions, it can be assumed that this is most likely developmental or tissue specific. If the pattern is dissimilar between B-deficient and B-sufficient conditions, it can be assumed that this is caused by B-deficiency. To discern between DEGs with B-deficiency-dependent transcript abundance patterns and those that were plant development dependent that became altered in expression due to the B-deficient state, k-means clusters were calculated (Figure S2; Appendix S1 Datasheet S10). In total, 40 k-means clusters were formed, examples of which can be found in Figure 4B-E. These clusters were analyzed for enrichment of GO-terms (Appendix S1 Datasheet S6). Cluster 21 (Figure 4B) represents an example of a gene set involved in normal B. napus development since expression was relatively consistent between B-deficient and B-sufficient samples across all sampling points but was higher specifically in mature flowers and green buds. GO-term analysis revealed that this cluster was enriched for such GO-terms as “developmental cell growth” (GO:0048588). Cluster 40 (Figure 4C), on the other hand, is an example of a cluster that was highly specific to the shoot head at BBCH 60–61 plus 4 days under B-deficient conditions. This cluster was enriched for “response to chitin” (GO:0010200), “defense response” (GO:0006952), “immune response” (GO:0006955) and other response to biotic stress terms. Clusters 19 (Figure 4D) and 7 (Figure 4E) form examples of DEGs specific to BBCH 60–61 and 60–61 plus 4 days, respectively, in each case upregulated under B-deficient conditions. In cluster 19, response to low fluence blue light stimulus (GO:0010244) is the only significantly enriched BP term according to our cutoffs. Meanwhile in cluster 7, GO-terms including “immune response” (GO:0006955), “defense response” (GO:0006952) and “regulation of response to biotic stimulus” (GO:0002831) were enriched, along with various terms related to cell wall organization. In total, we deemed 28 of the clusters to comprise genes that are generally B-responsive, 18 clusters developmental-specific, and 6 showing features of both, based on patterns of relative expression (Appendix S1, Datasheet S13). In general, GO-terms resembling a response to pathogen or herbivore attack were among the most enriched terms for the B-deficiency-specific clusters (observed in 9 out of 22 clusters), but not for the development-specific clusters (observed in 3 out of 12 clusters; Appendix S1 Datasheets S6, S13).
3.6 Differential expression within MapMan biotic-stress related functional bins indicates progressive defense-type response
Over 6,500 genes were differentially expressed between B-deficient and B-sufficient shoot heads at growth stage BBCH 60–61 plus 4 days, yet less than 10% of this number were differentially expressed in shoot heads at earlier stages, despite only a few days falling between sampling points (Figure 4A). To characterize changes leading to this temporal shift in expression and to build on results from the GO-term enrichment analysis, we screened significant DEGs in shoot heads at all developmental stages investigated here and isolated those which fell within MapMan bins linked to biotic stress. MapMan bins each comprise a list of genes of similar biological function or context, allowing elucidation of patterns of changes in transcript abundances based on function rather than phylogeny (Usadel et al., 2009; Schwacke et al., 2019).
At growth stage BBCH 51, 106 DEGs were detected in shoot heads, 42 of which fell within at least one MapMan biotic stress bin (Appendix S1 Datasheet S7). Whilst some bins were very highly enriched due to the overall low number of DEGs overall, most bins contained no DEGs (Figure S4). In addition, the majority of DEGs at this growth stage were downregulated under B-deficiency, in contrast to the mixed up- and down-regulation of DEGs within biotic stress bins at later growth stages. A larger number of DEGs fell within biotic stress bins at BBCH 60–61: 270 out of the 592 DEGs detected overall at this stage. Almost all bins contained a larger number of DEGs at this stage than at the earlier stage, indicating a growing defence-type response (Figure S4). For comparison, no additional B transporters of the NIP or BOR families were significantly differentially expressed at BBCH 60–61 compared to BBCH 51. Finally, of the 6,585 DEGs in shoot heads at BBCH 60–61 plus 4 days, 2,125 fell within at least one selected MapMan biotic stress bin (Figure 5). Multiple specific bins were enriched, including the “jasmonic acid” bin (4.60-fold enriched), the “WRKY” bin (3.60-fold enriched) and the “wounding” bin (3.28-fold enriched). Jasmonic acid is a positive regulator of immunity against necrotrophic pathogens and herbivory (Berens et al., 2017). It has also recently been implicated in negatively regulating growth in response to B-deficiency in A. thaliana seedlings (Huang et al., 2021a,b) and increased jasmonic acid biosynthesis under B-limitation has been reported in B. napus (Zhou et al., 2016) and other species (Chen et al., 2022). WRKY transcription factors have also been shown to play diverse roles in plant defence, involved in both detection and the response to pathogens and other biotic stresses (Sarris et al., 2015; Jiang et al., 2017). They have also been reported to respond to B-deficiency in both A. thaliana (Kasajima et al., 2010) and B. napus (Feng et al., 2020), with one WRKY transcription factor implicated in activating expression of a NIP5;1 B-transporter orthologue (Feng et al., 2020). Six additional NIPs were also differentially expressed between B-deficient and B-sufficient samples at this growth stage, as well as four BORs. However, the number of additional differentially expressed NIPs or BORs at this compared to earlier growth stages is less prevalent than the growth in pathogen or pest attack related genes. This builds on the hypothesis that the plants grown under B-deficient conditions reach a critical point at which they switch from focusing on trying to uptake as much B as possible to more of a defence-type response. This seems particularly convincing since four out of the nine differentially expressed NIPs and two out of the four differentially expressed BORs were actually downregulated under B-deficiency at BBCH 60–61 plus 4 days.
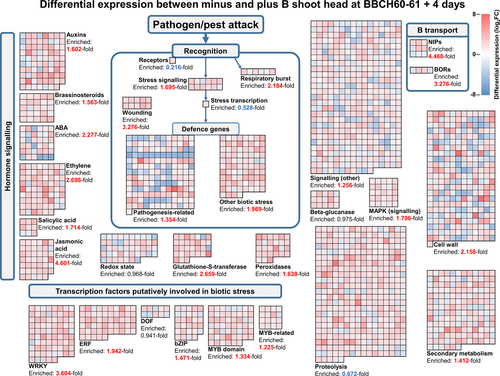
3.7 Metacaspases and asparagine-rich proteins among candidates for progressive B-deficiency symptoms leading to flowering-without-seed-setting
To identify specific candidate genes responsible for the “flowering-without-seed-setting “phenotype, we screened highly differentially expressed genes that fell within the MapMan biotic stress bins described above. Among these, several metacaspase (MC) encoding genes, which fell within the proteolysis bin, were significantly upregulated under B-deficiency in a tissue-specific manner (Figure 6). Metacaspases are a group of proteins that are conserved across plants, fungi and protozoa (Tsiatsiani et al., 2011). In A. thaliana, MC members are variously involved in orchestrating programmed cell death (PCD), some positively and others negatively. Most notably, we identified two orthologues of A. thaliana MC5, found on B. napus chromosomes A02 and C02, which were highly upregulated under B-deficiency in mature flowers at growth stages BBCH 60–61 and BBCH 60–61 plus 4 days and in green buds and shoot heads at BBCH 60–61 plus 4 days. Both of these genes were almost exclusively expressed in mature flowers under B-sufficient conditions, albeit at lower levels than those observed in B-deficient conditions. Across 30 metacaspase encoding genes found to be expressed in inflorescence tissues in B. napus, 11 were preferentially expressed in mature flowers, regardless of B status (Figure S5). These were orthologues of A. thaliana MC family members MC3, MC5, MC6 and MC9. This suggests that some metacaspases may help to regulate the normal developmental fate of B. napus flowers under nutrient-replete conditions, possibly through breaking down mature flower tissue in preparation for the generation of siliques. However, under prolonged B-deficiency, some metacaspases appear to be additionally expressed in green buds and shoot heads before mature flowers have developed. Whilst the underlying signal for upregulation of some metacaspases in these tissues is not known, it may be that their expression in immature inflorescence tissues plays a role in the observed drying out of buds before they can open under B-deficiency and that their increased expression in mature flowers leads to abortion of flowers before they can form fertile siliques. This theory requires further testing to confirm, but the metacaspases indicated here form suitable candidates for controlling the flowering-without-seed-setting phenotype of B-deficient B. napus.
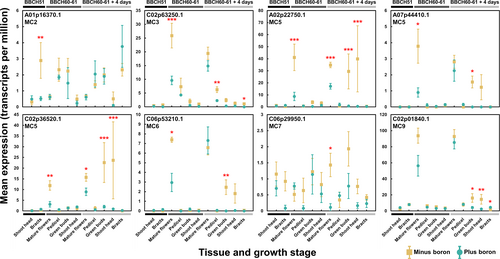
In addition to the metacaspases, four orthologues of A. thaliana NRP, encoding asparagine-rich proteins, were very highly upregulated in shoot heads under minus B, particularly at growth stage BBCH 60–61 plus 4 days (Figure S6). These genes all fell within the MapMan “wounding” bin, which was very highly enriched in shoot heads at all growth stages analyzed here (Figure 5, Figure S4). NRP was first characterized as a marker for the hypersensitive response in soybean, being strongly induced by a Pseudomonas syringae infection (Ludwig and Tenhaken, 2001). It has since additionally been implicated in the induction of PCD and senescence and the general stress response in plants more widely (Costa et al., 2008; Hoepflinger et al., 2011; Reis et al., 2016). Several other genes within the MapMan wounding bin also grew in expression with time, particularly in shoot heads, further demonstrating a growing defense-type response exhibited by B. napus plants grown under B-deficiency. This contrasts with the expression of known orthologues of the B transporters NIP5;1 and other known B transporters, which were generally already upregulated under B-deficiency from BBCH 51.
4 DISCUSSION
4.1 A growth medium carefully titrated for B concentration allows the study of B-deficient inflorescences on a phenotypically unaffected shoot
The lack of research to identify the mechanisms underlying the “flowering-without-seed-setting” phenotype or other B-deficiency related symptoms in B. napus inflorescences is mostly due to experimental obstacles. For example, B is a trace element found essentially everywhere in nature, with a very narrow range between deficient, sufficient, and toxic concentrations in plants (Brdar-Jokanović, 2020). To date, hydroponic growth systems have mostly represented the method of choice for adjusting B supply in a repeatable manner (Asad et al., 1997; Stangoulis et al., 2000; Savić et al., 2013). However, root system architecture, as well as molecular and physiological root functioning in hydroponics, do not resemble those in a soil environment. In this study, we have used a soil-based substrate containing undetectable B concentrations, carefully adjusted to induce B-deficiency at a defined developmental time point corresponding to early flowering. This has avoided the disadvantages of hydroponic systems while still avoiding secondary defects typically experienced when subjecting soil-grown plants to B limitations, which may arise due to metabolically harmful B-deficiency symptoms having formed at earlier developmental stages.
4.2 Known B transporters upregulated in a time and tissue-specific manner in response to low exogenous B
Across all members of the NIP and BOR transport families, many of which have previously been shown to transport B (Takano et al., 2006, 2008; Routray et al., 2018), only a subset showed a clear transcriptomic response to B status (Figure 3). In particular, expression of four out of the six NIP5;1 homologs identified in our dataset was higher in almost all tissues under B-deficiency compared to B-sufficient conditions (Figure S6). This is consistent with the striking B-responsiveness of NIP5;1 homologs in A. thaliana and other plant species (Takano et al., 2006; Pommerrenig et al., 2015). Across all NIPs, the NIP7;1 homologs had the highest maximum expression, although this was largely restricted to the shoot heads (Figure 3B). In A. thaliana, expression of NIP7;1 is mostly limited to anthers within a narrow temporal window, and Atnip7;1 T-DNA mutants show B-dependent sterility and defective pollen development (Routray et al., 2018). These findings, coupled with our observations, indicate a crucial role for NIP7;1 in B homeostasis during early flower development in B. napus. Interestingly, temporal expression patterns of three of the NIP3;1 homologues contrasted between B treatments: whilst expression in shoot heads increased with time in B-sufficient conditions, expression decreased with time in B-deficient conditions. We hypothesize that in conditions of sufficient available B, B is allocated to developing flowers in increasing amounts with time through increasing B importer expression. Meanwhile, under conditions of insufficient available B, we propose that B transporters are initially increased in expression to try to increase B allocation to developing flowers, which in critical conditions are later reduced in expression as plants change their strategy away from investing in flowers that would later likely terminate due to B-deficiency-induced infertility. Such observations indicate that our low B soil substrate induced an increasingly severe B-deficiency with time, as per the intended experimental design, which in turn led to a progressive transcriptome response across sampling time-points.
Amongst the BORs, the most notable expression changes were the upregulation of two BOR1 transporters in shoot heads at growth stage BBCH 60–61 plus 4 days under minus B conditions. Expression of one of these genes (here named BnaC04.BOR1c) was previously observed in flowers of B. napus, and overexpression of this gene was found to alleviate shoot B-deficiency symptoms by distributing more B from roots to shoots (Chen et al., 2018). In contrast, in the same study, the expression of the other BOR1 homolog shown here to be B-responsive in shoot heads (here named BnaA05.BOR1c) was mostly localized to root tissue. BOR1 has long been known to contribute to B homeostasis in Arabidopsis, acting as an efflux-type transporter for xylem loading (Takano et al., 2002). However, a possible role of BOR1 homologues in inflorescence B homeostasis has only recently emerged. In general, across many NIP and BOR Brassica genome homeologous pairs, developmental expression patterns followed a similar trend between A- and C-sub-genome copies (Appendix S1 Datasheet S3).
4.3 Overall B-deficient inflorescence transcriptome is a combination of B-specific response and more generic biotic stress-type responses
We detected only a limited common response to B-deficiency between tissues and time points among differentially expressed genes (DEGs). The majority of DEGs were specific to particular or small groups of different tissues (Figure 4A). However, we detected large changes representing thousands of genes within specific tissues and time points, especially in tissues forming as B-deficiency became progressively worse. In addition, whilst specific DEGs generally differed between samples, the types of response were often highly similar, as indicated by shared enriched GO-terms. The overall response appears to have comprised a limited specific B-deprivation response, developmental delays, and a more generic biotic stress-type response. The response specific to B was manifested in upregulation of multiple known B transporters of the NIP and BOR families under B-deficiency across tissues, as was apparent from relatively early in the progression of the deficiency (from BBCH 51 onwards) and discussed above (Figure 3; Figure S6). The transcriptome response of the developmental gene groups was likely due to delays in the development of plants suffering from B-deficiency compared to those grown in B sufficient conditions (Figure 4B). Whilst plants were visually at the same developmental stage between B-deficient and B-sufficient conditions when sampled for RNA extraction, slight differences in the timing and duration of specific plant processes between healthy and stressed plants were likely unavoidable.
Especially as development continued, a more generic biotic stress-type response became apparent (Figure 4C-F). GO-terms, including responses to chitin, wounding and biotic stimuli, were most notably enriched in later responses, particularly at growth stage BBCH 60–61 plus 4 days. (Appendix S1 Datasheet S4). Such GO-terms were less enriched at the other growth stages analyzed, despite only 10 days falling between BBCH 51 and BBCH 60–61 plus 4 days. In some tissues, terms related to cell wall organization and formation were enriched upon B-deficiency (Figure 4F), representing the known role of B in stabilizing the primary cell wall. Such responses to B-deficiency have been observed previously, though not before, in reproductive tissues (Lewis, 1980a,b; Peng et al., 2012; Kobayashi et al., 2018). We hypothesize that the biotic stress-type reaction was not necessarily a response specific to B-deficiency but instead caused by the physiological consequences of B-deficiency during flower formation. Boron has a major structural role in plants through the cross-linking of RG-II monomers by borate esters in the pectin fraction of primary cell walls (O'Neill et al., 2001). It is feasible that insufficient B in plant cells could lead to instability of cell walls in a manner that physiologically, structurally and biochemically resembles wounding experienced during pathogen or pest attack. In response, the plants would reasonably respond with pathogen or pest-specific differential gene expression. Given that the GO-term “response to chitin” was present in almost all the comparisons between B-deficient and B-sufficient samples, there appears to be a crossover between the detection of chitin-derived PAMPs and signals originating from B-deficiency. The latter signals could result from the accumulation of RG-II monomers (Chormova et al., 2014) or altered lignin or phenolic compounds (Lewis, 1980b) in B-deprived tissues, or from associated cell wall defects which can act as damage-associated molecular patterns (DAMPs; Tang et al., 2017). This hypothesis matches the observed enrichment of GO-terms linked to pathogen response under B-deficiency (Figure 4F, Appendix S1 Datasheet S4-S6), the enrichment of genes falling within MapMan biotic stress bins (Figure 5, Figure S4) and the incidence of pathogen or pest attack genes among DEGs in shoot heads the later growth stages analyzed. Many of the enriched GO terms observed here, most notably responses to chitin and wounding, were also highly enriched in A. thaliana roots exposed to B-deficiency for 1 hour, and PCD in root tips was also suggested to result from B-deficiency (Kobayashi et al., 2018). This suggests a conserved response across species and tissues, though the exact origin of the signal leading to cell death under B-deficiency remains to be identified.
4.4 A model of the inflorescence response to B-deficiency in B. napus
On a physiological scale, the present study suggests that the response of B. napus to B-deficiency at the flowering stage can be discriminated into two distinct levels. Level one, presented by a reduced but sustainable exogenous B concentration, begins by causing no permanent damage to vegetative or inflorescence tissues (Figure 1A-B). A temporal spike of unsustainably low exogenous B concentrations, which can cause damage to the developing inflorescence (Figure 1C), defines the second level. If this state endures, for example, longer than a flowering period, it will eventually prevent the plant from finalizing its reproductive cycle (Figure 1D). Exogenous B concentrations and timeframes required to elicit such reactions will vary between plant species and B-efficient and B-inefficient cultivars (Yuan et al., 2017; Brdar-Jokanović, 2020).
On a transcriptomic scale, the first level is characterized by the response of B-specific transporters and channels (Takano, 2006; Tanaka et al., 2011; Chen et al., 2018; Diehn et al., 2019). Under normal B concentrations, these, together with passive diffusion, control B uptake, fine-tuning of which is through tissue-specific expression changes of these transporters. This level corresponds to the early flowering stages in our study, characterized by early upregulation of NIP and BOR transporters in response to low exogenous B concentrations (Figure 3), in the absence of obvious developmental defects (Figure 1A-B). Independent of the subsequent severity and time period of deficiency, we propose that these genes always form part of the initial response. During a severe or prolonged deficiency, suitable concentrations of B within the plant cannot be maintained through increased expression of transporters. At this point, we propose that B-deficiency induced DAMPs start to accumulate, leading to the second level of the B-deficiency response. On a transcriptomic scale, this level is characterized by “defence triggering”, manifested through increased expression of gene sets typically observed with PCD in response to pathogen or pest attack, as discussed above.
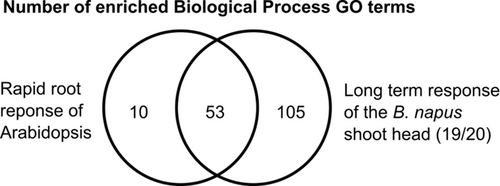
The response we observed in the second level of the B-deficiency response is supported by observations in other species (Lewis, 1980a,b; Peng et al., 2012; Kobayashi et al., 2018). In particular, most of the GO terms enriched in A. thaliana roots under short-term but severe B-deficiency overlap with those observed in severely B-deficient shoot heads here (Figure 7, Appendix S1 Datasheet S12; Kobayashi et al., 2018). In addition, in tobacco BY-2 cells, oxidative damage and cell death were observed 12 hours after exposure to B-deprivation (Koshiba et al., 2009). The authors of the latter study (Koshiba et al., 2009) argued that despite the morphological appearance of cells exposed to B-deficiency resembling those undergoing PCD, the cell death observed is unlikely to be PCD due to the absence of other typical features, and due to the fact that observed defects were rescuable by reapplying B. However, a later study by the same group (Kobayashi et al., 2018) suggested that rapid cell death in B-deficient A. thaliana roots may indeed be a PCD-type response, as indicated by shared transcriptomic responses and since they observed an accumulation of nitric oxide in B-deprived roots, which is required for execution of PCD. The overlapping GO terms observed between the study by Kobayashi et al. (2018) and our study indicate that a PCD-type response could be responsible for early flower abortion in B-deficiency B. napus. To our knowledge, this is the first study to link this molecular response to the physiological consequence of a premature abortion of inflorescence tissues.
4.5 Evolutionary origins and the arbiters of B-deficiency induced cell death
Further evolutionary investigations are required to elucidate whether the cell death response in inflorescences is an unintentional reaction to the accumulation of B-deficiency-induced DAMPs leading to activation of defense-type gene sets that limit development. Alternatively, a hypothetical PAMP-triggered immunity (PTI)-independent response pathway that uses a PTI-like gene set may have evolved specifically to respond to prolonged B-deficiencies. A selection pressure for this latter scenario may have resulted from the potential benefits of sparing resources, realized by preemptively aborting inflorescence tissues that may later prove to be unviable due to even longer-term B unavailability. Whichever scenario is correct, we hypothesize that this defence-type response is responsible for the flower abortion observed under longer-term B-deficiency, which can be phenotypically explained as a PCD event. There are likely to be multiple individual genes involved in the detection and signalling cascade leading to the “flowering-without-seed-setting” phenotype, candidates of which are indicated in our transcriptome dataset. Of particular note is the upregulation of several metacaspase family members under B-deficiency. Plant metacaspases have been shown to regulate various biological processes, including PCD in response to stress, in multiple species (reviewed in Tsiatsiani et al., 2011; Fagundes et al., 2015). Metacaspases, therefore, form an interesting candidate gene family for controlling B-deficiency-induced flower abortion. Within this family, a putative metacaspase-5 orthologue (A02p22750.1_Bnadar; Figure 6) was most prominently upregulated and might be considered a candidate arbiter of PCD in B-deprived B. napus inflorescences. Arabidopsis thaliana MC5 is considered a type II metacaspase (Tsiatsiani et al., 2011), and B. napus type II metacaspase orthologues, in particular, have been shown to accumulate in stress-treated microspores (Berenguer et al., 2020). Additional experiments will be required to confirm the role of MC5 and other metacaspases in the response to B-deficiency in B. napus. Further indications pointing towards an orchestrated death event from our dataset were: upregulation of autophagy-related genes and downregulation of ribosomal biogenesis (Minina et al., 2014), regulation of BON-interacting PCD co-suppressors and vacuolar processing enzymes (Vorster et al., 2019); Appendix S1 Datasheet S8). Overall, the data presented in this study point towards a PCD-type response to prolonged B-deficiency, triggering changes in the transcriptome that eventually lead to the costly “flowering-without-seed-setting” phenotype.
The work presented here provides new evidence that the inflorescence transcriptome response to B-deficiency in B. napus resembles that of a pathogen or pest attack. Whether this evolved as a deliberate response, utilizing already existing cell death pathways to abort affected tissues, or is a result of unintentional signalling through DAMPs produced in response to B-deficiency-induced cell wall damage, remains to be investigated. Regardless, the findings here indicate new pathways and candidate genes that might be further dissected and used to develop more B-efficient crop cultivars. In addition, this study provides a comprehensive transcriptome atlas for the B. napus response to B-deficiency, specifically during inflorescence development, without the vegetative plant body phenotypically and physiologically suffering from B-deficiency, which can be exploited for future research.
AUTHOR CONTRIBUTIONS
B.V. and T.D.A. performed research, performed bioinformatics analyses and analyzed most data. AF submitted raw data and supported bioinformatics. C.S., M.D.B., Z.L., and G.P.B. performed plant experiments and analyzed data. A.B. and G.B.P. analyzed data, designed the experiments, and supervised writing of the manuscript. All authors contributed to writing the manuscript and approved the final version.
ACKNOWLEDGEMENTS
The authors thank Sandra Driesslein and Ines Walde for their expert technical work during library construction and Illumina sequencing. The authors are thankful to Jacqueline Fuge and Benjamin Pommerrenig for their expert support during growth condition establishment and RNA work and to Yudelsy Tandron Moja for inductively coupled plasma mass spectrometry analyses (all IPK-Gatersleben, Germany). The authors are thankful to Dr. Abbadi and the Norddeutsche Pflanzenzucht Hans-Georg Lembke KG (NPZ) for the provision of Darmor-PBY018 seeds and their helpful comments and advice. This work was supported by the Emmy Noether grant 1668/1-1 and 1668/1-2 from the Deutsche Forschungsgemeinschaft (to G.P.B.). Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Author Guidelines are: Gerd Patrick Bienert (responsible for the plant experimentation) ([email protected]) and Andrea Bräutigam (responsible for the bioinformatics analyses) ([email protected]). Raw data of this study are deposited in the European Nucleotide Archive (ENA) under the study accession number: PRJEB48627.




